Introduction
Inflammatory bowel disease (IBD), comprising Crohn's
disease and ulcerative colitis, is multifactorial and results from
an interaction between genetic, microbial, autoimmune and
environmental factors (1). The
incidence of IBD is associated with dietary compositions, saturated
fats, depression, impaired sleep and serum vitamin D concentrations
(2–4). The pathogenesis of IBD is a chronic
relapsing inflammatory disorder leading to neutrophil accumulation,
gastrointestinal inflammation with villus atrophy and loss of
crypts, and is accompanied by diarrhea, blood in stool, weight
loss, disturbed intestinal barriers and dysfunction of tight
junctions (5,6). As inflammation is closely associated
with the generation of free radical species, oxidative stress has
been proposed as a mechanism underlying the pathophysiology of
various inflammation-associated diseases, including IBD (7,8).
Experimental and clinical evidence suggests that antioxidant
compounds may serve as the potential therapeutic modalities of
human IBD (8–11).
Soy isoflavones are diphenolic compounds that are
present in plants such as soybeans, red clover and kudzu root. It
has been demonstrated that they have antioxidant properties via
detoxifying free radical species and upregulating antioxidant genes
(12). Soy isoflavones are also
associated with cell survival, cell cycle, inflammation and
apoptosis, and they suppress nuclear factor (NF)-κB and other
signaling pathways (12). However,
the effects of soy isoflavones on dextran sulfate sodium
(DSS)-induced IBD remain unknown. Thus, the aim of the present
study was to investigate the protective effect of soy isoflavones
in DSS-challenged mice via assessing morphology and performing
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analyses.
Materials and methods
Animal model and groups
A total of 40, 5-week old female ICR mice weighing
22.66±0.12 g were obtained from Changsha Well-Bio (Changsha,
China). Mice were divided into four groups (n=10 in each): Control
(Cont), in which mice were fed a basic diet (13) and tap water; a DSS-treated group
(DSS), in which mice had ad libitum access to 5% DSS
solution (Kayon Biotechnology, Co., Ltd., Shanghai, China) supplied
as drinking water; a soy isoflavonones supplemented group (SIF), in
which 0.5% soy isoflavones were mixed in the feeding diet (Xi'an
Rongsheng Biotechnology, Co., Ltd., Shaanxi, China) according to
previous studies (14); and a soy
isoflavones and DSS-treated group (SDS), in which mice were treated
with DSS and soy isoflavones as previously described. All mice were
housed in polycarbonate cages in a room with a controlled
temperature of 25±3°C, a humidity of 50±5% and a 12-h light/dark
cycle. Mice were allowed ad libitum access to laboratory
strip chows throughout the experimental period.
Following the 7-day experimental period, all mice
were sacrificed via general anesthetic with Zoletil 50 (10 mg/kg
diluted in saline; Virbac S.A., Carros, France). Subsequently,
colons were harvested and colon length was measured (n=10). Prior
to sacrifice, 10 blood samples from each group were collected via
orbital vein bleeding after mice were sedated. In addition, colon
tissues from each mouse were harvested and immediately frozen in
liquid nitrogen and stored at −70°C for subsequent gene expression
analysis (n=6). The present study was conducted according to the
guidelines of the Declaration of Helsinki and all procedures
involving animal subjects were approved by the Animal Welfare
Committee of the Jiangsu Food and Pharmaceutical Science College
(Huaian, China).
Histomorphometry determination
The morphological evaluation following treatment
with DSS was performed using hematoxylin and eosin (H&E)
staining. Briefly, one section of each colon sample (0.5 cm) was
fixed in 4% neutral buffered formalin, processed using routine
histological methods and mounted in paraffin blocks (room
temperature). Each sample was cut into 6 µm-thick sections and
stained with H&E. All specimens were examined under a light
microscope (Nikon Corporation, Toyko, Japan). Villus height and
crypt depth were measured using Image-Pro Plus version 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA) (15).
Serum oxidative indexes
Harvested blood samples were stored at 4°C for 4 h,
when serum samples were separated from blood via centrifugation at
3,500 × g and 4°C for 15 min. Malondialdehyde (MDA) and total
antioxidant capability (T-AOC) were measured using assay kits in
accordance with the manufacturer's instructions (MDA, A003-1;
T-AOC, A0015-1; both Nanjing Jiancheng Bioengineering Institute,
Nanjing, China).
cDNA synthesis and quantification of
mRNA by RT-qPCR
Total RNA was isolated from liquid nitrogen
pulverized tissues using TRIzol reagent according to the
manufacturer's protocol (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) and then treated with DNase I (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Synthesis of the first strand (cDNA) was performed with
oligo (dT) 20 and Superscript II reverse transcriptase using the
following program: 42°C for 2 min; 37°C for 15 min; followed by
85°C for 5 sec (Invitrogen; Thermo Fisher Scientific, Inc.).
Primers were designed with Primer 5.0 according to
the gene sequence of mouse (www.ncbi.nlm.nih.gov/nuccore/?term=Mus+musculus) to
produce an amplification product. The primer sets used are
presented in Table I. The protocol
of RT-qPCR was completed using the SYBR ExScript RT-qPCR kit
(Takara Biotechnology Co., Ltd.). A total reaction system of 10 µl
contained 1 µl cDNA from the aforementioned PCR, 5 µl SYBR Premix
EX Taq, 0.5 µl of each of the primers (10 µM), and 3 µl
ddH2O. The PCR program was as follows: Denaturation at
95°C for 2 min; followed by 45 cycles at 95°C for 10 sec, 59°C for
20 sec and 72°C for 30 sec according to previous studies (16,17).
Relative gene expression was normalized to β-actin and expressed as
a ratio to the expression in control group using the formula
2−(∆∆Cq) (18), where
∆∆Cq=(CqTarget-Cqβ-actin)treatment-(CqTarget-Cqβ-actin)control.
Therefore, relative expression of target genes in the control group
was: Relative gene expression levels represented the comparison vs.
control group; results were reported as a fold-change from the
control value.
 | Table I.Polymerase chain reaction primer
sequences: F and R primers used in the present study. |
Table I.
Polymerase chain reaction primer
sequences: F and R primers used in the present study.
| Gene | Accession no. | Nucleotide sequence
of primers, 5′-3′ | Size, bp |
|---|
| β-Actin | NM_007393.3 | F:
GTCCACCTTCCAGCAGATGT | 117 |
|
|
| R:
GAAAGGGTGTAAAACGCAGC |
|
| Occludin | NM_008756.2 | F:
ACTGGGTCAGGGAATATCCA | 193 |
|
|
| R:
TCAGCAGCAGCCATGTACTC |
|
| ZO1 | XM_006540786.1 | F:
ACTCCCACTTCCCCAAAAAC | 166 |
|
|
| R:
CCACAGCTGAAGGACTCACA |
|
| Caludin1 | NM_016674.4 | F:
AGACCTGGATTTGCATCTTGGTG | 126 |
|
|
| R:
TGCAACATAGGCAGGACAAGAGTTA |
|
| CAT | XM_006498624.1 | F:
AATATCGTGGGTGACCTCAA | 243 |
|
|
| R:
CAGATGAAGCAGTGGAAGGA |
|
| ZnCuSOD | NM_011434.1 | F:
CCACTGCAGGACCTCATTTT | 216 |
|
|
| R:
CACCTTTGCCCAAGTCATCT |
|
| Gpx1 | NM_008160.6 | F:
GGTTCGAGCCCAATTTTACA | 199 |
|
|
| R:
CCCACCAGGAACTTCTCAAA |
|
| Gpx2 | NM_030677.2 | F:
GTGTGATGTCAATGGGCAGAA | 241 |
|
|
| R:
ACGTTTGATGTCAGGCTCGAT |
|
| Gpx3 | NM_008161.3 | F:
GATGTGAACGGGGAGAAAGA | 152 |
|
|
| R:
CCCACCAGGAACTTCTCAAA |
|
| Gpx4 | NM_001037741.3 | F:
CTCCATGCACGAATTCTCAG | 117 |
|
|
| R:
ACGTCAGTTTTGCCTCATTG |
|
| IL-1β | NM_008361.3 | F:
CTGTGACTCGTGGGATGATG | 213 |
|
|
| R:
GGGATTTTGTCGTTGCTTGT |
|
| IL-6 | NM_031168.1 | F:
TGCAAGAGACTTCCATCCAGT | 116 |
|
|
| R:
GTGAAGTAGGGAAGGCCG |
|
| IL-10 | NM_010548.2 | F:
ACAGCCGGGAAGACAATAAC | 116 |
|
|
| R:
CAGCTGGTCCTTTGTTTGAAAG |
|
| IL-17 | NM_010552.3 | F:
TACCTCAACCGTTCCACGTC | 119 |
|
|
| R:
TTTCCCTCCGCATTGACAC |
|
| TNF-α | NM_013693.2 | F:
AGGCACTCCCCCAAAAGAT | 192 |
|
|
| R:
TGAGGGTCTGGGCCATAGAA |
|
| TLR4 | NM_021297.3 | F:
TTTGCTGGGGCTCATTCACT | 164 |
|
|
| R:
GACTCGGCACTTAGCACTGT |
|
| Myd88 | NM_010851.2 | F:
CTCGCAGTTTGTTGGATGCC | 185 |
|
|
| R:
GGCCACCTGTAAAGGCTTCT |
|
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Group comparisons
were performed using a one-way analysis of variance to assess the
homogeneity of variances via Levene's test and followed with
Tukey's multiple comparison test. Data are expressed as the mean ±
standard error of the mean. P<0.05 was considered to represent a
statistically significant difference.
Results
Effects of soy isoflavones on body
weight and colonic length in DSS-challenged mice
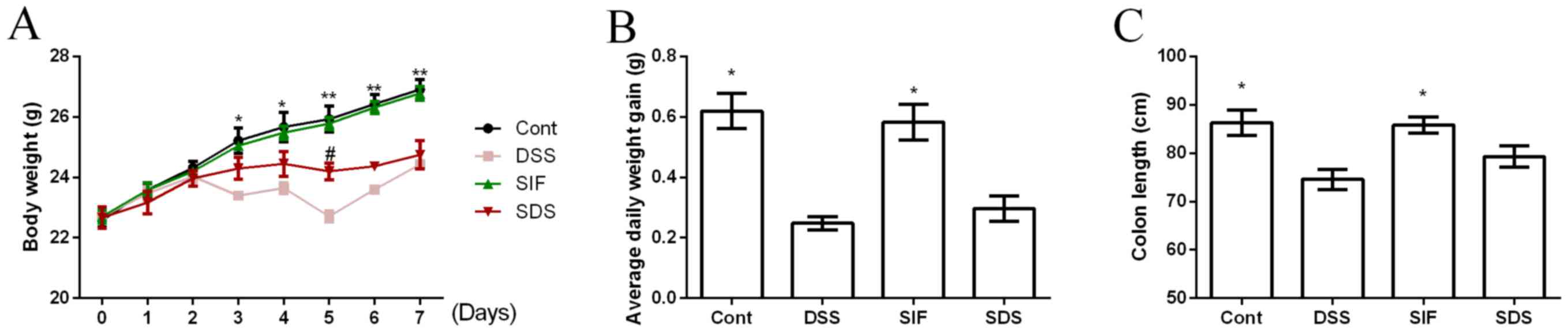
Body weight was recorded daily and is presented in
Fig. 1A. On days 3 to 7, DSS
treatment significantly reduced body weight in mice compared with
the control group (P<0.05). Dietary soy isoflavones
significantly increased body weight at day 5 in DSS-challenged mice
compared with the DSS group (P<0.05). Average daily weight gain
was significantly lower in the DSS compared with the control group
(Fig. 1B; P<0.05), while dietary
soy isoflavones failed to affect body weight gain in DSS-challenged
mice (Fig. 1B; P>0.05).
Colonic length has been used as a clinical index for
colonic inflammation and the present study demonstrated that DSS
exposure significantly decreased colonic length (P<0.05;
Fig. 1C). Dietary soy isoflavones
(SIF group) tended to alleviate DSS-induced colonic atrophy,
however the difference was not significant.
Effects of soy isoflavones on
inflammation in DSS-challenged mice
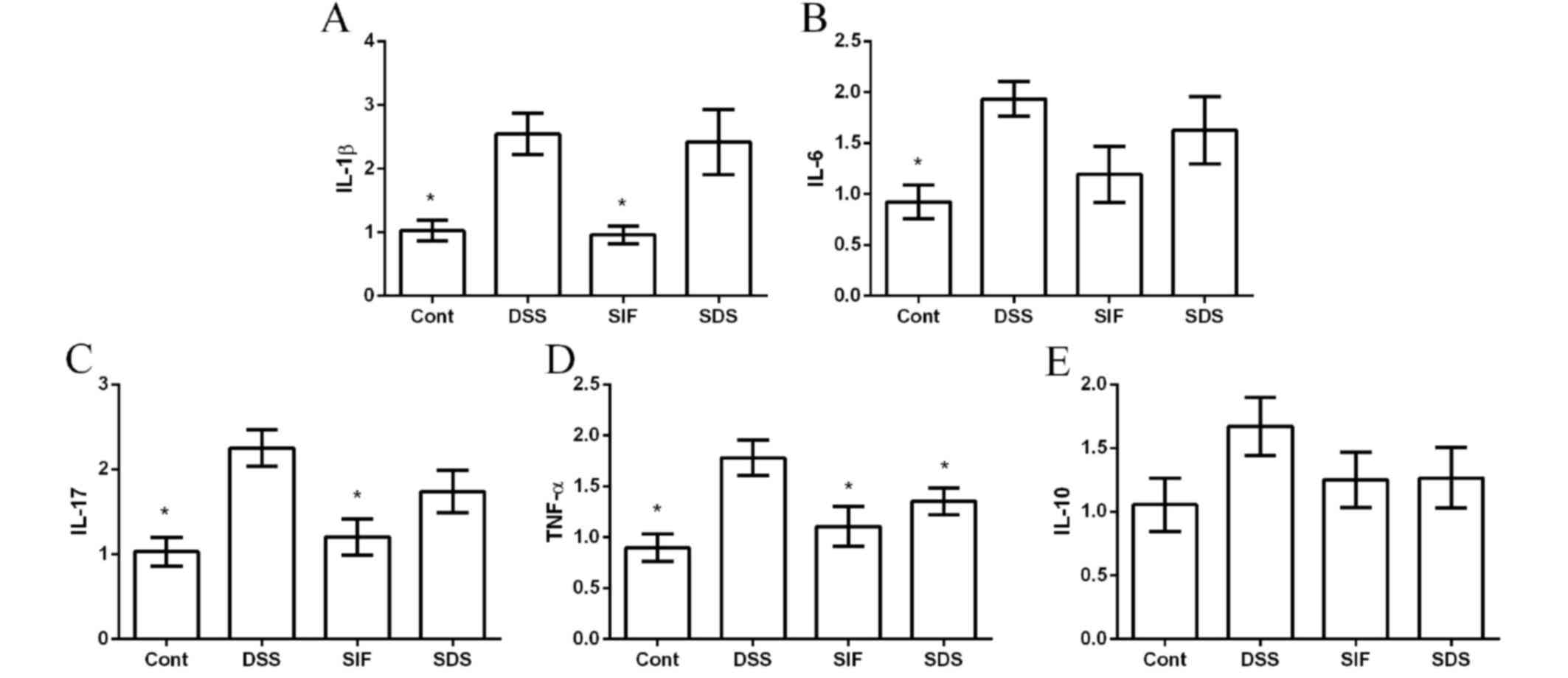
The mRNA abundances of interleukin (IL)-1β, IL-6,
IL-10, IL-17 and tumor necrosis factor (TNF)-α were measured via
RT-qPCR to investigate the anti-inflammatory effect of soy
isoflavones in DSS-challenged mice. The present data demonstrated
that DSS treatment significantly induced colonic inflammation,
indicated by the upregulation of IL-1β, IL-6, IL-17 and TNF-α
expression (Fig. 2A-D). IL-10 was
also tested in this study; however, no significant difference was
indicated (P>0.05; Fig. 2E).
Dietary supplementation with isoflavones downregulated the
expression of TNF-α, compared with the DSS group (P<0.05;
Fig. 2D).
Effects of soy isoflavones on
oxidative stress in DSS-challenged mice
MDA and T-AOC have been widely used as makers for
oxidative stress and are indicated to be associated with
inflammatory diseases. In the present study, DSS exposure
significantly increased serum MDA concentration and decreased serum
T-AOC activity compared with the control group (P<0.05; Fig. 3A and B, respectively), suggesting
that oxidative stress is associated with IBD. Although dietary soy
isoflavones failed to significantly alleviate DSS-caused MDA
generation, T-AOC activity in the SDS group was significantly
higher than that in DSS group (P<0.05; Fig. 3B). Furthermore, RT-qPCR results
(Fig. 3C-H) demonstrated that DSS
significantly inhibited zinc-copper superoxide dismutase (ZnCuSOD;
Fig. 3C) and glutathione peroxidase
1 (GPX1; Fig. 3D) gene expression
levels (both P<0.05), whereas dietary soy isoflavones markedly
upregulated ZnCuSOD, GPX1 and GPX4 mRNA expression levels
(P<0.05; Fig. 3C, D and G,
respectively). However, gene expression levels of GPX2, GPX3 and
CAT were not significant between groups (P>0.05; Fig. 3E, F and H, respectively).
 | Figure 3.Effects of dietary soy isoflavones on
oxidative stress following DSS exposure. The effects of dietary soy
isoflavones on (A) MDA, (B) T-AOC, (C) ZnCuSOD, (D) GPX1, (E) GPX2,
(F) GPX3, (G) GPX4 and (H) CAT. Data are presented as mean ±
standard error of the mean (n=6 or 8). *P<0.05 vs. DSS group.
DSS, dextran sulfate sodium; MDA, Malondialdehyde; T-AOC, total
antioxidant capability; ZnCuSOD, zinc-copper superoxide dismutase;
GPX, glutathione peroxidase; CAT, catalase; Cont, control group;
SIF, soy isoflavones supplemented group; SDS, soy isoflavones and
DSS-treated group. |
Effects of soy isoflavones on colonic
morphology and tight junctions in DSS-challenged mice
No abnormal morphology was observed in the colon of
mice in the control group (Fig. 4A).
By contrast, villus height in the challenged mice exhibited a
marked scattering and desquamation (Fig.
4B-D). Villus height in the DSS group (82.53±4.20 µm) was
significantly lower than that in the control group (103.25±3.77 µm;
P<0.05) and soy isoflavones (108.70±5.15 µm) significantly
alleviated DSS-induced colonic villus injury (P<0.05; Table II).
 | Table II.Effects of dietary soy isoflavones on
villus height (µm) and crypt depth (µm) in the colon following
exposure to DSS. |
Table II.
Effects of dietary soy isoflavones on
villus height (µm) and crypt depth (µm) in the colon following
exposure to DSS.
| Item | Control | DSS | SIF | SDS |
|---|
| Villus height |
103.25±3.77a | 82.53±2.43 | 108.70±5.15a |
100.05±3.56a |
| Crypt depth | 32.15±4.07 | 31.57±1.68 | 32.60±2.69 | 34.60±3.01 |
| V/C | 3.39±0.51 | 2.64±0.20 | 3.40±0.32 | 2.96±0.26 |
The present study further determined the expression
of Occludin, zonula occludens-1 and Claudin1 (Fig. 4E-G) following DSS exposure, and the
results demonstrated that DSS significantly downregulated the
expression of Occludin and soy isoflavones enhanced the Occludin
mRNA level (P<0.05; Fig. 4E).
Effects of soy isoflavones on toll
like receptor 4 (TLR4)/myeloid differentiation primary response
gene 88 (Myd88) in DSS-challenged mice
TLR4/Myd88 is associated with various inflammatory
responses (19). The present study
demonstrated that DSS significantly enhanced the expression of
colonic TLR4/Myd88 mRNA (P<0.05; Fig.
5), suggesting that DSS activates the TLR4/Myd88 signal.
Although soy isoflavones tended to inhibit the TLR4 expression
caused by DSS, the difference was not significant. However, dietary
supplementation with soy isoflavones significantly inactivated
Myd88 following DSS exposure (P<0.05; Fig. 5B).
Discussion
Soybeans, most widely used in Asian countries, are a
rich source of biologically active isoflavones, such as genistein
(4,5,7-trihydroxyisoflavone) and daidzein (4,7-dihydroxyisoflavone)
known to have a spectrum of biological activities. Although soy
isoflavones have been demonstrated to exert protective effects
against a series of diseases in vitro and in vivo
(20,21), to the best of our knowledge no
reports are available regarding the evaluation of soy isoflavones
in inflammation and specifically in IBD. The present study
demonstrated that dietary soy isoflavones reduced the severity and
extent of progressive chronic colonic damage induced by a 7-day
exposure of DSS.
Although the etiology of IBD remains essentially
obscure, it has been suggested that the development and pathology
may be associated with an abnormal inflammatory response in the
gastrointestinal tract (22). Kim
et al (22) reported that
disease severity is often associated with an increase in higher
levels of pro-inflammatory cytokines in experimental colitis.
Pro-inflammatory cytokines are important mediators of inflammation
and have distinguished roles in cancer development. The present
study determined that there is an abundance of IL-1β, IL-6, IL-10,
IL-17 and TNF-α mRNA in the colon using RT-qPCR analysis and the
results demonstrated that DSS-induced colonic inflammation. This
was indicated by the upregulation of IL-1β, IL-6, IL-17 and TNF-α
gene expression. However, in DSS-challenged mice, dietary soy
isoflavones downregulated the gene expression of TNF-α. Similarly,
soy isoflavones have also been demonstrated to alleviate
inflammation induced by the generation of IL-1β, IL-6 and TNF-α
(23). IL-1β, IL-6 and TNF-α have
been implicated in a number of cellular and molecular mechanisms
associated with the majority of inflammation-associated chronic
human diseases, including IBD (23).
Thus, the present study speculated that soy isoflavones have
evident anti-inflammatory potential in the IBD model.
Studies on the antioxidant function of soy
isoflavones have suggested a free radical scavenging ability, an
ability to reduce low-density lipoprotein and DNA susceptibility to
oxidative stress, and an ability to boost the activity and
expression of antioxidant enzymes (14). Due in part to their potential
antioxidant activities; soy isoflavones have been linked to a
decreased risk of cardiovascular disease, osteoporosis,
endocrine-responsive cancer and inflammatory diseases (24,25). MDA
and T-AOC have been widely used as makers for oxidative stress
(26,27). In the present study, DSS exposure
significantly induced oxidative stress, indicated by the elevated
MDA level and decreased T-AOC activity. Although dietary soy
isoflavones failed to alleviate DSS-induced MDA generation, soy
isoflavones enhanced serum T-AOC activity, suggesting an
antioxidant function of soy isoflavones in the DSS-induced IBD
model. To investigate the antioxidant mechanism of soy isoflavones,
the present study further determined colonic antioxidant gene
expression. The results demonstrated that dietary soy isoflavones
upregulated the gene expression levels of ZnCuSOD, GPX1 and GPX4 in
DSS-challenged mice. In the exercise-induced oxidative stress
model, Yoon and Park (14) reported
that soy isoflavones significantly enhanced SOD activity and
alleviated oxidative injury. GPX1 and GPX4 have been revealed to be
involved in IBD via regulating T cell function and oxidative stress
(28,29). Thus, the antioxidant function of soy
isoflavones in IBD model may be associated with increasing the
expression of ZnCuSOD, GPX1 and GPX4.
Dysfunction of the gastrointestinal barriers is a
major characteristic symptom in the pathophysiology of IBD
(30). In the present study, dietary
soy isoflavones significantly alleviated DSS-induced colonic villus
injury and upregulated occludin expression in DSS-challenged mice.
This indicated a beneficial role in intestinal morphologic
structure and barrier function. Kiatprasert et al (31) reported that treatment with soy
isoflavones may promote and restore the impaired endometrial
barrier function by increasing the gene expression of tight
junction-associated genes in lipopolysaccharide-induced
inflammation.
TLR4/Myd88 signalling is associated with various
inflammatory diseases, including IBD (32,33). Cao
et al (33) demonstrated that
TLR4/Myd88 is able to regulate interferon-γ and IL-17 production by
inducing Foxp3+ regulatory T cells during intestinal
inflammation. It has also been suggested that TLR4/Myd88 is able to
mediate the NF-κB pathway and maintain the intestinal barrier
function (34). In the present
study, DSS activated TLR4/Myd88 and dietary soy isoflavones
significantly inhibited Myd88 expression. Genistein, a principal
soy isoflavone, has been revealed to mediate TLR4/Myd88 in various
inflammations (35,36). In addition, genistein attenuated the
initiation of intracellular signaling cascades by LPS through
inhibiting NF-κB activation by inhibiting the binding of LPS to
TLR-4 on microglial cells (37).
In conclusion, DSS caused inflammation, oxidative
stress, intestinal barrier dysfunction in mice. However, findings
from the present study demonstrated that dietary soy isoflavones
alleviated DSS-induced inflammation in mice, which may be
associated with enhancing antioxidant function and inhibiting the
TLR4/MyD88 signal.
Acknowledgements
The present study was supported by Scientific
Research Program of Jiangsu Provincial Huaian Municipal Government
(grant no. HAN2014007).
References
|
1
|
Dubeau MF, Iacucci M, Beck PL, Moran GW,
Kaplan GG, Ghosh S and Panaccione R: Drug-induced inflammatory
bowel disease and IBD-like conditions. Inflamm Bowel Dis.
19:445–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ananthakrishnan AN: Epidemiology and risk
factors for IBD. Nat Rev Gastroenterol Hepatol. 12:205–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mileva S, Galunska B, Gospodinova M,
Gerova D and Svinarov D: Vitamin D3 status in children with acute
diarrhea. Integr Food Nutr Metab. 1:1–6. 2014.
|
|
4
|
Hirai F and Matsui T: Status of food
intake and elemental nutrition in patients with Crohn's disease.
Integr Food Nutr Metab. 2:148–150. 2015.
|
|
5
|
Naito Y, Takagi T and Yoshikawa T:
Neutrophil-dependent oxidative stress in ulcerative colitis. J Clin
Biochem Nutr. 41:18–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sann H, Erichsen JV, Hessmann M, Pahl A
and Hoffmeyer A: Efficacy of drugs used in the treatment of IBD and
combinations thereof in acute DSS-induced colitis in mice. Life
Sci. 92:708–718. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Piechota-Polanczyk A and Fichna J: Review
article: The role of oxidative stress in pathogenesis and treatment
of inflammatory bowel diseases. Naunyn Schmiedebergs Arch
Pharmacol. 387:605–620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu H and Li YR: Oxidative stress and
redox signaling mechanisms of inflammatory bowel disease: Updated
experimental and clinical evidence. Exp Biol Med (Maywood).
237:474–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Almenier HA, Al Menshawy HH, Maher MM and
Al Gamal S: Oxidative stress and inflammatory bowel disease. Front
Biosci (Elite Ed). 4:1335–1344. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shori AB and Baba AS: Fermented milk
derives bioactive peptides with antihypertensive effects. Integr
Food Nutr Metab. 2:178–181. 2015.
|
|
11
|
McCann MJ, Dalziel JE, Bibiloni R and
Barnett MPG: An integrated approach to assessing the bio-activity
of nutrients in vitro: The anti-oxidant effects of catechin and
chlorogenic acid as an example. Integr Food Nutr Metab. 2:197–204.
2015. View Article : Google Scholar
|
|
12
|
Mahmoud AM, Yang W and Bosland MC: Soy
isoflavones and prostate cancer: A review of molecular mechanisms.
J Steroid Biochem Mol Biol. 140:116–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin J, Wu M, Duan J, Liu G, Cui Z, Zheng
J, Chen S, Ren W, Deng J, Tan X, et al: Pyrrolidine dithiocarbamate
inhibits NF-KappaB activation and upregulates the expression of
Gpx1, Gpx4, occludin, and ZO-1 in DSS-induced colitis. Appl Biochem
Biotechnol. 177:1716–1728. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon GA and Park S: Antioxidant action of
soy isoflavones on oxidative stress and antioxidant enzyme
activities in exercised rats. Nutr Res Pract. 8:618–624. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yin J, Liu M, Ren W, Duan J, Yang G, Zhao
Y, Fang R, Chen L, Li T and Yin Y: Effects of dietary
supplementation with glutamate and aspartate on diquat-induced
oxidative stress in piglets. PLoS One. 10:e01228932015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin J, Duan J, Cui Z, Ren W, Li T and Yin
Y: Hydrogen peroxide-induced oxidative stress activates NF-κB and
Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Advances.
5:15479–15486. 2015. View Article : Google Scholar
|
|
17
|
Yin J, Wu MM, Xiao H, Ren WK, Duan JL,
Yang G, Li TJ and Yin YL: Development of an antioxidant system
after early weaning in piglets. J Anim Sci. 92:612–619. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doz E, Noulin N, Boichot E, Guénon I, Fick
L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF and
Couillin I: Cigarette smoke-induced pulmonary inflammation is
TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol.
180:1169–1178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pudenz M, Roth K and Gerhauser C: Impact
of soy isoflavones on the epigenome in cancer prevention.
Nutrients. 6:4218–4272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hillman GG and Singh-Gupta V: Soy
isoflavones sensitize cancer cells to radiotherapy. Free Radic Biol
Med. 51:289–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JJ, Shajib MS, Manocha MM and Khan WI:
Investigating intestinal inflammation in DSS-induced model of IBD.
J Vis Exp pii. 36782012.
|
|
23
|
Khan AQ, Khan R, Rehman MU, Lateef A,
Tahir M, Ali F and Sultana S: Soy isoflavones (daidzein &
genistein) inhibit 12-O-tetradecanoylphorbol-13-acetate
(TPA)-induced cutaneous inflammation via modulation of COX-2 and
NF-κB in Swiss albino mice. Toxicology. 302:266–274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arteel GE, Uesugi T, Bevan LN, Gäbele E,
Wheeler MD, McKim SE and Thurman RG: Green tea extract protects
against early alcohol-induced liver injury in rats. Biol Chem.
383:663–670. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yousef MI, Kamel KI, Esmail AM and
Baghdadi HH: Antioxidant activities and lipid lowering effects of
isoflavone in male rabbits. Food Chem Toxicol. 42:1497–1503. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin J, Ren W, Yang G, Duan J, Huang X,
Fang R, Li C, Li T, Yin Y, Hou Y, et al: l-Cysteine metabolism and
its nutritional implications. Mol Nutr Food Res. 60:134–146. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yin J, Ren W, Liu G, Duan J, Yang G, Wu L,
Li T and Yin Y: Birth oxidative stress and the development of an
antioxidant system in newborn piglets. Free Radic Res.
47:1027–1035. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Häuser F, Rossmann H, Laubert-Reh D, Wild
PS, Zeller T, Müller C, Neuwirth S, Blankenberg S and Lackner KJ:
Inflammatory bowel disease (IBD) locus 12: Is glutathione
peroxidase-1 (GPX1) the relevant gene? Genes Immun. 16:571–575.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HR, Lee A, Choi EJ, Kie JH, Lim W, Lee
HK, Moon BI and Seoh JY: Attenuation of experimental colitis in
glutathione peroxidase 1 and catalase double knockout mice through
enhancing regulatory T cell function. PLoS One. 9:e953322014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amasheh M, Grotjohann I, Amasheh S, Fromm
A, Söderholm JD, Zeitz M, Fromm M and Schulzke JD: Regulation of
mucosal structure and barrier function in rat colon exposed to
tumor necrosis factor alpha and interferon gamma in vitro: A novel
model for studying the pathomechanisms of inflammatory bowel
disease cytokines. Scand J Gastroenterol. 44:1226–1235. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiatprasert P, Deachapunya C, Benjanirat C
and Poonyachoti S: Soy isoflavones improves endometrial barrier
through tight junction gene expression. Reproduction. 149:269–280.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma W, Wang J, Li Y, Hu X, Shi F and Wang
X: Enhancing pentose phosphate pathway in Corynebacterium
glutamicum to improve l-isoleucine production. Biotechnol Appl
Biochem. 63:877–885. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao AT, Yao S, Stefka AT, Liu Z, Qin H,
Liu H, Evans-Marin HL, Elson CO, Nagler CR and Cong Y: TLR4
regulates IFN-γ and IL-17 production by both thymic and induced
Foxp3+ Tregs during intestinal inflammation. J Leukoc Biol.
96:895–905. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang W, Xia T and Yu X: Wogonin suppresses
inflammatory response and maintains intestinal barrier function via
TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm Res.
64:423–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dijsselbloem N, Goriely S, Albarani V,
Gerlo S, Francoz S, Marine JC, Goldman M, Haegeman G and Berghe W
Vanden: A critical role for p53 in the control of
NF-kappaB-dependent gene expression in TLR4-stimulated dendritic
cells exposed to Genistein. J Immunol. 178:5048–5057. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu HL, Li XY, Zhou X, Yuan LH, Ma WW, Xi
YD, Zhao X, Wu J and Xiao R: Beta amyloid peptide (25–35) leading
to inflammation through Toll-like receptors and the
anti-inflammatory effect of genistein in BV-2 cells. J Mol
Neurosci. 51:771–778. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jeong JW, Lee HH, Han MH, Kim GY, Kim WJ
and Choi YH: Anti-inflammatory effects of genistein via suppression
of the toll-like receptor 4-mediated signaling pathway in
lipopolysaccharide-stimulated BV2 microglia. Chem Biol Interact.
212:30–39. 2014. View Article : Google Scholar : PubMed/NCBI
|