Introduction
Congenital branchial cleft anomalies are the second
most common lesions in the head and neck in children (1). The term branchial cyst was first
mentioned by Ascherson in 1832 (2).
First branchial cleft anomalies (FBCA) account for 1 to 8% of all
types of anomalies. Four theories have been proposed for the
development of FBCA, which include incomplete obliteration of the
branchial clefts, persistence of vestiges of the precervical sinus,
the thymopharyngeal theory and the cervical lymph node theory
(3). The most widely accepted theory
is the incomplete obliteration of the branchial clefts during
embryogenesis. According to the varying degree of closure, the
lesion could present sinus, fistula or cyst. Six pairs of branchial
arches appear during the 4th week of human embryological
development, and these arches are separated from each other by 5
pharyngeal pouches internally (endoderm) and the 5 branchial clefts
(ectoderm) externally. In the embryonic period at seven weeks, the
arches fuse and the clefts obliterate. FBCA are due to incomplete
fusion of the ventral portion of the first and second arches.
During development, the closure time of the cleft is concurrent
with the migration of the facial nerve and emergence of the
developing parotid gland, which originates from the second
branchial arch; thus, FBCA have a close relationship between these
structures. As obliteration of the cleft proceeds from ventral to
dorsal, lesions occur more often near the ear and parotid gland
area than at the hyoid region (4).
FBCA can occur in any part of the external auditory
canal (EAC) to the mandibular angle, including the parotid gland
area. The complete removal of the lesion is the only way to treat
FBCA, and one of the key factors for complete excision is keeping
the tract, cyst, and any fistula or scar tissue intact (5–7).
Fistulas are often closely associated with the facial nerve, which
is prone to damage during the operation, thus causing facial
paralysis postoperatively and decreasing the quality of life in
children. Therefore, the surgical treatment of FBCA is challenging.
This study analyzes all the cases of FBCA operated at Shanghai
Children's Hospital (Shanghai, China) during the past 7 years.
Materials and methods
This study was conducted at the Pediatric ENT
Department at Shanghai Children's Hospital in China. All children
who underwent surgery for first branchial cleft sinus or fistula
from June 2009 to June 2016 were included in this study. The data
included patient demographics, operative details, histopathological
sections and postoperative course. The data revealing the
relationship between the facial nerve and the branchial cleft were
also analyzed.
Results
The summary of patients was depicted in Table I. Thirty patients (11 male and 19
female) with anomalies of FBCA were diagnosed. The ages ranged from
1 to 13 years (median, 3 years). The lesions were on the right side
in 10 cases and on the left in 20. Bilateral lesions were not
recorded. Seven cases had no previous intervention, and 23 cases
had received a previous intervention. 22 cases had been treated by
abscess incision and drainage, 7 cases had received 2 abscess
incision and drainage procedures, and 4 cases had received abscess
incision and drainage three times. Four cases had a history of
surgical resection, and all underwent one surgery. Olsen et
al classified the defects as cysts, sinuses, or fistulas in
1980 (8). Three cases were cysts.
Fourteen cases involved sinus defects. There were 13 cases with
fistulas, which had external and internal openings. In 17 cases,
the lesions were only located in the retroauricular groove. In 3
cases, the lesions were only located in the cheek. The lesions of 3
cases were both located in the cheek and the retroauricular groove,
and the lesions of 5 cases were only located near the mandibular
angle. In 2 cases, the lesions were both located near the
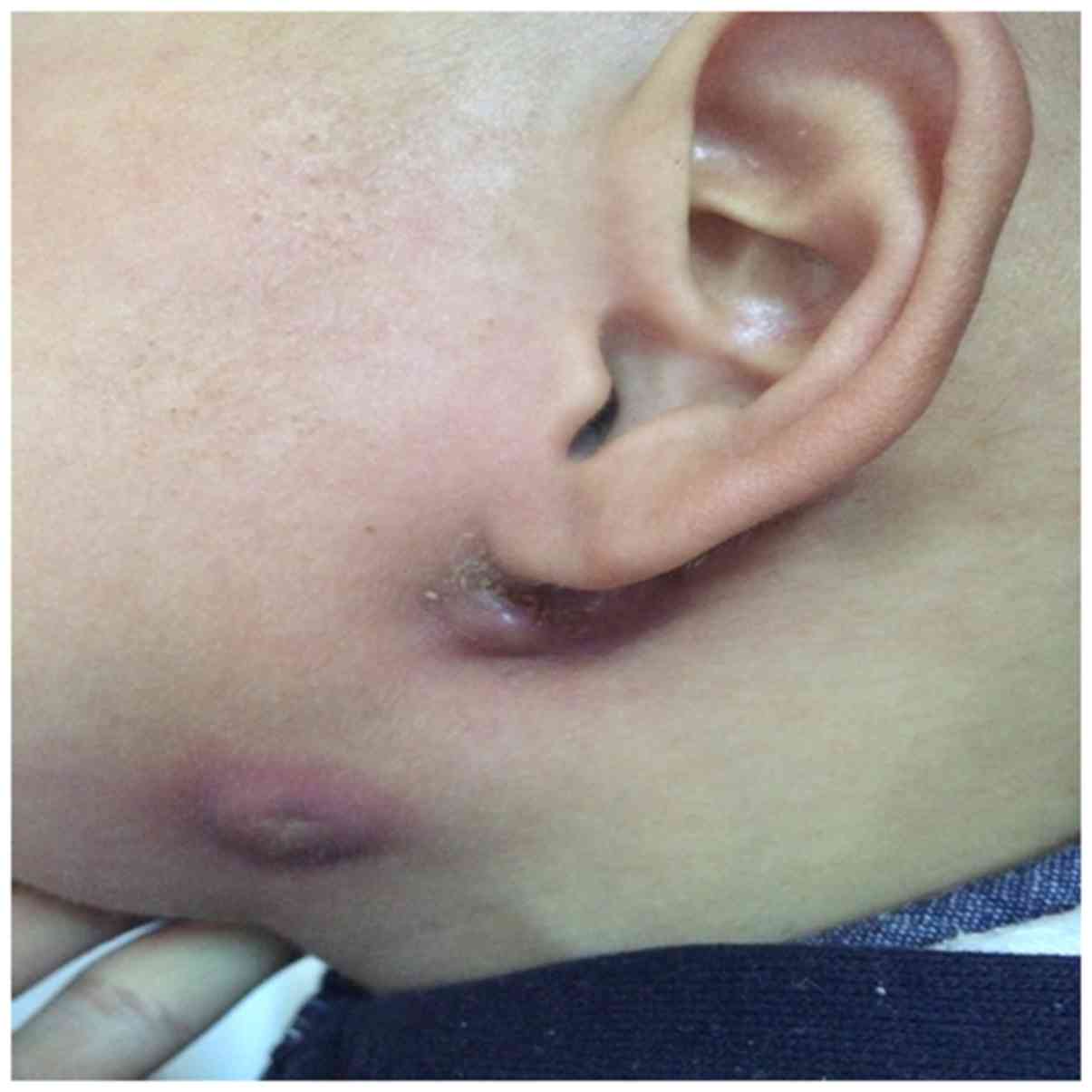
mandibular angle and retroauricular groove (Fig. 1). There were two skin openings of the
fistula in some cases, which mostly resulted from abscess incision
and drainage. One patient had pinna malformation, and one patient
had stenosis of the EAC.
 | Table I.Features of 30 cases with FBCA. |
Table I.
Features of 30 cases with FBCA.
| Characteristic | Number | % |
|---|
| Sex |
|
|
| Male | 11 | 36.7 |
|
Female | 19 | 63.3 |
| Age (year) |
|
|
| 1–4 | 17 | 56.7 |
| 5–8 | 9 | 30.0 |
| 9–11 | 4 | 13.3 |
| Side |
|
|
| Left | 10 | 33.3 |
|
Right | 20 | 66.7 |
| Previous
treatment |
|
|
| Incision
and drainage | 22 | 73.3 |
| Surgical
resection | 4 | 13.3 |
| Non | 7 | 23.3 |
| Olsen
classification |
|
|
|
Cysts | 3 | 10.0 |
|
Sinuses | 14 | 46.7 |
|
Fistulas | 13 | 43.3 |
| Anatomical site |
|
|
|
Retroauricular groove | 17 | 56.7 |
|
Cheek | 3 | 10.0 |
|
Retroauricular groove and
cheek | 3 | 10.0 |
|
Mandibular angle | 5 | 16.7 |
|
Mandibular angle and
retroauricular groove | 2 | 6.7 |
| Work
classification |
|
|
| Work
I | 18 | 60.0 |
| Work
II | 12 | 40.0 |
| Relation of tract to
facial nerve |
|
|
|
Superficial | 21 | 70.0 |
| Between
branches | 6 | 20.0 |
| Deep | 3 | 10.0 |
| Complications |
|
|
| Temporary
facial paralysis | 2 | 6.7 |
| Permanent
facial paralysis | 1 | 3.3 |
|
Recurrence | 1 | 3.3 |
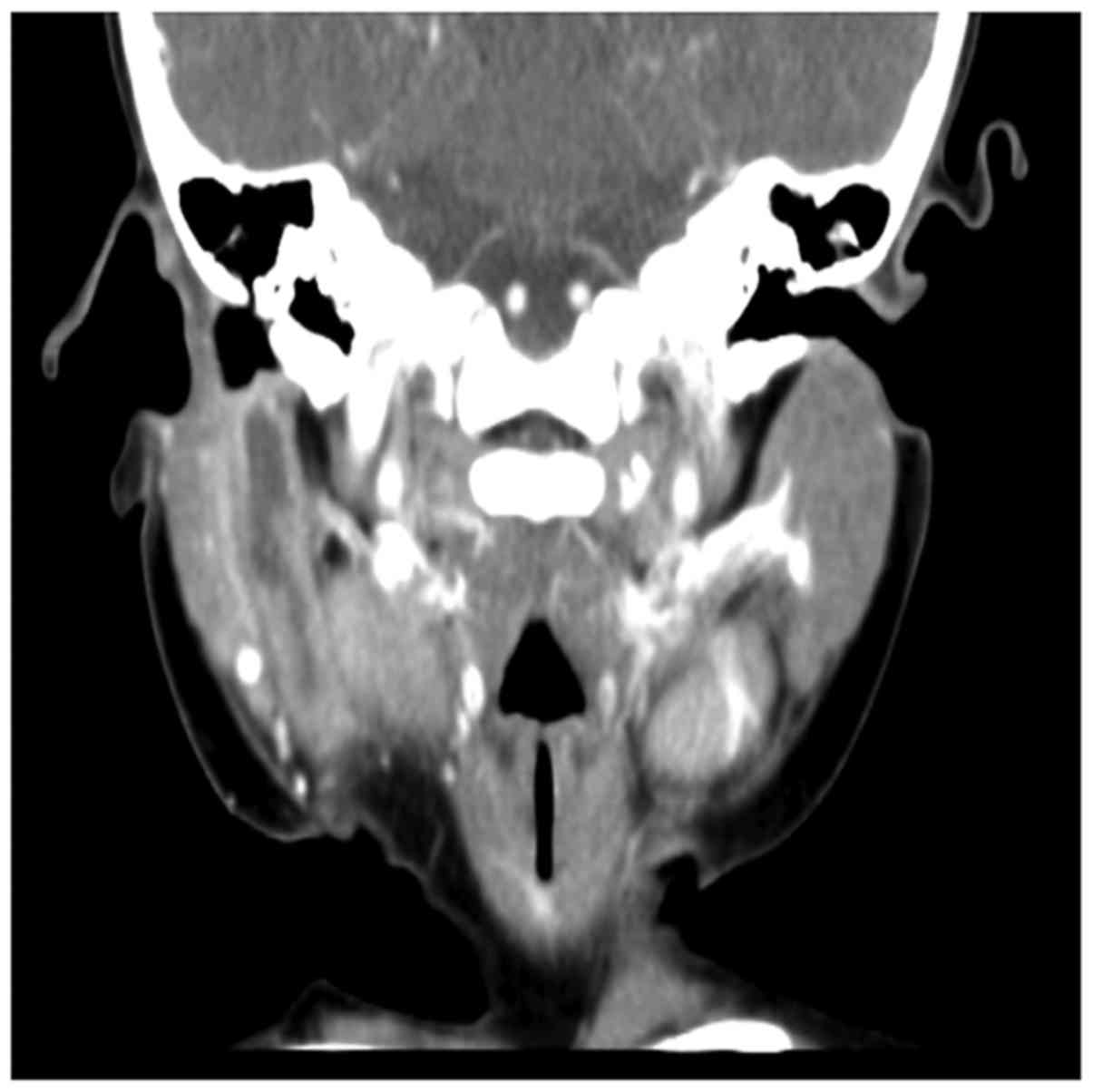
All patients were examined by enhanced CT, which
showed cystic lesions or soft tissue shadows in the parotid gland
parenchyma or in the surrounding area. The relationship between the
fistula and EAC was also clear, and 11 patients were diagnosed with
FBCA. Ultrasound scans (US) were performed in 10 cases, but only 1
patient was diagnosed with FBCA, so the positive detection of US is
low, but US is the preferred method for neck abscess because it is
not radioactive or traumatic.
All children underwent branchial fistula or cyst
excision under general anesthesia. The fistulas ended in EAC in 13
cases, in the bottom wall of the EAC in 10 cases, in the front wall
in 1 case, in the back wall in 2 cases, and in auricle cartilage in
4 cases. The relation of the anomalies to the facial nerve varied.
In reviewing the histopathological results and applying the Work
classification from 1972, we found 18 cases of type I and 12 cases
of type II. The tract ran deep to the facial nerve in 3 cases,
superficial to it in 21 cases, and passed between the branches of
the nerve in 6 cases. Twenty cases had a close relationship with
the parotid gland. The facial nerve was identified in 20 of the 30
patients. The facial nerve was not identified in ten patients, as
the tract was superficial to it. There were 2 cases of
postoperative temporary facial paralysis (2/30, 6.7%). The symptoms
gradually improved after one month; 1 case had permanent facial
paralysis (1/30, 3.3%), and 1 case had postoperative recurrence
(1/30, 3.3%).
Discussion
FBCA is the most unusual type (8 to 10%) of all
branchial cleft deformities and accounts for 17% of all cervical
masses in children (9,10). We found 87 cases of branchial cleft
deformity from 2009–2016, and the proportion of FBCA was 34.5%
(30/87, 34.5%). The number of cases was more than the second
branchial fistula (11/87, 12.6%). The annual incidence of FBCA has
been reported as 1 per 1,000,000, and they occur more frequently in
females (69%) compared with males (31%). The lesions are more
likely to occur on the left side (5). We found 19 female cases (19/30, 63.3%)
and 11 male cases (11/30, 36.7%). Twenty cases involved the left
side (20/30, 66.7%), and 10 cases involved the right side (10/30,
33.3%). Our study's findings were similar to those of previous
literature.
According to the anatomic classification, Arnot in
1972 divided FBCA into two types: Type I mainly occurs in early or
middle adult life as a defect in the parotid region, and type II
defects mainly occur during childhood and appear in the anterior
cervical region (11). The Arnot
classification in FBCA of our cases were depicted in Table II. Our department found 7 cases of
type II, and we found that type II FBCA involved the EAC. The route
of the tract was longer than that of type I defects, and 3 lesions
were located deep to the facial nerve, while 4 lesions passed
between the branches of the facial nerve. Therefore, type II had a
close relationship with the facial nerve, and the facial nerve
should be identified during operations. There were 23 cases of type
II according to the Arnot classification. Twenty-one cases were
superficial to the facial nerve, and 2 cases passed between the
branches of the facial nerve, so type I usually did not require
identifying the facial nerve (10/23, 43.5%). The surgery for type
II is generally more difficult than that for type I.
 | Table II.The Aront classification in FBCA. |
Table II.
The Aront classification in FBCA.
| Arnot
classification | Number | Relation of tract to
facial nerve | Identify facial
nerve |
|---|
| Aront I | 23 | Superficial (21) | 13 |
|
|
| Between the branches
(2) | 2 |
|
|
| Deep (0) | 0 |
| Aront II | 7 | Superficial (0) | 0 |
|
|
| Between the branches
(4) | 4 |
|
|
| Deep (3) | 3 |
Work classified FBCA into two types in 1972 based on
clinical features and histopathology (12). Type I anomalies are purely ectodermal
and often present a cystic mass. Pathology shows squamous
epithelium but no cartilage or skin adnexa. Type II anomalies are
ectodermal and esodermal and involve a cyst, sinus or fistula
tract. Histology shows squamous epithelium with cartilage or skin
adnexa. In 3 cases, the cystic mass was type I. There was no
difference between the sinus or fistula tract forms.
Almost all FBCA occur in the Pochet's triangle area,
which consists of the EAC, the hyoid body and the mandibular angle,
particularly in the retroauricular groove or parotid region. The
lesions of the triangle area usually present repeatedly with
swelling, pain, and purulent discharge of the skin fistula.
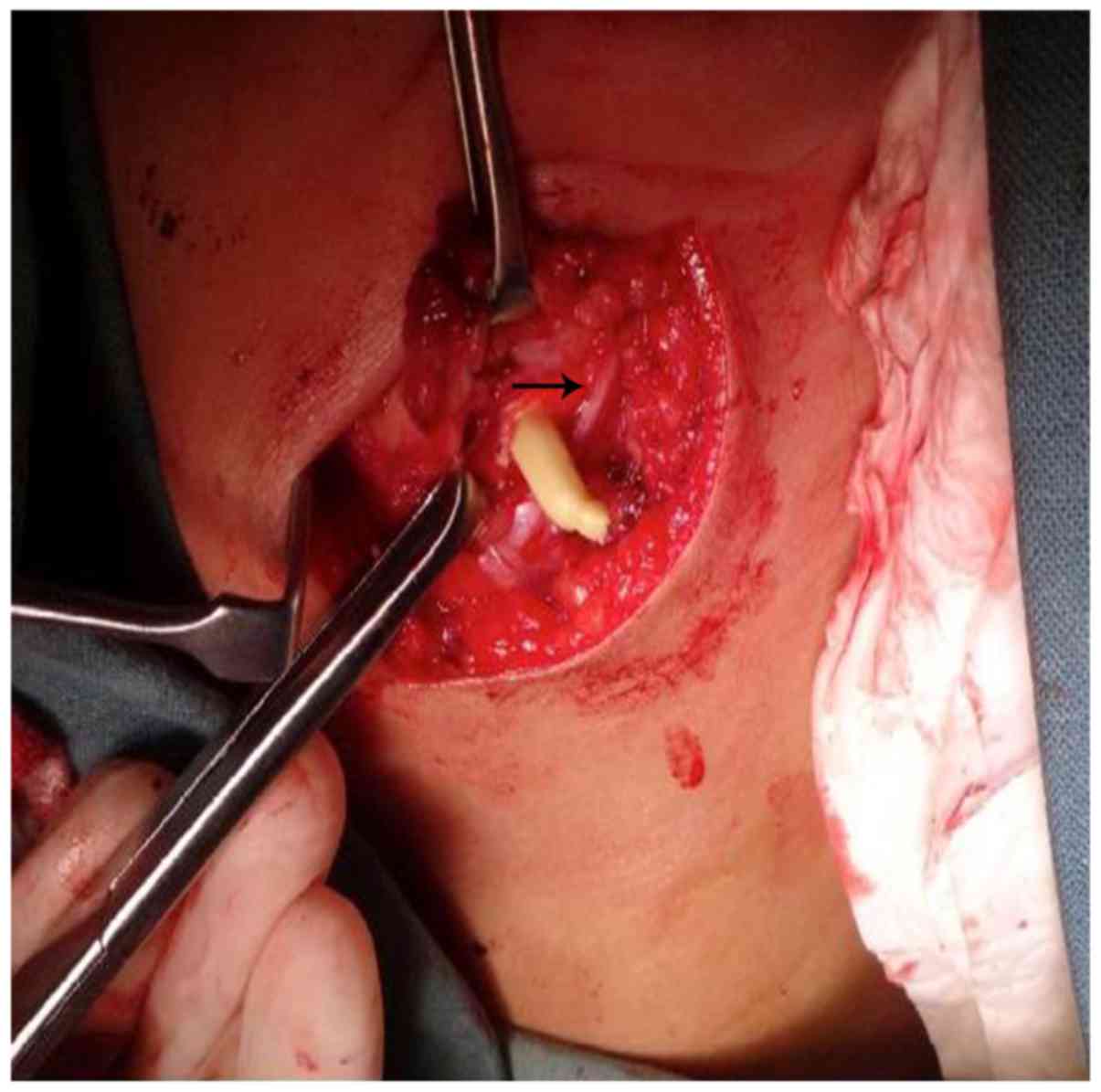
Preoperative enhancement CT examination can help define the size of
the lesion and anatomic relationship to important surrounding
structures, such as the facial nerve, EAC and parotid gland
(Fig. 2), this information can be
helpful for determining the surgical approach, and ultrasonic
examination, as the preferred therapy for head and neck tumors, can
display the mass as substantive or cystic. FBCA is characterized by
low-echo area or cystic appearance.
FBCA is easily misdiagnosed, and Triglia et
al reported a delay of 3.5 years between the onset of the first
clinical symptom and when adequate treatment is received, with
almost 50% of patients not receiving successful treatment (13). Shinn et al noted that more
than half of the children had received an incision and drainage
before the surgical removal of the fistula, and more than a quarter
of the patients had a history of surgery (14). Of our cases, 22 (22/30, 73.3%)
patients had a history of incision and drainage. Only 4 cases
received surgery (4/30, 13.3%), so the inflammation phase of the
FBCA was difficult to diagnose, and most children underwent abscess
incision and drainage because of swelling and pain. We generally
conduct surgery to remove a fistula when inflammation is controlled
for one month. Guo and Guo (15)
recommended that the most appropriate timing for surgery on FBCA is
after 4 years of age because surgery will be easier after complete
maturation of the facial nerve, with a decreased risk in damaging
the facial nerve. However, the ages in our cases ranged from 1 to
13 years, and the median age was 3 years. Early diagnosis and
surgical treatment is suggested in our experience. The risk of
facial palsy during the surgical removal of FBCA is higher if a
patient has a history of recurrent infections and inadequate
treatment (incision, drainage or incomplete excision) that may make
the tract have adhesions with the facial nerve, so immediate
surgery once the diagnosis has been confirmed can minimize the risk
of scarring.
The course of the tract varies and has different
relationships with the facial nerve. We can divide these variations
into 3 types: superficial, deep to the facial nerve, or between the
branches of the nerve. The surgical approaches differ according to
the various types. A review of 83 patients with FBCA analyzed the
relationship between the facial nerve and tract, and 47 cases were
superficial, 25 cases were deep to the facial nerve, and 11 cases
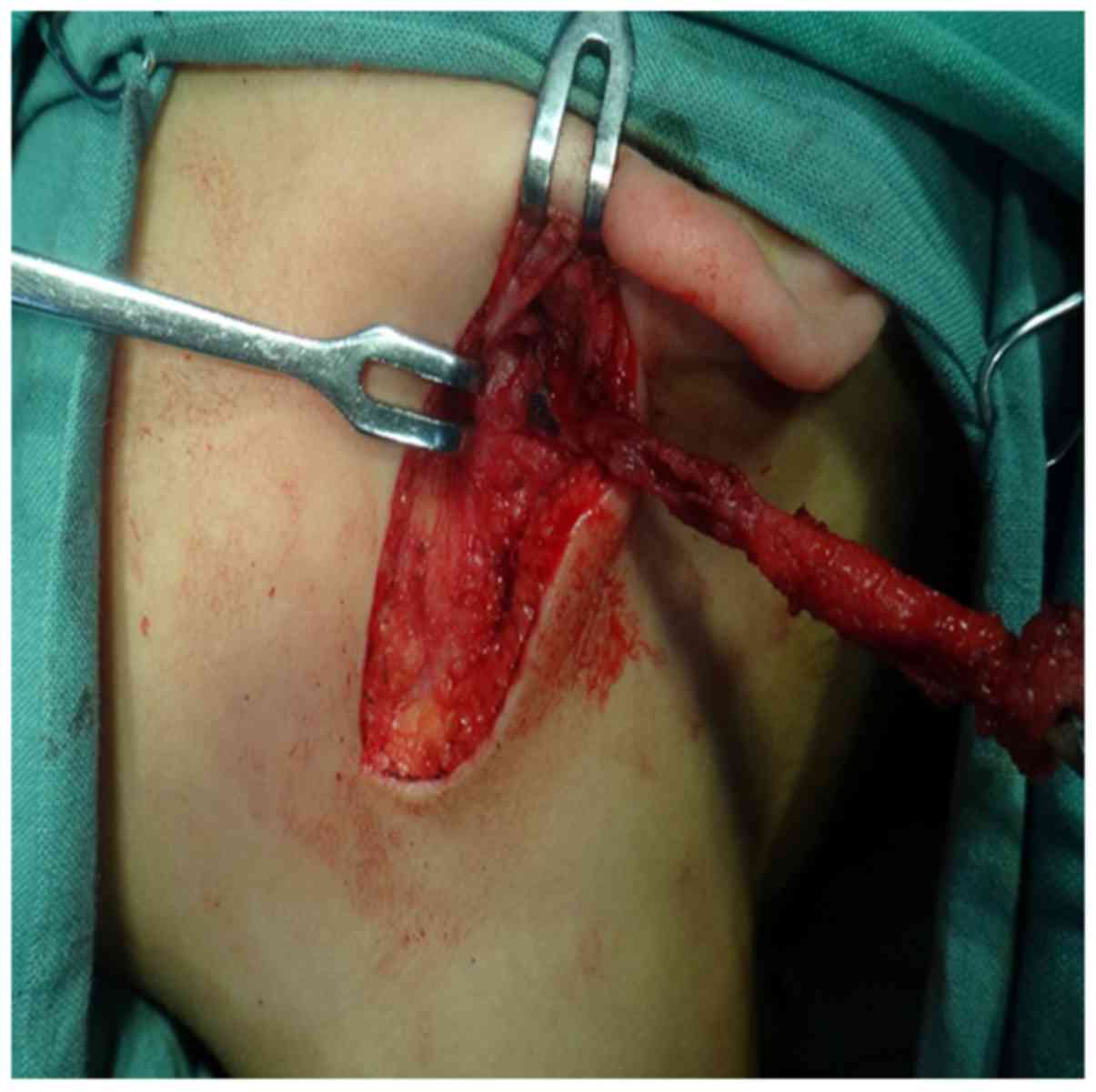
were between the branches of the nerve (5). In our patients, the lesions of 21 cases
lay superficial to the facial nerve, which occurs most often, and
we usually did not need to identify the nerve during surgery
(10/21, 47.6%). Instead, we tracked the tract from Pochet's
triangle to the EAC (Fig. 3). Parts
of the lesion had superficial adhesions with the parotid (11/21,
52.4%), so we needed to partially excise the parotid and identify
the facial nerve during the operation. The lesions of 3 cases were
deep to the facial nerve, which is the rarest case, and we found
that these tracts were thick. It was easy to identify these cases
as belonging to the Work II type. Guo and Guo reported that this
type of tract needed to receive a superficial parotidectomy and
exposure of the facial nerve and its branches to ensure safe
recovery (15). However, the lesions
of 3 cases were located in the mandibular angle, and a spindle
incision for the lesion was made. We separated the fistula tract in
the direction of the EAC. The tract was tracked from the gap, which
is surrounded by the deep lobe of parotid gland, the bottom of the
EAC and sternocleidomastoid, this method avoided excessive
resection of the parotid gland, and we did not need to completely
identify the facial nerve to reduce the risk of facial palsy
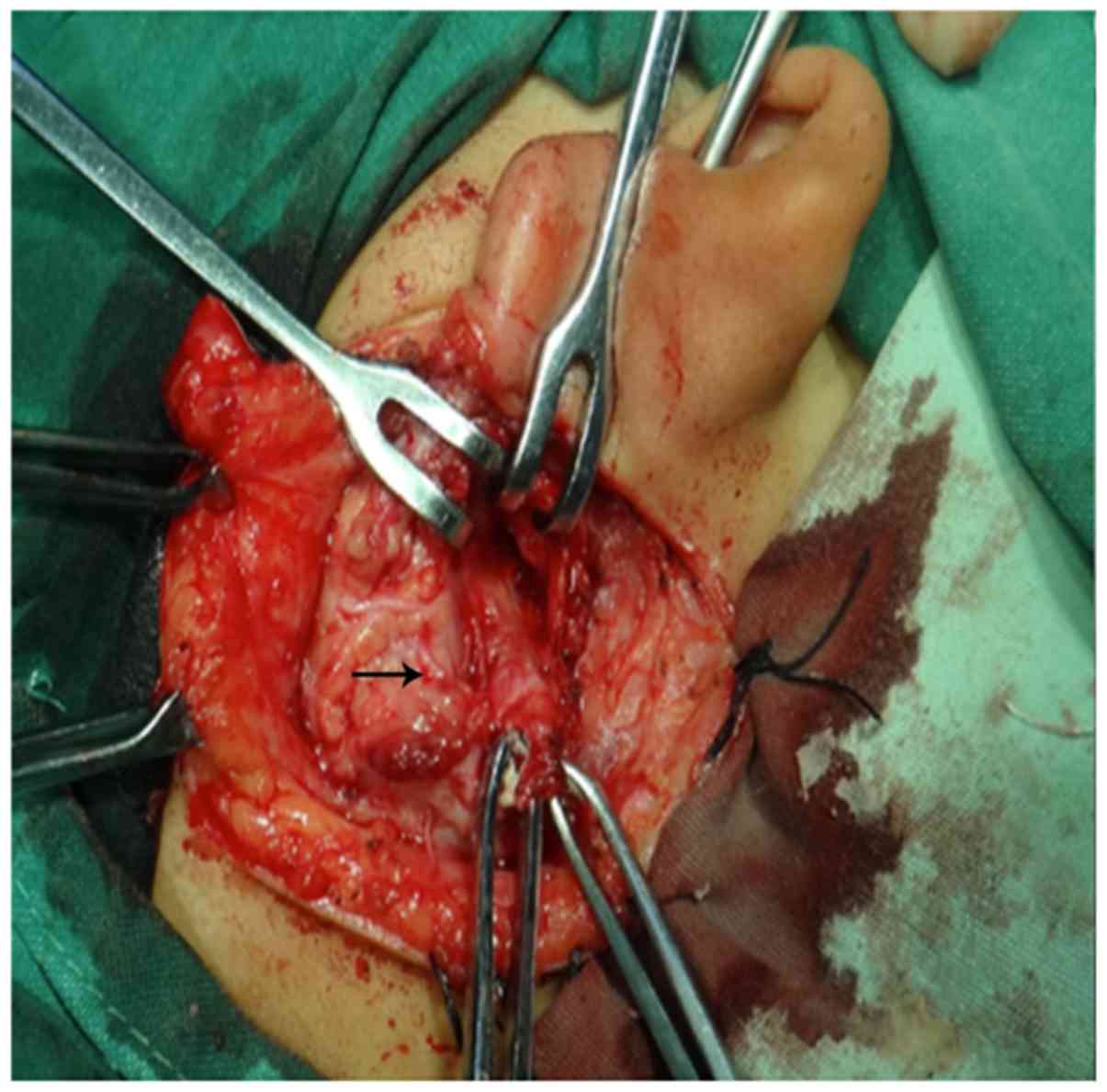
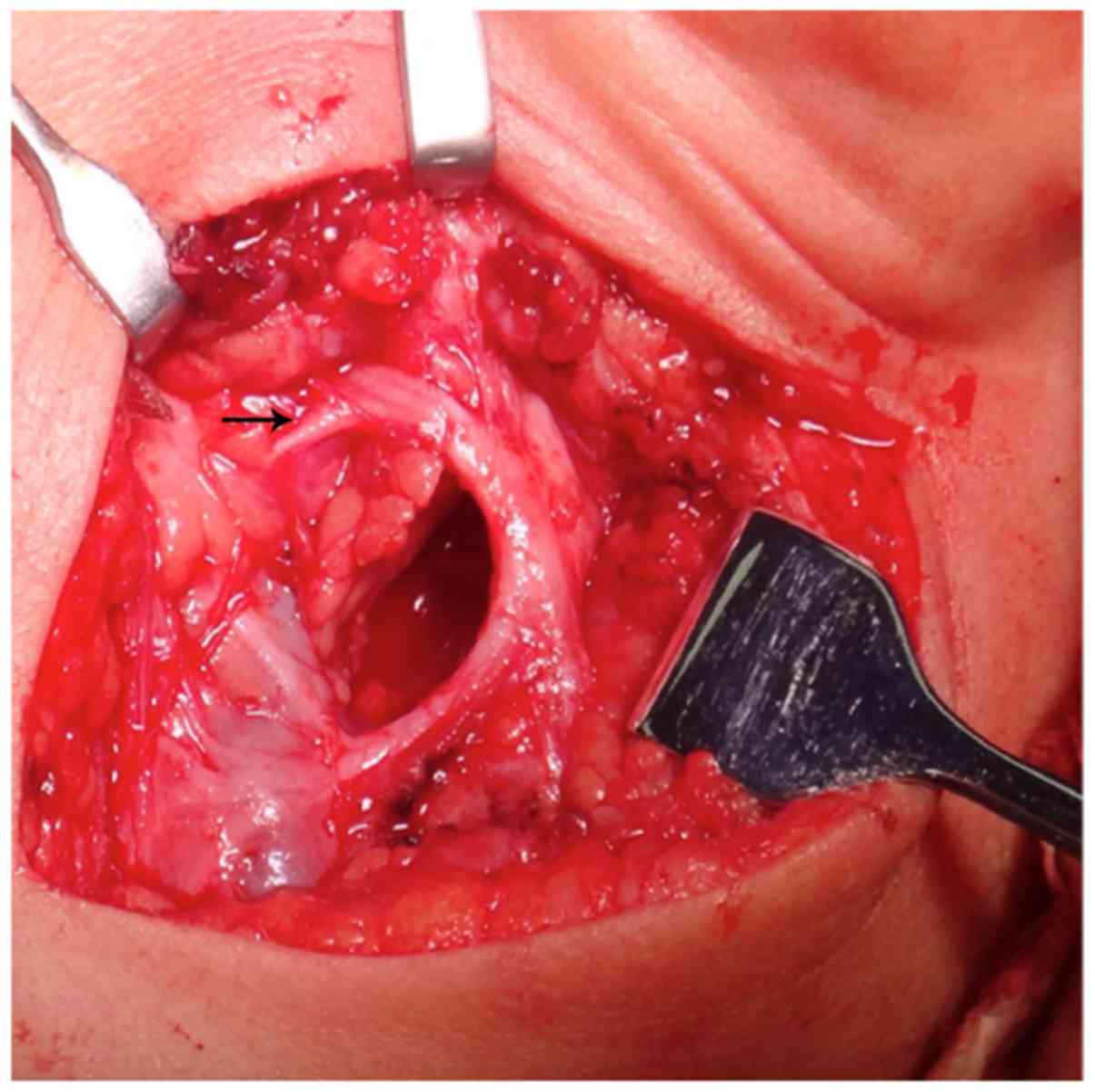
(Fig. 4). The lesions of 6 cases
were between the branches of the nerve (Fig. 5). The surgery for this type was the
most difficult, and the trunk and each branch of the facial nerve
should be identified (Fig. 6), a
superficial parotidectomy approach with a wide exposure was
performed to preserve facial nerve. For the cases who had received
the previous intervention, such as abscess incision and surgical
resection, the scar tissue formation around the fistula could
result in the displacement of facial nerve trunk. but the position
of marginal mandibular branches was relatively stable, we could
reverse the anatomy of facial nerve trunk through the anatomy of
marginal mandibular branches in the first place. For the cases who
had no previous intervention, the anatomy of the facial nerve trunk
was firstly considered. In addition, as the tract adheres with the
facial nerve, the surgeon does not have a solid foundation to
identify the facial nerve, and it is easy to relapse for the
residual fistula or injure the facial nerve, which influences the
patient's quality of life after surgery.
D'Souza et al reported 87 cases of FBCA in
which they identified the facial nerve, 18 cases of patients with
temporary facial paralysis (18/87, 20.7%), 1 case with permanent
facial paralysis (1/87, 1.1%), and 12 cases with recurrence (12/87,
13.8%) (5). Triglia et al
reported 39 patients received surgery for FBCA, of which 5 cases
had temporary facial paralysis (5/39, 12.8%), 1 case had permanent
facial paralysis (1/39, 2.6%), and postoperative recurrence
occurred in 3 patients who required reoperation (3/39, 7.7%)
(13). In our patients, 2 cases had
postoperative temporary facial paralysis (2/30, 6.7%), 1 case had
permanent facial paralysis (1/30, 3.3%), and 1 cases had
postoperative recurrence (1/30, 3.3%). This result occurred because
this patient had a history of incision and drainage due to
infection. The fistula with scar adhesion was very obvious during
the operation. We could not find and remove the fistula completely,
so complete removal of the tract and protection of the facial nerve
was challenging.
Some authors suggest injecting methylene blue to
display the tract of the fistula (15,16). We
do not use this approach because the dye may leak into neighboring
tissues and obscure the surgical area, even if it is impossible to
identify the facial nerve. The cyst type of FBCA with no skin
fistula cannot be injected with methylene blue. In some patients
with repeated abscess incision and drainage procedures, the fistula
may be closed because of scar adhesion, and methylene blue also
cannot be injected.
As we intraoperatively track the tract of FBCA to
the EAC, the part of the EAC wall that adheres to the fistula tract
must be removed. Fourteen cases in our study had a burst of the EAC
during the operation, and if resection is too great, it can cause
hyperplasia of granulation tissue in the EAC wall, even causing
stenosis of the EAC. If resection is too slight, the missing
residual tract and epithelial tissue may cause recurrence. We
considered that filling the EAC with iodoform gauze for two weeks
after the operation can prevent growth of granulation tissue and
stenosis of the EAC. For patients who have growth of granulation
tissue, we can shave the granulation tissue and use hormone
ointment to daub the EAC, which is effective.
FBCA is rare in the clinical setting and is easily
misdiagnosed. Clinical manifestations include repeated swelling or
discharge of the skin fistula in Pochet's triangle area. Complete
excision of the tract is the only way to manage FBCA, but the
course of the tract varies and has different relationships with the
facial nerve (3 types: Superficial, deep to the facial nerve,
between the branches of the nerve). Therefore, surgical approaches
differ according to the various types, and careful preoperative
planning and protection of the facial nerve during resection of the
tract are essential.
References
|
1
|
Bajaj Y, Ifeacho S, Tweedie D, Jephson CG,
Albert DM, Cochrane LA, Wyatt ME, Jonas N and Hartley BE: Branchial
anomalies in children. Int J Pediatr Otorhinolaryngol.
75:1020–1023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitroi M, Dumitrescu D, Simionescu C,
Popescu C, Mogoantă C, Cioroianu L, Surlin C, Căpitănescu A and
Georgescu M: Management of second branchial cleft anomalies. Rom J
Morphol Embryol. 49:69–74. 2008.PubMed/NCBI
|
|
3
|
Chandler RJ and Mitchell B: Branchial
cleft cysts, sinuses, and fistulas. Otolaryngol Clin North Am.
14:175–186. 1981.PubMed/NCBI
|
|
4
|
Benson MT, Dalen K, Mancuso AA, Kerr HH,
Cacciarelli AA and Mafee MF: Congenital anomalies of the branchial
apparatus: Embryology and pathologic anatomy. Radiographics.
12:943–960. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Souza AR, Uppal HS, De R and Zeitoun H:
Updating concepts of first branchial cleft defects: A literature
review. Int J Pediatr Otorhinolaryngol. 62:103–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rattan KN, Rattan S, Parihar D, Gulia JS
and Yadav SP: Second branchial cleft fistula: Is fistulogram
necessary for complete excision. Int J Pediatr Otorhinolaryngol.
70:1027–1030. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quintanilla-Dieck L, Virgin F, Wootten C,
Goudy S and Penn E Jr: Surgical approaches to first branchial cleft
anomaly excision: A case series. Case Rep Otolaryngol.
2016:39029742016.PubMed/NCBI
|
|
8
|
Olsen KD, Maragos NE and Weiland LH: First
branchial cleft anomalies. Laryngoscope. 90:423–436. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tham YS and Low WK: First branchial cleft
anomalies have relevance in otology and more. Ann Acad Med
Singapore. 34:335–338. 2005.PubMed/NCBI
|
|
10
|
Ford GR, Balakrishnan A, Evans JN and
Bailey CM: Branchial cleft and pouch anomalies. J Laryngol Otol.
106:137–143. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arnot RS: Defects of the first branchial
cleft. S Afr J Surg. 9:93–98. 1971.PubMed/NCBI
|
|
12
|
Work WP: Newer concepts of first branchial
cleft defects. Laryngoscope. 82:1581–1593. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Triglia JM, Nicollas R, Ducroz V, Koltai
PJ and Garabedian EN: First branchial cleft anomalies: A study of
39 cases and a review of the literature. Arch Otolaryngol Head Neck
Surg. 124:291–295. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shinn JR, Purcell PL, Horn DL, Sie KC and
Manning SC: First branchial cleft anomalies: Otologic
manifestations and treatment outcomes. Otolaryngol Head Neck Surg.
152:506–512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo YX and Guo CB: Relation between a
first branchial cleft anomaly and the facial nerve. Br J Oral
Maxillofac Surg. 50:259–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piccioni M, Bottazzoli M, Nassif N,
Stefini S and Nicolai P: Intraoperative use of fibrin glue dyed
with methylene blue in surgery for branchial cleft anomalies.
Laryngoscope. 126:2147–2150. 2016. View Article : Google Scholar : PubMed/NCBI
|