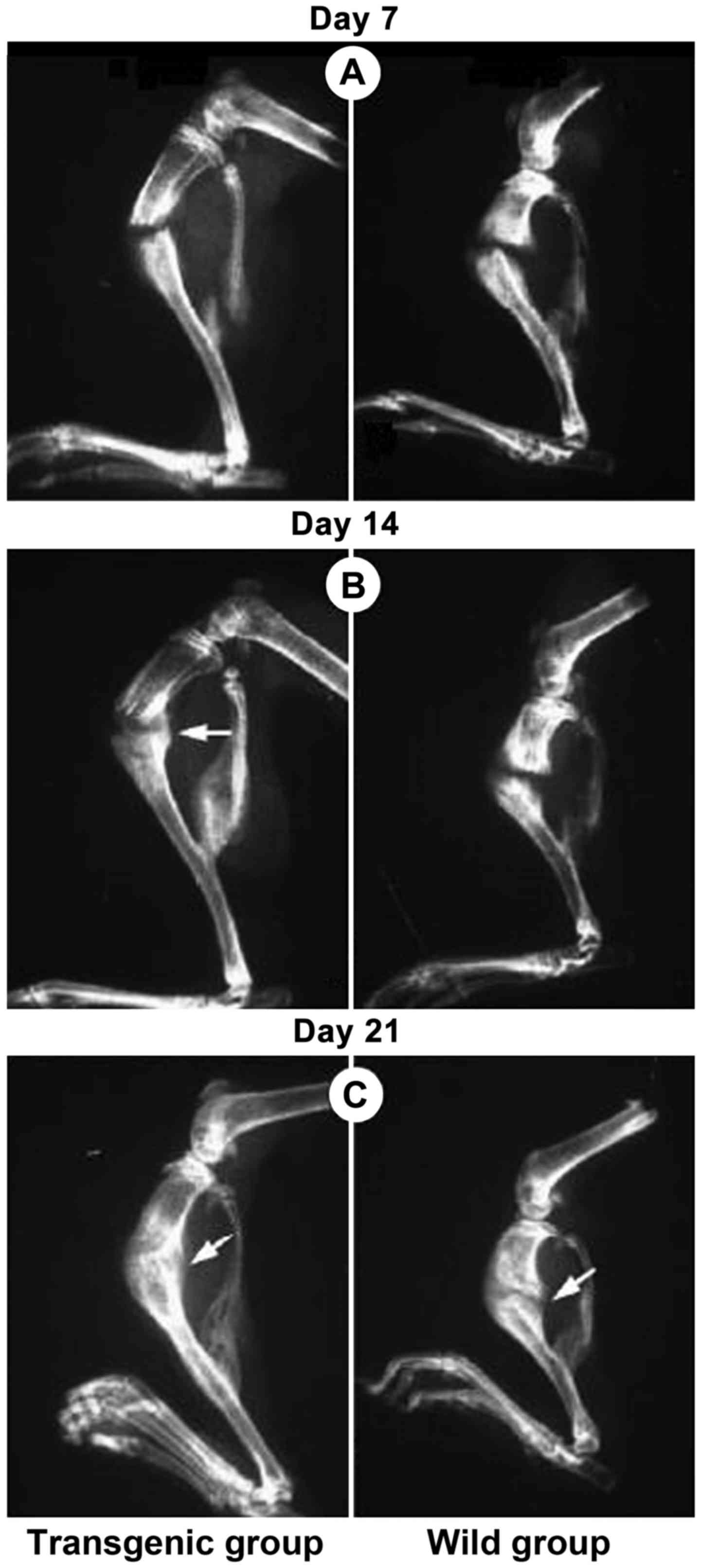
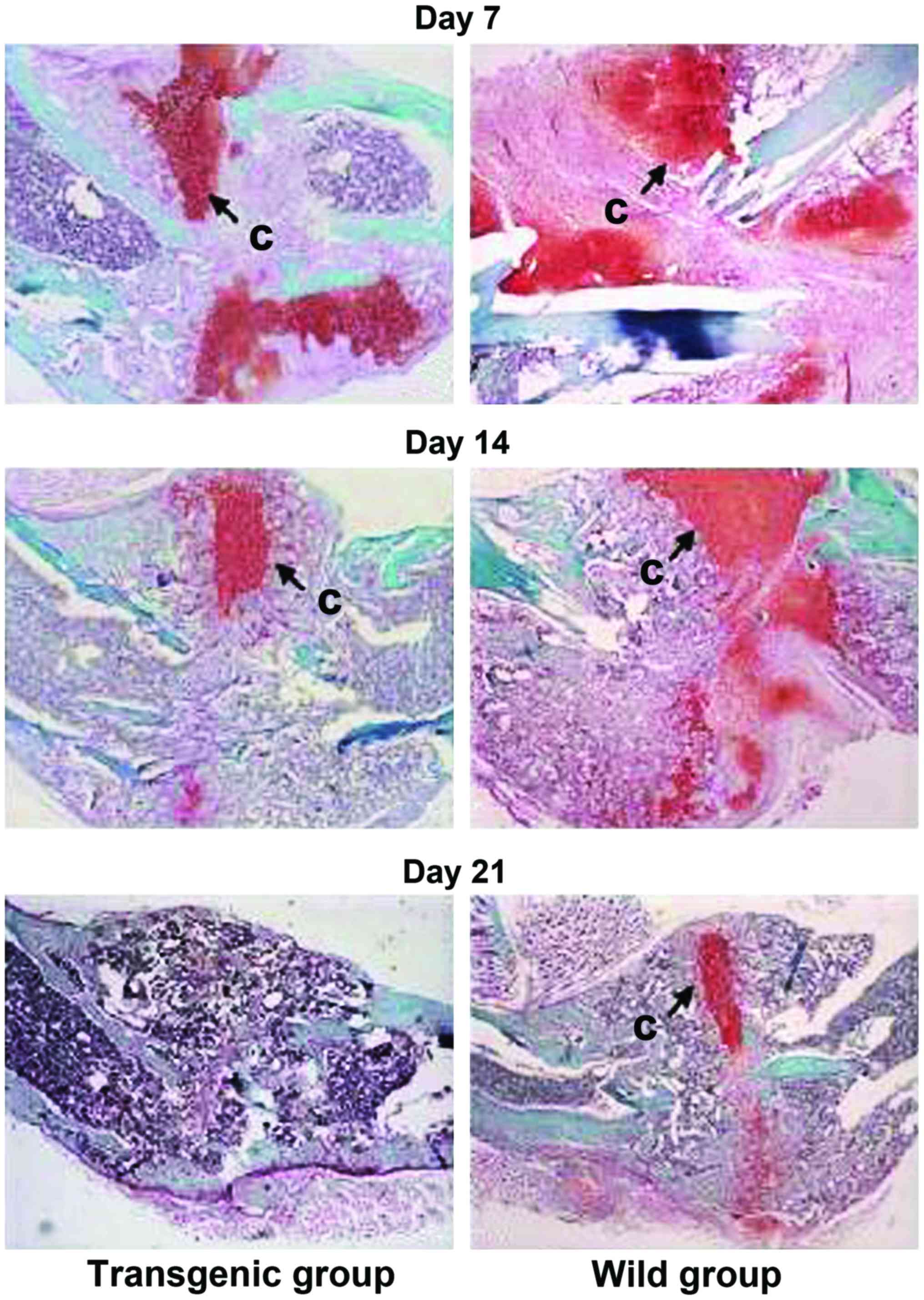
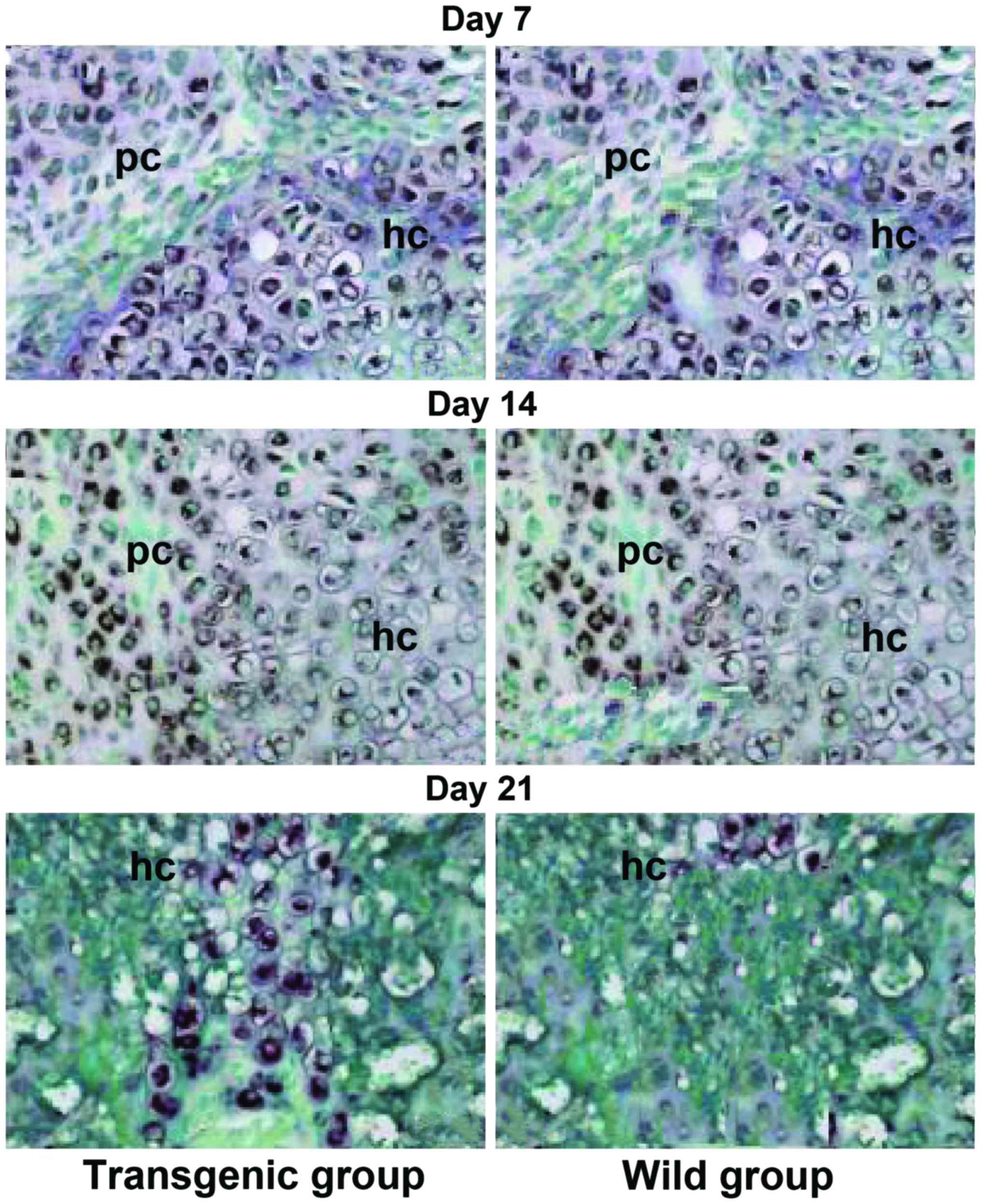
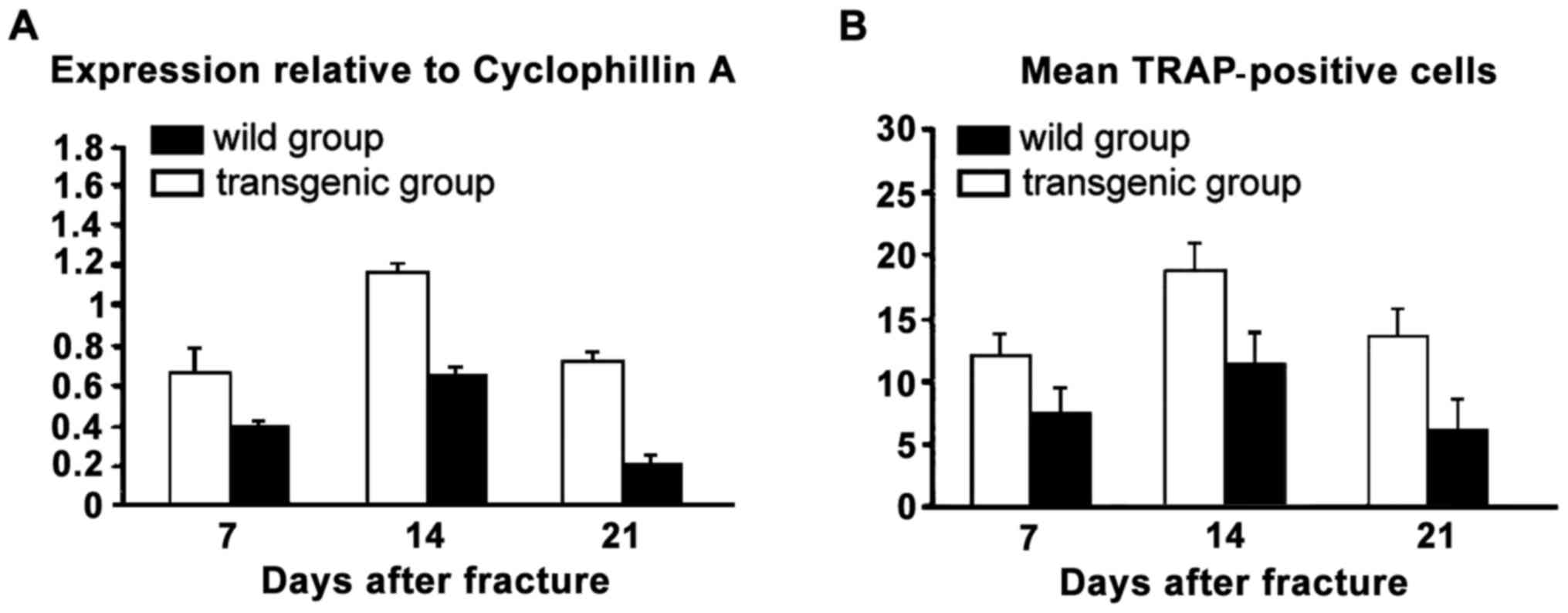
|
1
|
Turner JE and Bosch JA: Closing the Border
on a New Frontier: The problem with salivary nerve growth factor.
Psychosom Med. 78:114–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Majuta LA, Longo G, Fealk MN, McCaffrey G
and Mantyh PW: Orthopedic surgery and bone fracture pain are both
significantly attenuated by sustained blockade of nerve growth
factor. Pain. 156:157–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grills BL, Schuijers JA and Ward AR:
Topical application of nerve growth factor improves fracture
healing in rats. J Orthop Res. 15:235–242. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Zhou S, Liu B, Lei D, Zhao Y, Lu C
and Tan A: Locally applied nerve growth factor enhances bone
consolidation in a rabbit model of mandibular distraction
osteogenesis. J Orthop Res. 24:2238–2245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhuang YF and Li J: Serum EGF and NGF
levels of patients with brain injury and limb fracture. Asian Pac J
Trop Med. 6:383–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rapp AE, Kroner J, Baur S, Schmid F,
Walmsley A, Mottl H and Ignatius A: Analgesia via blockade of
NGF/TrkA signaling does not influence fracture healing in mice. J
Orthop Res. 33:1235–1241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SJ, Shin SJ, Choi NH and Cho SK:
Arthroscopically assisted treatment of avulsion fractures of the
posterior cruciate ligament from the tibia. J Bone Joint Surg Am.
83-A:698–708. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Zhao D, Wang W, Wang B, Liu Z,
Zhang Y and Li Z: Nerve growth factor modulates bone morphogenetic
protein expression in rabbit fracture. Zhonghua Yi Xue Za Zhi.
94:1825–1828. 2014.(In Chinese). PubMed/NCBI
|
|
9
|
Frenkel SR, Guerra LA, Mitchell OG and
Singh IJ: Nerve growth factor in skeletal tissues of the embryonic
chick. Cell Tissue Res. 260:507–511. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo TZ, Wei T, Li WW, Li XQ, Clark JD and
Kingery WS: Immobilization contributes to exaggerated neuropeptide
signaling, inflammatory changes, and nociceptive sensitization
after fracture in rats. J Pain. 15:1033–1045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghilardi JR, Freeman KT, Jimenez-Andrade
JM, Mantyh WG, Bloom AP, Bouhana KS, Trollinger D, Winkler J, Lee P
and Andrews SW: Sustained blockade of neurotrophin receptors TrkA,
TrkB and TrkC reduces non-malignant skeletal pain but not the
maintenance of sensory and sympathetic nerve fibers. Bone.
48:389–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yasui M, Shiraishi Y, Ozaki N, Hayashi K,
Hori K, Ichiyanagi M and Sugiura Y: Nerve growth factor and
associated nerve sprouting contribute to local mechanical
hyperalgesia in a rat model of bone injury. Eur J Pain. 16:953–965.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bei C, Lin Z, Yang Z, Zhao J, Su W, Sha K,
Wei Q, Hua Q and Bo Z: Study on effect of NGF on fracture healing.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 23:570–576. 2009.(In
Chinese). PubMed/NCBI
|
|
14
|
Ishihara A and Bertone AL: Cell-mediated
and direct gene therapy for bone regeneration. Expert Opin Biol
Ther. 12:411–423. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mo Y, Yang Z, Zhao J, Su W, Sha K, Wei Q,
Yang F, Hua Q and Ding X: Preliminary study on appropriate
concentration gradient of nerve growth factor in promoting fracture
healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 25:575–581.
2011.(In Chinese). PubMed/NCBI
|
|
16
|
Wang T, Wang Y, Menendez A, Fong C, Babey
M, Tahimic CG, Cheng Z, Li A, Chang W and Bikle DD:
Osteoblast-Specific Loss of IGF1R Signaling Results in Impaired
Endochondral Bone Formation During Fracture Healing. J Bone Miner
Res. 30:1572–1584. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang RC, Chen MH, Chen PY, Chen CY, Tsai
SF, Cheng CK and Sun JS: A mutation of the Col2a1 gene (G1170S)
alters the transgenic murine phenotype and cartilage matrix
homeostasis. J Formos Med Assoc. 113:803–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Liang DC, Bai JY, Kang N, Feng JY
and Yang ZQ: Overexpression of Sox9 gene by the lentiviral vector
in rabbit bone marrow mesenchymal stem cells for promoting the
repair of cartilage defect. Zhongguo Gu Shang. 28:433–440. 2015.(In
Chinese). PubMed/NCBI
|
|
19
|
Pecchi E, Priam S, Gosset M, Pigenet A,
Sudre L, Laiguillon MC, Berenbaum F and Houard X: Induction of
nerve growth factor expression and release by mechanical and
inflammatory stimuli in chondrocytes: Possible involvement in
osteoarthritis pain. Arthritis Res Ther. 16:R162014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Liu C, Xu F, Cui L, Tan S, Chen R,
Yang L and Huang J: Effects of neuritin on the migration,
senescence and proliferation of human bone marrow mesenchymal stem
cells. Cell Mol Biol Lett. 20:466–474. 2015. View Article : Google Scholar : PubMed/NCBI
|