Introduction
The pain-depression dyad is characterized by
widespread pain, tenderness to palpation and various concomitant
symptoms, including affective disorders such as depression
(1). This syndrome is becoming
increasingly widespread in the clinic and is attracting increasing
amounts of attention (2–5). It is usually treated with
antidepressants and antiepileptics. However, due to heavy side
effects of antidepressants, it is difficult for patients to adhere
to the medication for a long duration (6). Researchers have begun to focus on
non-pharmaceutical therapies such as music therapy and
psychotherapy, in an attempt to identify an ideal and systemic
therapy for the pain-depression dyad.
Acupuncture, particularly electro-acupuncture (EA),
has been approved worldwide to be effective for pain or emotional
problems (7–9). Low-frequency EA can relieve pain mainly
at the supraspinal level and the spinal cord, while high-frequency
EA mainly acts via the spinal cord (10). Low-frequency EA also effectively
attenuates depression (11).
Considering that the pain-depression dyad is associated to lesions
at the supraspinal level (1,12), it was hypothesized that low-frequency
EA is also more effective than high-frequency EA in treating the
pain-depression dyad. However, by comparing the effects of 2-, 50-,
100- and 2/100-Hz EA on the pain-depression dyad, a previous study
by our group found that only 100-Hz EA effectively alleviated
allodynia and depression (13).
Consequently, the present study was performed to investigate the
underlying mechanism of this effect of 100-Hz EA.
Accumulating evidence has shown that the
serotonergic system in the dorsal raphe nucleus (DRN) participates
in the descending modulation of pain (14,15). It
also has an important role in the induction and manipulation of
emotional disorders (16,17). Studies have shown that the
serotonergic system in DRN has a role in the effects of EA on pain
or emotional disorders (18,19). Whether the serotonergic system in DRN
is also involved in the effects of 100-Hz EA on the pain-depression
dyad has remained elusive, which was therefore the focus of the
present study.
Materials and methods
Animals
A total of 50 male Sprague-Dawley rats (weight,
250±20 g; age, 8 weeks old) were purchased from the Shanghai
Laboratory Animal Center (Shanghai, China) and housed in 40×50×25
cm cages at room temperature (25±1°C) with ad libitum access to
food and water. The animals were housed in groups of 5–6 rats with
a 12-h light/dark cycle. All animal experiments were performed in
accordance with the regulations of the State Science and Technology
Commission for the Care and Use of Laboratory Animals (no. 2,
1988). The present study was approved by the Ethics Committee of
Zhejiang Chinese Medical University (Hangzhou, China).
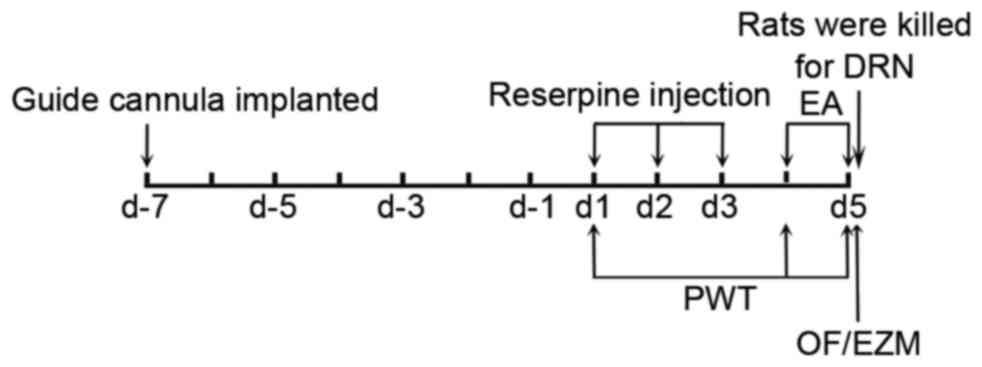
Experimental design
All animals were implanted with a guide cannula and
allowed to recover for seven days. The animals were then randomly
assigned to a normal group or a reserpine treatment group. The
reserpine treatment group included the following subgroups: Model
group, EA group and pCPA + EA group. Mechanical allodynia was
assessed via paw withdrawal threshold (PWT) to a mechanical
stimulus. The reserpine solution was subcutaneously injected once
daily for three consecutive days [day(d)1-d3]. The PWT was assessed
1 day after the last reserpine injection (d4).
Intracerebroventricular (i.c.v.) injection of a
para-chlorophenylalanine (pCPA) or sterile saline was administrated
immediately after the PWT tests. Then EA treatment was applied on
the same day. Another PWT test was performed 30 min after EA
treatment. On d5, the micro-injection and EA treatment were
repeated. PWT, open field (OF) and elevated zero maze (EZM) tests
were performed in sequence 30 min after EA treatment. All rats were
immediately sacrificed after the behavioral tests to obtain DRN
samples (Fig. 1).
Guide cannula implantation
The surgery was performed as described previously
(20), with slight modifications. On
the day of surgery, the animals were anesthetized with 0.5 ml/kg of
7% chloral hydrate (Sinopharm Chemical Reagent Co., Ltd, Shanghai,
China). Each rat was then placed in a stereotaxic apparatus (68002;
RWD Life Science Co., Ltd, Shenzhen, China). A small incision was
made to expose the skull and a burr hole was drilled. And i.c.v.
guide cannula (62001; RWD Life Science Co., Ltd) was implanted
according to the following coordinates, which were based on a
standard rat brain stereotaxic atlas (21): 0.9 mm posterior to the bregma, 1.5 mm
lateral to the midline and 3.8 mm ventral from the surface of the
skull. The guide cannula was affixed to the skull using two
stainless steel screws and dental cement. All of the animals were
allowed to recover for seven days after surgery.
Induction of the pain-depression
dyad
Reserpine powder (100 mg; Sigma-Aldrich, Merck KGaA,
Darmstadt, Germany) was dissolved in 400 µl glacial acetic acid and
then diluted with distilled water to 20 ml. The animals in the
reserpine treatment group were injected subcutaneously with a
reserpine solution (0.2 ml/kg daily) for three consecutive days as
previously described (13). The rats
in the normal group were injected subcutaneously with a vehicle
solution (2% solution of glacial acetic acid in distilled
water).
Micro-injection of pCPA
In the pCPA + EA group, pCPA (20 mg/ml;
Sigma-Aldrich; Merck KGaA), an inhibitor of serotonin
(5-hydroxytryptamine; 5-HT) resynthesis, was injected directly into
the lateral ventricle. An injection cannula was connected to a
250-µl Hamilton syringe with polyethylene tubing [outer diameter,
0.85 mm; inner diameter (ID), 0.42 mm; RWD Life Science Co., Ltd]
and back-filled with the pCPA solution. The injection cannula was
inserted into the guide cannula and 10 µl of the pCPA solution was
injected (i.c.v.) using a microsyringe infusion pump (UMP3; World
Precision Instruments Inc., Sarasota, FL, USA) at a rate of 1
µl/min. The injection cannula was kept in the guide cannula for 10
min after injection. The rats in other groups were injected with 10
µl sterile saline.
EA treatment
The bilateral Zusanli (ST 36, 5 mm lateral to the
anterior tubercule of the tibia) and Sanyinjiao (SP 6, 10 mm
proximal to the prominence of medial malleolus) acupoints were
selected as in the previous study by our group (13). Stainless steel acupuncture needles
(0.25 mm in diameter, 13 mm in length) were inserted into the
acupoints at a depth of 5 mm. The two ipsilateral needles were
connected to the output terminals of a Han's Acupoint Nerve
Stimulator (LH-202H; Huawei Co. Ltd., Beijing, China). The EA
parameters were adopted as follows: Square wave current output
(pulse width, 0.2 msec); stimulation intensities of 1.0, 1.5 and
2.0 mA, each for 15 min in sequence; stimulation frequency of 100
Hz. Animals were awake and calmed by placing their heads in black
hoods with no physical restraint during EA treatment. EA was
performed on d4 and d5.
Assessment of mechanical
allodynia
Mechanical allodynia was measured using an
electronic von Frey instrument (EVF-3; Bioseb In Vivo
Research Instruments, Chaville, France) (22,23).
Rats were placed on an elevated metal mesh floor and allowed to
adapt for 15 min. The stimulus was applied to the left hind paw for
5 sec. The plastic rod was pushed against the left hind paw with
linear ascending force until a robust and immediate withdrawal
occurred. The PWT was calculated as the mean of three tests with
intervals of 30 sec.
Behavioral tests of depression and
associated emotional disorders
Emotional behavior was quantified using the OF test
and EZM test, which are generally used to evaluate depression and
anxiety (24,25). All exterior lights were blocked and
the ambient noise in the testing room was maintained below 40 db;
abrupt loud noises that may have altered locomotion or produce
prolonged immobility were avoided during testing. The room
temperature was maintained at ~25°C.
The OF test was performed as follows (13): Four square OF arenas (100 cm in
diameter, 100 cm in width and 100 cm in height) constructed with
black plexiglass were placed together to form the apparatus. The
entire apparatus was wiped with 75% ethanol prior to each trial.
Animals were placed in the testing room 1 h before the test. Each
animal was placed in the center of the arena for 20 sec at the
beginning of the trial to adapt to the environment and the behavior
was then videotaped for 5 min and quantified by the SMART 3.0
system (Panlab Harvard Apparatus, Barcelona, Spain).
The EZM test was performed as follows (13): A maze with a black metallic annular
platform (100 cm in diameter, 25 cm in width and 55 cm in height)
was equally divided into four quadrants. Two opposite quadrants
(closed arms) were enclosed by black metallic walls (30 cm in
height) on the inner and outer edges of the platform, while the
remaining two opposite quadrants (open arms) remained uncovered
(24). The animals were placed in
the testing room 1 h prior to the test. The entire apparatus was
wiped with 75% ethanol prior to each trial. The animal was placed
in the center of a closed arm for 20 sec to adapt to the
environment and its behavior was then videotaped for 5 min and
quantified by the SMART 3.0 system.
Detection of 5-HT levels by
high-performance liquid chromatography and electrochemistry
detection (HPLC-ECD)
HPLC-ECD was performed as described previously
(26), with slight modifications. A
stock solution of 5-HT (H9523; Sigma-Aldrich; Merck KGaA) at a
concentration of 1 µg/ml was prepared as a standard. 5-HT powder
was added to 0.1 mol/l hydrochloric acid, which was then dissolved
in 0.1 mol/l perchloric acid including 1 mol/l EDTA (Sigma-Aldrich;
Merck KGaA). The working standard solutions were prepared by
serially diluting the stock solutions to concentrations of 160, 80,
40, 20, 10, 5 and 2.5 ng/ml. All solutions were filtered through a
0.22-µm Millipore filter prior to injection into the HPLC-ECD
system (Agilent 1200 HPLC system; Agilent Technologies, Inc., Santa
Clara, CA, USA) equipped with an ESA Coulochem III detector (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Standard solutions (10
µl) were injected using an autosampler to generate a standard curve
by HPLC-ECD analysis.
Samples were prepared in accordance with the
procedure of Pani et al (27). The animals were anesthetized and
transcardially perfused with ice-cold saline to remove circulating
blood. The brains were quickly removed from the calvaria and placed
in a cooled rat brain matrix (68711; RWD Life Science Co., Ltd).
The DRN was dissected (21), weighed
and stored at −80°C. The samples were ultrasonically homogenized in
0.1 mol/l perchloric acid containing 1 mol/l EDTA (10 µl solution
for each milligram of tissue). The homogenate was centrifuged at
25,300 × g at 4°C for 15 min. The supernatant was filtered through
a 0.22-µm millipore filter and stored at −80°C.
The following working conditions were maintained in
the HPLC-ECD system for the detection of 5-HT: Gradient elution;
mobile phase: 75 mmol/l
NaH2PO4·H2O; 1.7 mmol/l sodium
octane sulfonate; 100 µl/l triethyl amine; 25 mmol/l EDTA; 10%
acetonitrile; pH 3.0; C18 reversed-phase column (inner diameter,
3.2 mm; length, 150 mm; MD-150 ODS; Thermo fisher Scientific,
Inc.); flow rate, 0.6 ml/min; temperature, 33°C; injection volume,
10 µl; detector Model, 5014B (analytical cell) and 5020 (guard
cell); cell potentials E1, E2 and
EGC: −150, +220 and +270 mV, respectively; full
scale/range, 100 nA; signal output voltage, 1.0 V. The 5-HT levels
are expressed as ng/g of wet tissue.
Immunofluorescence of 5-HT expression
in the DRN
After the behavioral tests, the animals were
anesthetized with chloral hydrate (3.5 mg/kg, intraperitoneal
injection) and transcardially perfused with 150 ml pre-cooled
saline followed by 400 ml of a 4% paraformaldehyde solution. The
brains were removed and post-fixed in paraformaldehyde for 24 h
prior to being placed in a 15% sucrose solution overnight. The
brains were transferred to a 30% sucrose solution and incubated for
72 h prior to embedding in optimal cutting temperature matrix.
Cryostat sections were cut at 30 µm around the DRN region (bregma
−7.50, 8.00 and 8.50 mm) on a sliding microtome and blocked in 5%
donkey serum (ab7475; Abcam, Cambridge, UK) in Tris-buffered saline
containing Tween-20 (TBST)/Triton for 60 min. The sections were
then incubated at 4°C overnight in TBST containing an anti-5-HT
primary antibody (1:100 dilution; cat. no. ab10385; Abcam).
Immunoreactivity to the antigen was visualized using an Alexa Fluor
488-conjugated secondary antibody (1:1,000 dilution; cat. no.
103715; Jackson ImmunoResearch Laboratories, Inc., Bar Harbor, ME,
USA). Images were obtained using a fluorescence microscope (Olympus
IX71; Olympus, Tokyo, Japan) equipped with Image-Pro Insight 8.0
software (Media Cybernetics, Rockville, MD, USA).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was
used for all data analysis. Statistical analysis was performed by
one-way analysis of variance followed by a post-hoc test of the
least significant differences for multiple comparisons. The data
were analyzed using a two-tailed student's t-test for the behavior
results, 5-HT levels and 5-HT cell expression levels. P<0.05 was
considered to indicate a statistically significant difference.
Results
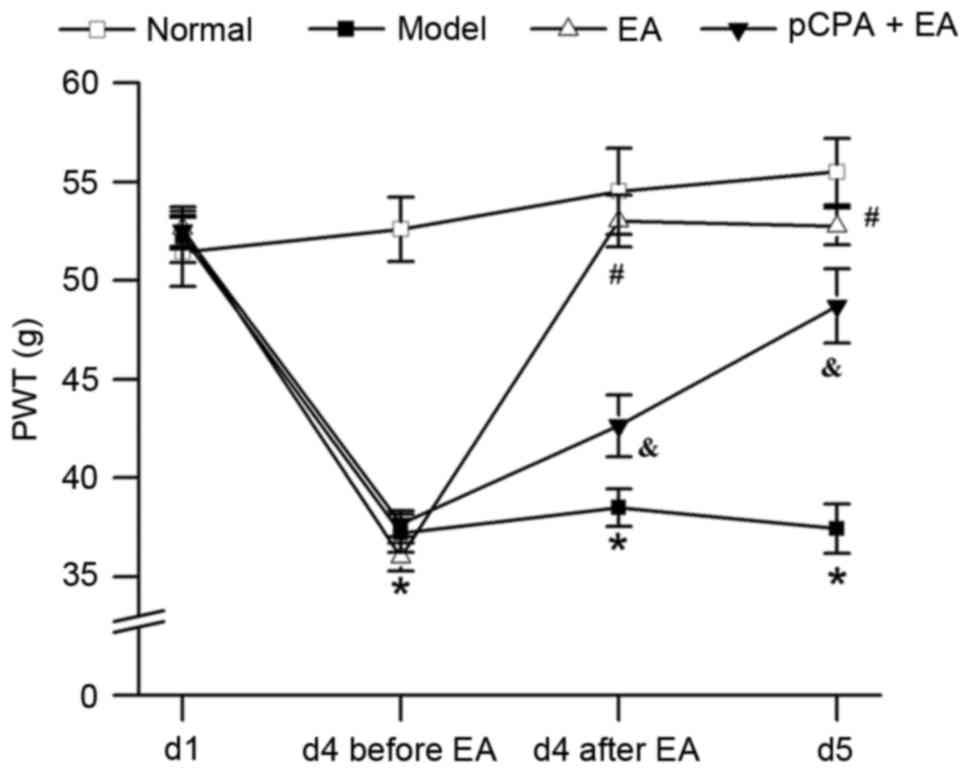
EA increases the PWT in rats with
pain-depression dyad via 5-HT
As is shown in Fig.
2, repeated injection of reserpine resulted in a significant
decrease in the PWT of rats (P<0.05). 100-Hz EA significantly
increased the PWT in reserpine-injected rats (P<0.05, vs. the
model group). Injection of pCPA (i.c.v.) significantly restrained
the effect of 100-Hz EA on PWT (P<0.05, vs. the EA group).
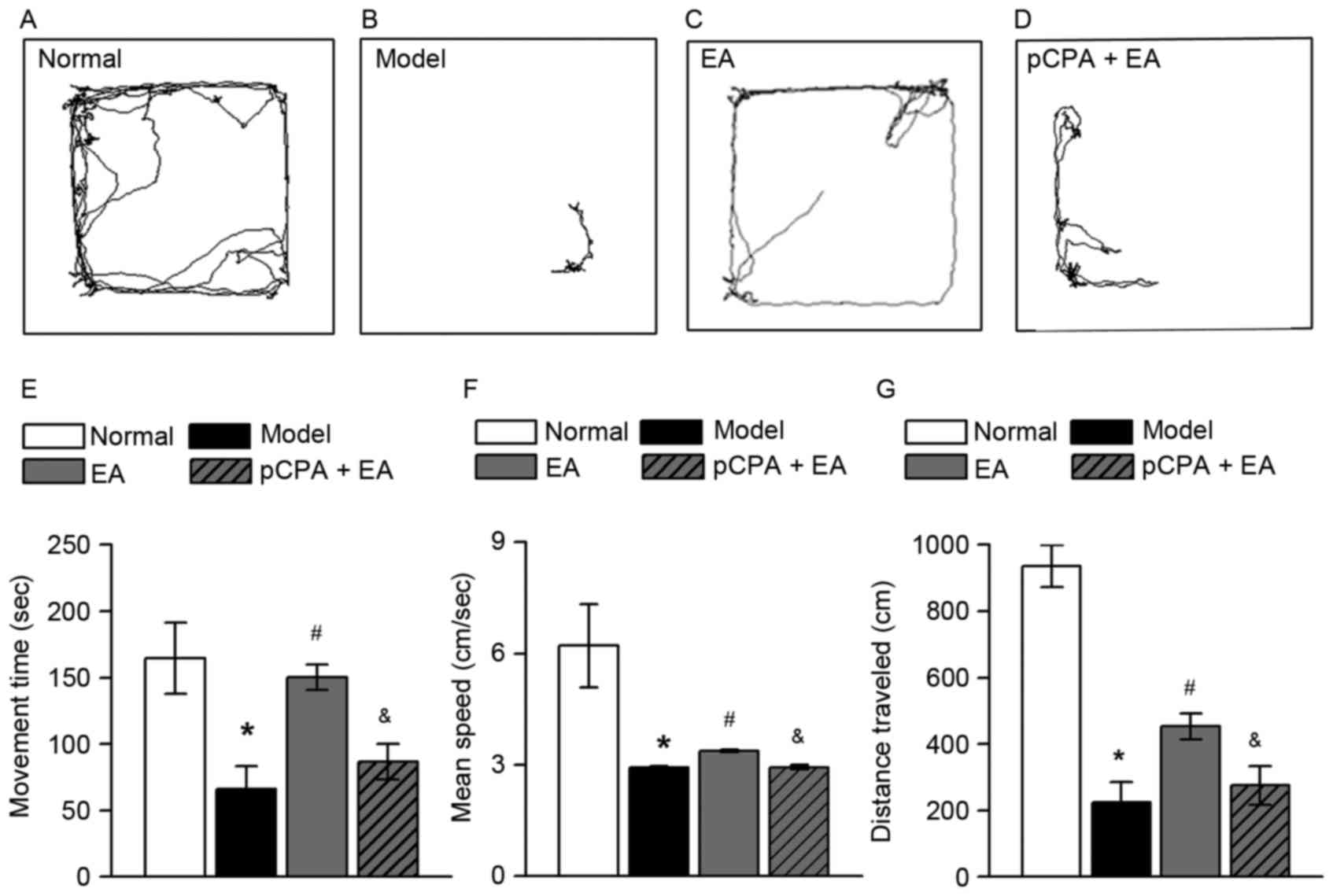
EA reduces depressive-like behavior in
rats with pain-depression dyad via 5-HT
The OF test was performed to observe depressive-like
behavior in rats. Representative trajectories from the OF test for
the normal group, model group, EA group and pCPA + EA group are
presented in Fig. 3A-D,
respectively. Repeated injection of reserpine significantly reduced
movement time, mean speed and distance traveled in rats (Fig. 3E-G, respectively; P<0.05).
Movement time, mean speed and distance traveled were significantly
increased by 100-Hz EA (P<0.05, vs. the model group). Injection
of pCPA (i.c.v.) significantly restrained the effect of 100-Hz EA
on the three parameters mentioned above (P<0.05, vs. the EA
group).
EA reduces anxiety-like behavior in
rats with pain-depression dyad via 5-HT
In certain rodent models of depression, anxiety-like
responses are observed (28,29). To determine whether anxiety-like
behaviors accompany the pain-depression dyad, rats were subjected
to the EZM test. Three-dimensional activities in the EZM test of
animals from the normal group, model group, EA group, and pCPA + EA
group are presented in Fig. 4A-D,
respectively. Repeated injection of reserpine resulted in
significant decreases in entries in the open arms, stretching time
and distance traveled in rats (Fig.
4E-G, respectively; P<0.05). 100-Hz EA significantly
increased the number of entries in the open arms, stretching time
and distance traveled in rats with pain-depression dyad (P<0.05,
vs. the model group). Although a declining trend of entries into
the open arms existed in the pCPA + EA group, no significant
difference was found between the EA group and the pCPA + EA group
(Fig. 4E). Injection of pCPA
(i.c.v.) significantly restrained the effects of 100-Hz EA on
stretching time and distance traveled (Fig. 4F and G, respectively; P<0.05, vs.
the EA group).
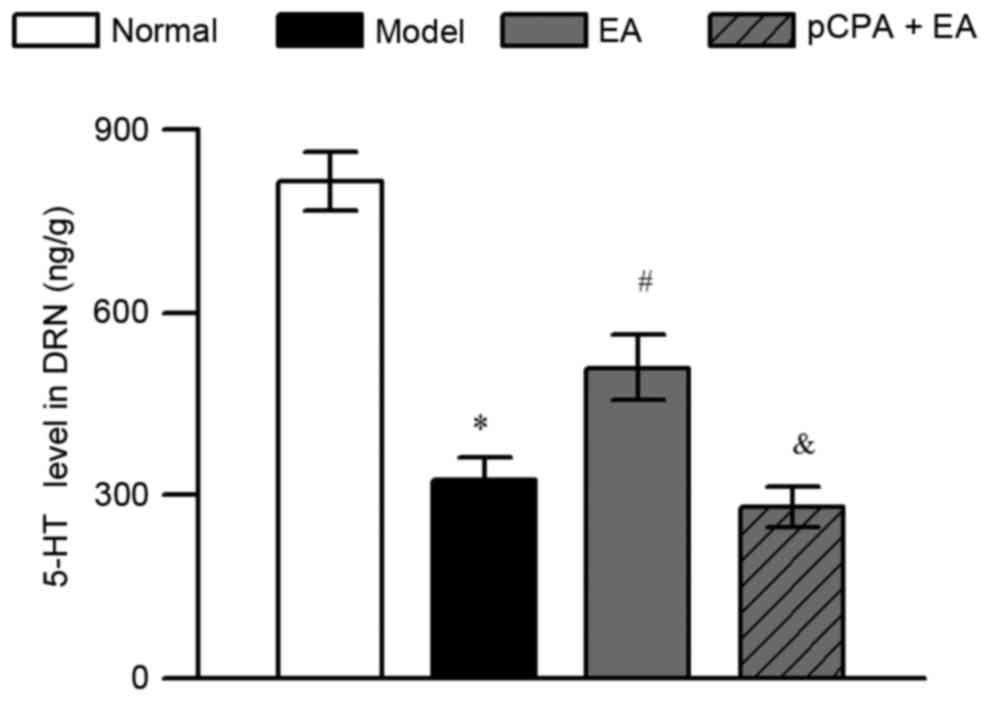
EA increases 5-HT in the DRN of rats
with pain-depression dyad
HPLC-ECD was adopted to determine the effect of
100-Hz EA on 5-HT levels in the DRN in rats with pain-depression
dyad. Repeated injection of reserpine significantly decreased the
5-HT levels of DRN in rats (Fig. 5;
P<0.05). 100-Hz EA significantly increased the 5-HT levels of
DRN in reserpine-injected rats (P<0.05, vs. the model group).
The upregulation of 5-HT levels in DRN by 100-Hz EA was completely
abrogated by injection of pCPA (i.c.v.) in the pCPA + EA group
(P<0.05, when compared to the EA group).
EA enhances the number of
5-HT-immunoreactive cells in the DRN of rats with pain-depression
dyad
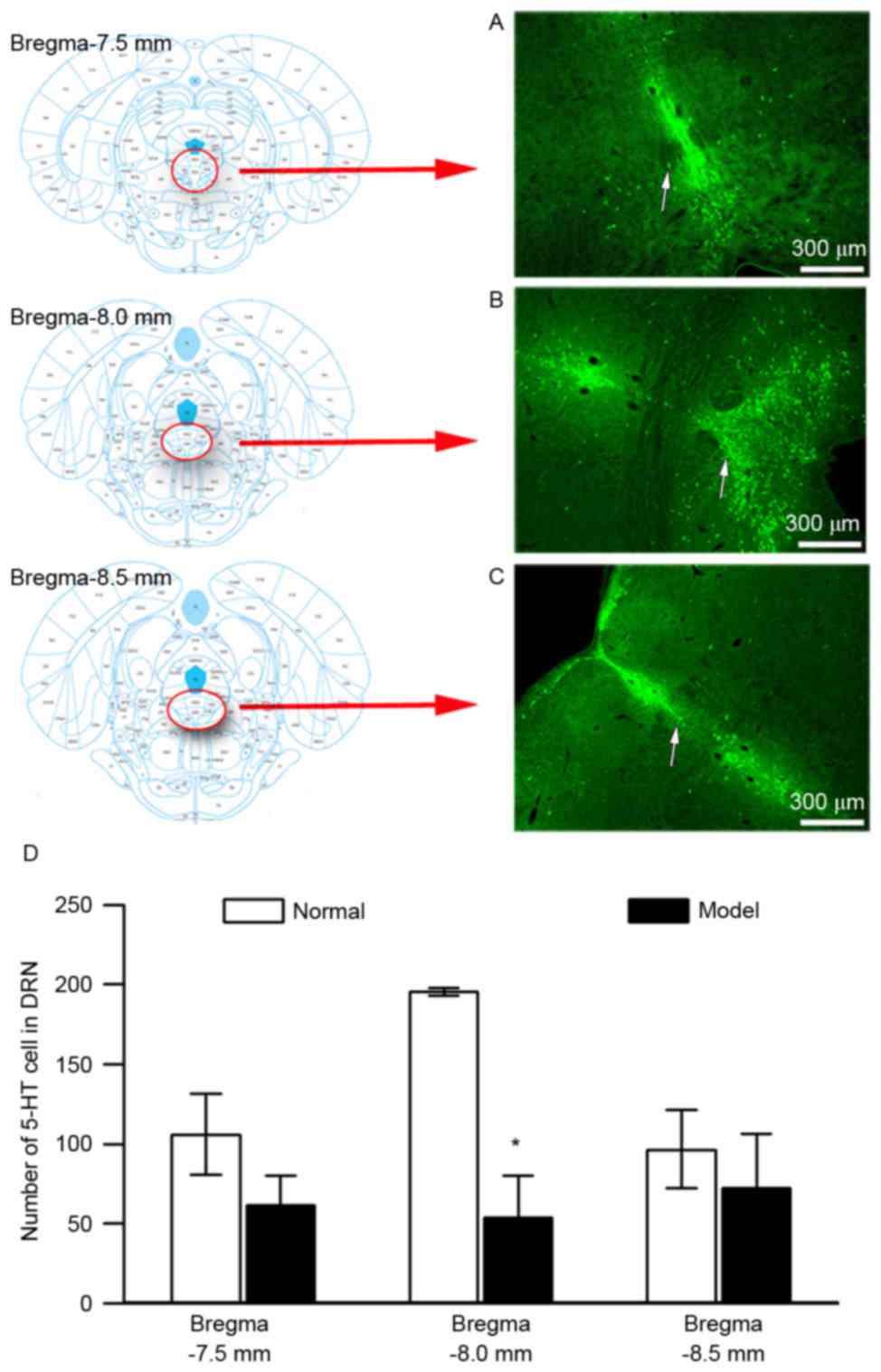
Distribution of 5-HT-immunoreactive cells in the DRN
of rats was investigated at bregma −7.5, −8.0 and −8.5 mm (Fig. 6A-C, respectively). The abundance of
5-HT-immunoreactive cells in the DRN was higher at bregma −8.00 mm
than at bregma −7.50 and −8.50 mm. A significant decline of
5-HT-immunoreactive cells in the DRN compared with the normal group
was found at bregma −8.00 mm (Fig.
6D, P<0.05), which was greater than that observed at the
other points. Bregma −8.0 mm was therefore determined to be the
optimal location for examining the effect of 100-Hz EA on
5-HT-immunoreactive cells in the DRN of rats with pain-depression
dyad.
Representative images of 5-HT-immunoreactive cells
in the DRN for the normal group, model group, EA group and pCPA +
EA group are shown in Fig. 7A-D,
respectively. As is shown in Fig.
7E, repeated injection of reserpine significantly reduced the
number of 5-HT-immunoreactive cells in the DRN of rats (P<0.05).
Of note, 100-Hz EA significantly increased the number of
5-HT-immunoreactive cells in the DRN in reserpine-injected rats
when compared to that in the model group (P<0.05). The
upregulation of the number of 5-HT-immunoreactive cells in DRN by
100-Hz EA was totally abrogated by injection of pCPA (i.c.v.) in
the pCPA + EA group (P<0.05, when compared to the EA group).
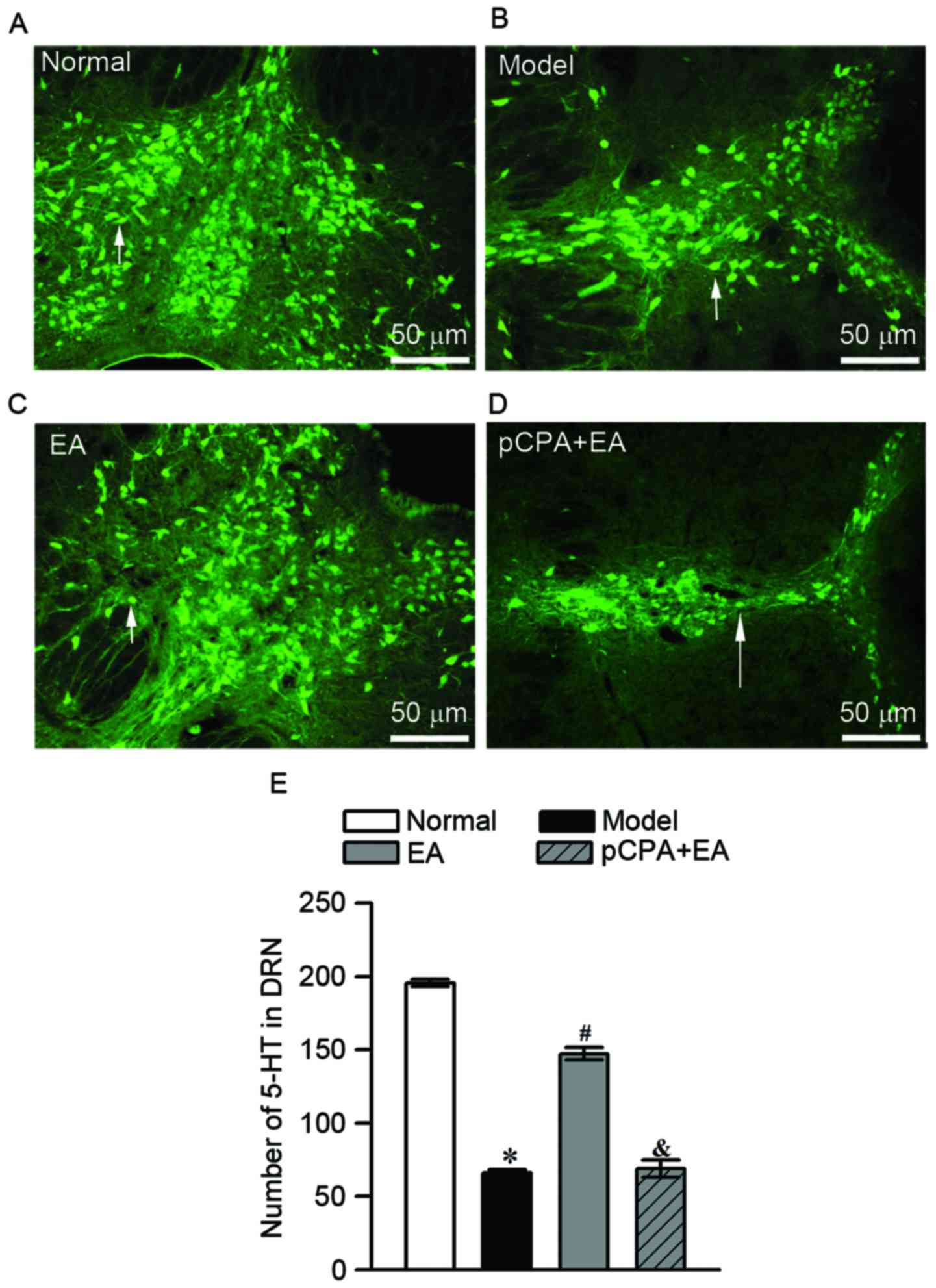 | Figure 7.Effect of EA on 5-HT-immunoreactive
cells in DRN assessed by immunofluorescence on day 5. (A-D)
Immunofluorescence detection of 5-HT in the DRN of (A) the normal
group, (B) model group, (C) EA group and (D) pCPA + EA group (scale
bar, 50 µm for all). White arrows indicate the 5-HT-immunoreactive
cells. (E) Quantified amount of 5-HT-immunoreactive cells in DRN.
Values are expressed as the mean ± standard error of the mean
(n=3). *P<0.05, vs. normal group; #P<0.05, vs.
model group; &P<0.05, vs. EA group. DRN, dorsal
raphe nucleus; EA, electroacupuncture; 5-HT, 5-hydroxytryptamine;
pCPA, para-chlorophenylalanine. |
Discussion
Pain-depression dyad is a complex illness with
symptoms of pain overlapping with emotional disorders such as
depression and anxiety (1). It has
been reported that 52% patients with chronic pain suffer from
depression, which is a costly health problem (30). While antidepressants and
antiepileptics substantially reduce the symptoms, numerous patients
cannot tolerate the side effects of their long-term administration.
This dissatisfaction compelled researchers to explore complementary
and alternative medicines (1,6,9). A previous study by our group initially
demonstrated that EA with high frequency but not low frequency
effectively relieves pain-depression dyad (13). The present study found that 5-HT in
the DRN was involved in the analgesic and anti-depressant effects
of 100-Hz EA.
Several modeling methods are used for studying the
mechanisms of the pain-depression dyad, including nerve injury
(31), stress load (32,33) and
administration of reserpine (34),
monosodium iodoacetate (35) and
Freund's complete adjuvant (36).
Reserpine-injected rats, an ideal model of pain-depression dyad,
display hyperalgesia, allodynia and depressive behaviors
accompanied with anxiety (28,34,37). The
response is also similar to that of pain-depression patients in the
clinic. This model was thus selected for use in the present study.
The results showed that reserpine injection resulted in mechanical
allodynia in rats. The forced swimming test, OF test and EZM test
have been widely used in animal psychology for decades (24,25,38). The
forced swimming and OF tests are commonly adopted to evaluate
depressive behaviors of animals (38). Considering that the forced swimming
test can influence pain sensitivity in rodents (39,40), the
present study used the OF test to evaluate depressive behaviors in
rats with reserpine-induced pain-depression dyad. As the
reserpine-injected rats exhibited not only depressive-like but also
anxiety-like behavior in the EZM test, it was indicated that the
pain-depression dyad model was successfully established.
5-HT has an important role in the central nervous
system in the descending control of pain or emotion (18,41–43). DRN
is abundant of serotonergic neurons (42). The present study found that
reserpine, a monoamine depletor, caused a decline of 5-HT in the
DRN, which contributes to reserpine-induced allodynia and
depressive behaviors in rats (37,44).
Acupuncture, particularly EA, is commonly used for pain or
emotional problems (7–9,19,45,46).
The present and a previous study by our group showed that 100-Hz EA
effectively improved mechanical allodynia and depressive behaviors
in rats caused by reserpine injection (13). However, the mechanism underlying the
effects of EA on the pain-depression dyad has been rarely assessed.
Studies have shown that high-frequency EA at the spinal cord is
highly effective and exerts its effects by segmental inhibition at
the spinal cord (10,42,47,48)
However, recent studies demonstrated that 100-Hz EA improved
Parkinson's disease (a typical brain-derived disease) and may exert
its effects through the cerebrum (49,50). In
the present study, 100-Hz EA upregulated 5-HT DRN in rats with
reserpine-induced pain-depression dyad. Furthermore, injection of
pCPA (i.c.v.), an inhibitor of 5-HT resynthesis, suppressed the
upregulation of 5-HT in the DRN by 100-Hz EA and partially
abrogated the analgesic and anti-depressive effects of 100-Hz EA.
These findings suggested that 100-Hz EA improves reserpine-induced
pain-depression dyad partially via 5-HT DRN. It may be assumed that
high-frequency EA on body parts other than the spinal cord is also
efficient.
In conclusion, the present study was the first, to
the best of our knowledge, to demonstrate that 5-HT in the DRN
participates in mediating the effects of 100 Hz EA on the
pain-depression dyad. The present study provided a scientific basis
for the value of high-frequency EA in treating
supraspinal-originating diseases.
Acknowledgements
We acknowledge Mr. Sheng-Jian Zhuang and Mr. Jie
Gong (postgraduate students) at Department of Neurobiology and
Acupuncture Research, The Third Clinical Medical College, Zhejiang
Chinese Medical University (Hangzhou, China) for their support with
the behavioral testing of all of the animals. The present study was
supported by the National Natural Science Foundation of China (nos.
81072855 and 81303039), the Zhejiang Provincial Natural Science
Foundation of China (nos. Z2100979 and LY12H27015) and the Key
Subject of State Administration of Traditional Chinese Medicine of
China (Acupuncture and Moxibustion). This manuscript has been
edited and proofread by Nature Publishing Group Language
Editing.
Glossary
Abbreviations
Abbreviations:
|
5-HT
|
5-hydroxytryptamine (serotonin)
|
|
DRN
|
dorsal raphe nucleus
|
|
EA
|
electro-acupuncture
|
|
PWT
|
paw withdrawal threshold
|
|
pCPA
|
para-chlorophenylalanine
|
|
OF
|
open field
|
|
EZM
|
elevated zero maze
|
|
ST 36
|
Zusanli, 5 mm lateral to the anterior
tubercule of tibia
|
|
SP 6
|
Sanyinjiao, 10 mm proximal to the
prominence of medial malleolus
|
|
HPLC-ECD
|
high-performance liquid chromatography
and electrochemistry detection
|
|
TBST
|
Tris-buffered saline and Tween-20
|
References
|
1
|
Goldenberg DL: Pain/depression dyad: A key
to a better understanding and treatment of functional somatic
syndromes. Am J Med. 123:675–682. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohayon MM and Schatzberg AF: Using chronic
pain to predict depressive morbidity in the general population.
Arch Gen Psychiatry. 60:39–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Minami M: Neuronal mechanisms underlying
pain-induced negative emotions. Brain nerve. 64:1241–1247. 2012.(In
Japanese). PubMed/NCBI
|
|
4
|
Wieser MJ, Gerdes AB, Reicherts P and
Pauli P: Mutual influences of pain and emotional face processing.
Front Psychol. 5:11602014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Keefe FJ, Lumley M, Anderson T, Lynch T,
Studts JL and Carson KL: Pain and emotion: New research directions.
J Clin Psychol. 57:587–607. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellato E, Marini E, Castoldi F,
Barbasetti N, Mattei L, Bonasia DE and Blonna D: Fibromyalgia
syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain
Res Treat. 2012:4261302012.PubMed/NCBI
|
|
7
|
Berman BM, Langevin HM, Witt CM and Dubner
R: Acupuncture for chronic low back pain. N Engl J Med.
363:454–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin JG, Lo MW, Wen YR, Hsieh CL, Tsai SK
and Sun WZ: The effect of high and low frequency electroacupuncture
in pain after lower abdominal surgery. Pain. 99:509–514. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber A, Werneck L, Paiva E and Gans P:
Effects of music in combination with vibration in acupuncture
points on the treatment of fibromyalgia. J Altern Complement Med.
21:77–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersson SA and Holmgren E: Pain
threshold effects of peripheral conditioning stimulationAdvances in
Pain Research Therapy. 1. Raven Press; New York, NY: pp. 761–768.
1976
|
|
11
|
Youn JI, Sung KK, Song BK, Kim M and Lee
S: Effects of electro-acupuncture therapy on post-stroke depression
in patients with different degrees of motor function impairments: A
pilot study. J Phys Ther Sci. 25:725–728. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lumley MA, Cohen JL, Borszcz GS, Cano A,
Radcliffe AM, Porter LS, Schubiner H and Keefe FJ: Pain and
emotion: A biopsychosocial review of recent research. J Clin
Psychol. 67:942–968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu YY, Jiang YL, He XF, Zhao XY, Shao XM,
Du JY and Fang JQ: Effects of electroacupuncture with dominant
frequency at SP 6 and ST 36 based on meridian theory on
pain-depression dyad in rats. Evid Based Complement Alternat Med.
2015:7328452015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ossipov MH, Dussor GO and Porreca F:
Central modulation of pain. J Clin Invest. 120:3779–3787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freitas RL, Bassi GS, de Oliveira AM and
Coimbra NC: Serotonergic neurotransmission in the dorsal raphe
nucleus recruits in situ 5-HT (2A/2C) receptors to modulate the
post-ictal antinociception. Exp Neurol. 213:410–418. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lowry CA, Johnson PL, Hay-Schmidt A,
Mikkelsen J and Shekhar A: Modulation of anxiety circuits by
serotonergic systems. Stress. 8:233–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lowry CA, Hollis JH, De Vries A, Pan B,
Brunet LR, Hunt JR, Paton JF, van Kampen E, Knight DM, Evans AK, et
al: Identification of an immune-responsive mesolimbocortical
serotonergic system: Potential role in regulation of emotional
behavior. Neuroscience. 146:756–772. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu SF: Pain and analgesiaNeurology. Wei L:
1. 2nd. Fudan University Press; Shanghai: pp. 330–349. 1999, (In
Chinese).
|
|
19
|
Yano T, Kato B, Fukuda F, Shinbara H,
Yoshimoto K, Ozaki A and Kuriyama K: Alterations in the function of
cerebral dopaminergic and serotonergic systems following
electroacupuncture and moxibustion applications: Possible
correlates with their antistress and psychosomatic actions.
Neurochem Res. 29:283–293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bertholomey ML, Henderson AN, Badia-Elder
NE and Stewart RB: Neuropeptide Y (NPY)-induced reductions in
alcohol intake during continuous access and following alcohol
deprivation are not altered by restraint stress in
alcohol-preferring (P) rats. Pharmacol Biochem Behav. 97:453–461.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paxinos G and Watson C: The rat brain in
stereotaxic coordinates. Academic Press; San Diego, CA: 1986
|
|
22
|
Thibault K, Calvino B, Rivals I, Marchand
F, Dubacq S, McMahon SB and Pezet S: Molecular mechanisms
underlying the enhanced analgesic effect of oxycodone compared to
morphine in chemotherapy-induced neuropathic pain. PLoS One.
9:e912972014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thibault K, Elisabeth B, Sophie D, Claude
FZ, Bernard R and Bernard C: Antinociceptive and anti-allodynic
effects of oral PL37, a complete inhibitor of
enkephalin-catabolizing enzymes, in a rat model of peripheral
neuropathic pain induced by vincristine. Eur J Pharmacol.
600:71–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shepherd JK, Grewal SS, Fletcher A, Bill
DJ and Dourish CT: Behavioural and pharmacological characterisation
of the elevated ‘zero-maze’ as an animal model of anxiety.
Psychopharmacology (Berl). 116:56–64. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gould TD, Dao DT and Kovacsics CE: The
open field testMood and anxiety related phenotypes in mice. Gould
TD: Humana Press; Totowa, NJ: pp. 1–20. 2009, View Article : Google Scholar
|
|
26
|
Singer S, Rossi S, Verzosa S, Hashim A,
Lonow R, Cooper T, Sershen H and Lajtha A: Nicotine-induced changes
in neurotransmitter levels in brain areas associated with cognitive
function. Neurochem Res. 29:1779–1792. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pani AK, Jiao Y, Sample KJ and Smeyne RJ:
Neurochemical measurement of adenosine in discrete brain regions of
five strains of inbred mice. PLoS One. 9:e924222014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukui M, Rodriguiz RM, Zhou J, Jiang SX,
Phillips LE, Caron MG and Wetsel WC: Vmat2 heterozygous mutant mice
display a depressive-like phenotype. J Neurosci. 27:10520–10529.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Vry J, Vanmierlo T, Martínez-Martínez
P, Losen M, Temel Y, Boere J, Kenis G, Steckler T, Steinbusch HW,
De Baets M and Prickaerts J: TrkB in the hippocampus and nucleus
accumbens differentially modulates depression-like behavior in
mice. Behav Brain Res. 296:15–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bair MJ, Robinson RL, Katon W and Kroenke
K: Depression and pain comorbidity: A literature review. Arch
Intern Med. 163:2433–2445. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alba-Delgado C, Llorca-Torralba M,
Horrillo I, Ortega JE, Mico JA, Sánchez-Blázquez P, Meana JJ and
Berrocoso E: Chronic pain leads to concomitant noradrenergic
impairment and mood disorders. Biol Psychiatry. 73:54–62. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rojas-Corrales MO, Berrocoso E,
Gibert-Rahola J and Micó JA: Antidepressant-like effects of
tramadol and other central analgesics with activity on monoamines
reuptake, in helpless rats. Life Sci. 72:143–152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang N, Shi M, Wang JY and Luo F:
Brain-network mechanisms underlying the divergent effects of
depression on spontaneous versus evoked pain in rats: A multiple
single-unit study. Exp Neurol. 250:165–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taguchi T, Katanosaka K, Yasui M, Hayashi
K, Yamashita M, Wakatsuki K, Kiyama H, Yamanaka A and Mizumura K:
Peripheral and spinal mechanisms of nociception in a rat
reserpine-induced pain model. Pain. 156:415–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stevenson GW, Mercer H, Cormier J, Dunbar
C, Benoit L, Adams C, Jezierski J, Luginbuhl A and Bilsky EJ:
Monosodium iodoacetate-induced osteoarthritis produces
pain-depressed wheel running in rats: Implications for preclinical
behavioral assessment of chronic pain. Pharmacol Biochem Behav.
98:35–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stein C, Millan MJ and Herz A: Unilateral
inflammation of the hindpaw in rats as a model of prolonged noxious
stimulation: Alterations in behavior and nociceptive thresholds.
Pharmacol Biochem Behav. 31:445–451. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arora V, Kuhad A, Tiwari V and Chopra K:
Curcumin ameliorates reserpine-induced pain-depression dyad:
Behavioural, biochemical, neurochemical and molecular evidences.
Psychoneuroendocrinology. 36:1570–1581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang CY, Guo HR, Tsai WC, Yang KL, Lin
LC, Cheng TJ and Chuu JJ: Subchronic arsenic exposure induces
anxiety-like behaviors in normal mice and enhances depression-like
behaviors in the chemically induced mouse model of depression.
Biomed Res Int. 2015:1590152015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ibironke GF and Rasal KS: Forced swimming
stress-related hypoalgesia: Nondependence on the histaminergic
mechanisms. Neurophysiology. 45:340–343. 2013. View Article : Google Scholar
|
|
40
|
Łapo IB, Konarzewski M and Sadowski B:
Analgesia induced by swim stress: Interaction between analgesic and
thermoregulatory mechanisms. Pflugers Arch. 446:463–469. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McDevitta RA and Neumaier JF: Regulation
of dorsal raphe nucleus function by serotonin autoreceptors: A
behavioral perspective. J Chem Neuroanat. 41:234–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han JS: 5-hydroxytryptamineNeuroscience.
Han ZG and Liu Y: 1. 3rd. Peking University Medical Press; Beijing:
pp. 382–351. 2009, (In Chinese).
|
|
43
|
Hale MW, Dady KF, Evans AK and Lowry CA:
Evidence for in vivo thermosensitivity of serotonergic neurons in
the rat dorsal raphe nucleus and raphe pallidus nucleus implicated
in thermoregulatory cooling. Exp Neurol. 227:264–278. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagakura Y, Oe T, Aoki T and Matsuoka N:
Biogenic amine depletion causes chronic muscular pain and tactile
allodynia accompanied by depression: A putative animal model of
fibromyalgia. Pain. 146:26–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sniezek DP and Siddiqui IJ: Acupuncture
for treating anxiety and depression in women: A clinical systematic
review. Med Acupunct. 25:164–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park H, Yoo D, Kwon S, Yoo TW, Park HJ,
Hahm DH, Lee H and Kim ST: Acupuncture stimulation at HT7
alleviates depression-induced behavioral changes via regulation of
the serotonin system in the prefrontal cortex of
maternally-separated rat pups. J Physiol Sci. 62:351–357. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ogata A, Sugenoya J, Nishimura N and
Matsumoto T: Low and high frequency acupuncture stimulation
inhibits mental stress-induced sweating in humans via different
mechanisms. Auton Neurosci. 118:93–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fung SJ and Chan SH: Primary afferent
depolarization evoked by electroacupuncture in the lumbar cord of
the cat. Exp Neurol. 52:168–176. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Du J, Sun ZL, Jia J, Wang X and Wang XM:
High-frequency electro-acupuncture stimulation modulates
intracerebral γ-aminobutyric acid content in rat model of
Parkinson's disease. Sheng Li Xue Bao. 63:305–310. 2011.PubMed/NCBI
|
|
50
|
Rui G, Guangjian Z, Yong W, Jie F, Yanchao
C, Xi J and Fen L: High frequency electro-acupuncture enhances
striatum DAT and D1 receptor expression, but decreases D2 receptor
level in 6-OHDA lesioned rats. Behav Brain Res. 237:263–269. 2013.
View Article : Google Scholar : PubMed/NCBI
|