Article
Open Access
The mechanism of human β-defensin 3 in MRSA-induced infection of implant drug-resistant bacteria biofilm in the mouse tibial bone marrow
- Authors:
- Chen Zhu
- Ni-Rong Bao
- Shuo Chen
- Jian-Ning Zhao
-
View Affiliations / Copyright
Affiliations:
Department of Orthopaedic Surgery, Jinling Hospital, Nanjing University School of Medicine, Nanjing, Jiangsu 210002, P.R. China
-
Pages:
1347-1352
|
Published online on:
February 9, 2017
https://doi.org/10.3892/etm.2017.4112
- Expand metrics +
Metrics:
Total
Views: 0
(Spandidos Publications: | PMC Statistics:
)
Metrics:
Total PDF Downloads: 0
(Spandidos Publications: | PMC Statistics:
)
This article is mentioned in:
Abstract
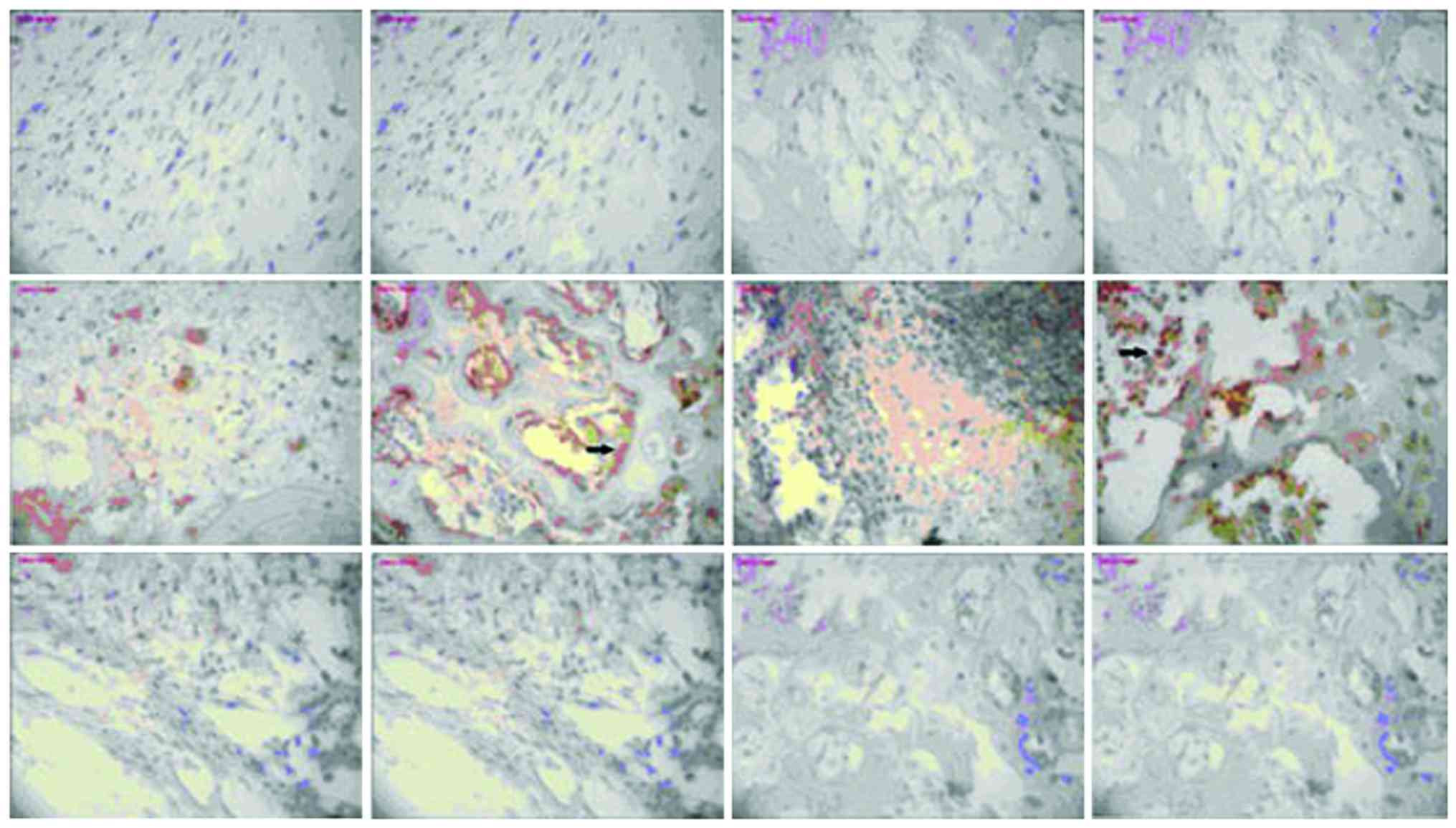
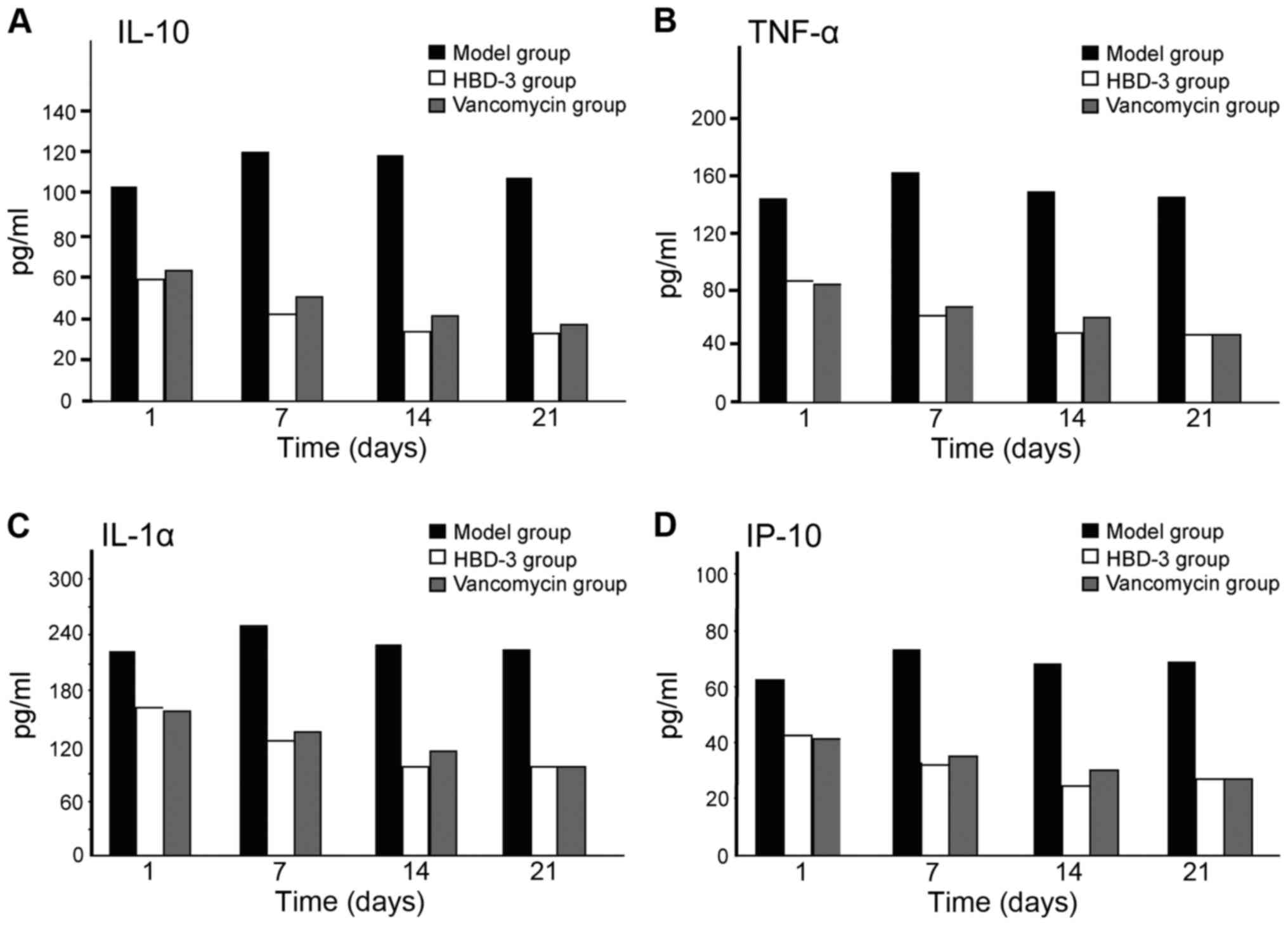
The mechanism of human β-defensin 3 (HBD-3) in methicillin-resistant Staphylococcus aureus (MRSA‑induced infection of implant drug-resistant bacteria biofilm in the mouse tibial bone marrow was studied. Healthy adult male Sprague-Dawley rats with average weight of 230 g were selected to construct the infection model of MRSA‑induced implant drug-resistant bacteria biofilm in the mouse left tibial bone marrow.The drugs were intraperitoneally injected after 24 h medullary cavity infection, and the experimental groups included the model group, HBD-3 group, and vancomycin group (20 rats in each group). The model group was injected with 10 ml saline, HBD-3 group was injected with 10 ml of 8 µg/ml (1 MIC) and vancomycin group was injected with 10 ml of 0.5 µg/ml (1 MIC), five animals in each group were sacrificed on the 1, 7, 14 and 21 days, respectively. Observation was carried out on whether there was swelling and purulent secretion on the local wound; 1 ml venous sinus blood of eye socket was collected for blood routine examination and blood culture, and the laser scanning confocal microscopy was used to observe the morphology of the biofilm on the implant surface and the number of viable bacteria. Immunohistochemical staining was adopted to test the expression of nuclear factor-κB (NF-κB) and toll‑like receptor 4 (TLR-4), and ELISA method was used to test interleukin-10 (IL-10), tumor necrosis factor-α (TNF‑α), IL-1α and interferon-γ (INF-γ)-inducible protein-10 (IP-10) expression levels. There was no death due to infection in the HBD-3 group or vancomycin group, 1 case with significant wound swelling was found, respectively, in each group, but there was no purulent secretion. The percentage of the total white blood cells and neutrophil granulocytes as well as the biofilm morphology and the number of viable bacteria in the model group was gradually increased with time, while those in the HBD-3 group and vancomycin group were decreased with time. The comparative difference among groups was statistically significant (P<0.05); those in the HBD-3 group and vancomycin group at each time-point was decreased significantly compared with the model group, and the difference among groups was statistically significant (P<0.05), but in terms of the comparison between the HBD-3 group and vancomycin group, the difference was not significantly different (P>0.05). The NF-κB and TLR-4 expressions in the model group and vancomycin group were not significantly changed at each time-point, those in the HBD-3 group began to increase on the 1st day, and reached the peak on the 7th day and began to decline on the 14th day, and the comparative difference at each time-point was statistically significant (P<0.05); those in the HBD-3 group were significantly higher than the model group and vancomycin group at each time-point and the difference was statistically significant (P<0.05). The IL-10, TNF-α, IL-1α, and IP-10 expressions in the model group at each time were significantly higher than the other two groups and the difference was statistically significant (P<0.05); in terms of the comparison between the HBD-3 group and vancomycin group, the difference was not statistically significant (P>0.05). In conclusion, β-defensin 3 can inhibit the bacterial growth by regulating inflammation and immune responses in the MRSA-induced implant drug-resistant bacteria biofilm infection in the mouse tibial bone marrow.
View References
|
1
|
Montanaro L, Speziale P, Campoccia D,
Ravaioli S, Cangini I, Pietrocola G, Giannini S and Arciola CR:
Scenery of Staphylococcus implant infections in orthopedics. Future
Microbiol. 6:1329–1349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batoni G, Maisetta G, Esin S and Campa M:
Human beta-defensin-3: a promising antimicrobial peptide. Mini Rev
Med Chem. 6:1063–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi Y, Song W, Feng ZH, Zhao YT, Li F,
Tian Y and Zhao YM: Disinfection of maxillofacial silicone
elastomer using a novel antimicrobial agent: recombinant human
beta-defensin-3. Eur J Clin Microbiol Infect Dis. 28:415–420. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu C, Tan H, Cheng T, Shen H, Shao J, Guo
Y, Shi S and Zhang X: Human β-defensin 3 inhibits
antibiotic-resistant Staphylococcus biofilm formation. J Surg Res.
183:204–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu C, He N, Cheng T, Tan H, Guo Y, Chen
D, Cheng M, Yang Z and Zhang X: Ultrasound-targeted microbubble
destruction enhances human β-defensin 3 activity against
antibiotic-resistant Staphylococcus biofilms. Inflammation.
36:983–996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh PK, Shiha MJ and Kumar A:
Antibacterial responses of retinal Müller glia: production of
antimicrobial peptides, oxidative burst and phagocytosis. J
Neuroinflammation. 11:332014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colavita I, Nigro E, Sarnataro D, Scudiero
O, Granata V, Daniele A, Zagari A, Pessi A and Salvatore F:
Membrane protein 4F2/CD98 is a cell surface receptor involved in
the internalization and trafficking of human β-Defensin 3 in
epithelial cells. Chem Biol. 22:217–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hinrichsen K, Podschun R, Schubert S,
Schröder JM, Harder J and Proksch E: Mouse beta-defensin-14, an
antimicrobial ortholog of human beta-defensin-3. Antimicrob Agents
Chemother. 52:1876–1879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Röhrl J, Yang D, Oppenheim JJ and Hehlgans
T: Identification and biological characterization of mouse
beta-defensin 14, the orthologue of human beta-defensin 3. J Biol
Chem. 283:5414–5419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bedran TB, Mayer MP, Spolidorio DP and
Grenier D: Synergistic anti-inflammatory activity of the
antimicrobial peptides human beta-defensin-3 (hBD-3) and
cathelicidin (LL-37) in a three-dimensional co-culture model of
gingival epithelial cells and fibroblasts. PLoS One. 9:e1067662014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Semple F, MacPherson H, Webb S, Cox SL,
Mallin LJ, Tyrrell C, Grimes GR, Semple CA, Nix MA, Millhauser GL,
et al: Human β-defensin 3 affects the activity of pro-inflammatory
pathways associated with MyD88 and TRIF. Eur J Immunol.
41:3291–3300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Funderburg N, Lederman MM, Feng Z, Drage
MG, Jadlowsky J, Harding CV, Weinberg A and Sieg SF:
Human-defensin-3 activates professional antigen-presenting cells
via toll-like receptors 1 and 2. Proc Natl Acad Sci USA.
104:18631–18635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ning R, Zhang X, Guo X and Li Q:
Staphylococcus aureus regulates secretion of interleukin-6 and
monocyte chemo-attractant protein-1 through activation of nuclear
factor kappaB signaling pathway in human osteoblasts. Braz J Infect
Dis. 15:189–194. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu C, Wang J, Cheng T, Li Q, Shen H, Qin
H, Cheng M and Zhang X: The potential role of increasing the
release of mouse β-defensin-14 in the treatment of osteomyelitis in
mice: a primary study. PLoS One. 9:e868742014. View Article : Google Scholar : PubMed/NCBI
|