Introduction
Acute cholecystitis is characterized by inflammation
of the gallbladder and typically occurs due to obstruction of the
gallbladder neck or cystic duct by a gallstone (1). Gallbladder infection may also spread to
the liver or pericholecystic cavity and lead to the development of
liver abscesses or peritonitis, respectively (2–4).
Therefore, prompt treatment with cholecystectomy, percutaneous
transhepatic gallbladder drainage or aspiration is typically
recommended following diagnosis of acute cholecystitis (5–7).
Accurate diagnosis is also critical for the appropriate management
of acute cholecystitis (8).
Diffusion-weighted whole-body magnetic resonance
imaging with background body signal suppression (DWIBS) is based on
a diffusion-weighted imaging (DWI) technique, which enables
visualization of the random movement of water molecules (Brownian
motion) for diagnostic imaging (9).
Images are acquired with DWIBS during free breathing of the patient
through the use of multiple-signal averaging, fat suppression and
heavy diffusion weighting (10). In
particular, a high signal intensity of DWIBS is a positive
indicator of cancer and inflammation. An advantage of DWIBS over
DWI is that it exhibits positive signals with strong contrast
against surrounding normal tissues. However, the evaluation of
positive signals to obtain anatomical information becomes difficult
when signals in the surrounding tissues are suppressed (11,12).
Images obtained from DWIBS are fused with their corresponding
T2-weighted images using a workstation (13–15).
DWIBS and T2-weighted image fusion (DWIBS/T2) enables anatomical
evaluation of the positive signals obtained from DWIBS (16). Therefore, the present study
retrospectively analyzed images obtained by DWIBS/T2 from patients
with acute cholecystitis to determine how useful this technique is
in the management of the disease.
Patients and methods
Patients
The current study was approved by the Ethics
Committee of the National Hospital Organization of Shimoshizu
Hospital (Yotsukaido, Japan). Consent was obtained from patients
prior to abdominal ultrasonography (US), though written informed
consent was waived as abdominal US is considered to be safe and
non-invasive. Written informed consent was obtained from patients
before all patients underwent magnetic resonance (MR)
cholangiopancreatography, DWIBS/T2 and computed tomography (CT)
with or without contrast enhancement. The current study was not
considered a clinical trial as procedures were performed as part of
routine clinical practice. Therefore, written informed consent for
inclusion in the study was waived. All patient records and
information were anonymized prior to analysis.
The medical records of patients who were diagnosed
with acute cholecystitis and underwent DWIBS/T2 between January
2013 and March 2014 were analyzed retrospectively. A total of 8 men
(mean age ± standard deviation: 69.8±4.0 years) and 3 women
(72.7±3.1 years) were enrolled in the current study. All patients
were recruited from the National Hospital Organization Shimoshizu
Hospital.
Diagnostic procedure for acute
cholecystitis
Patients were diagnosed with acute cholecystitis
according to the Updated Tokyo Guidelines for the Management of
Acute Cholecystitis (17). Acute
cholecystitis was suspected in patients with upper abdominal and/or
right upper quadrant pain, and elevated white blood cell count
and/or C-reactive protein levels, all of which suggested
inflammation of the abdominal organs. Upper abdominal and right
upper quadrant pain were identified by a Murphy's sign test, a
right upper quadrant mass, general pain and/or tenderness. The
diagnosis of acute cholecystitis was confirmed in these patients
according to characteristic features of abdominal CT and US scans
with or without contrast enhancement. These features were an
enlarged gallbladder, thickened gallbladder wall and
pericholecystic fluid (18).
Abdominal US was a reliable method of detecting Murphy's sign test,
as described previously (19).
Following diagnosis, DWIBS/T2 was performed. Patients were excluded
from the present study if they were unable to undergo at least one
of either US, CT or DWIBS/T2.
MR imaging (MRI)
All MRI examinations were performed with a 1.5-Tesla
scanner using Achieva 3.2.2 software (both from Philips Medical
Systems B.V., Eindhoven, The Netherlands). Patients were placed in
a supine headfirst position on an extended table platform to allow
for full-body scanning from the head to the lower legs. The imaging
protocol of DWIBS/T2 consisted of unenhanced T1-weighted,
T2-weighted, DWI and DWIBS scans. MRI pulse sequences are presented
in Table I. Images obtained from
DWIBS were acquired in an axial plane with a Q-body coil under free
breathing conditions using an echo-planar imaging single-shot pulse
sequence. DWI gradients were applied along the X, Y, and Z axes
prior to and following a pre-pulse 180° inversion, which was
performed to obtain fat-saturated isotropic images with DWI
sensitivity using the following parameters for a single stack:
b-value, 0 and 800 mm2/sec; repetition, echo and
inversion times, 6,960/79/150 msec; acquisition matrix, 176×115;
reconstruction matrix, 256; right/left field of view, 530 mm;
anterior/posterior field of view, 349 mm; feet/head field of view,
226 mm; slice thickness, 6 mm; size of the reconstructed voxel,
2.07×2.08×6 mm3; and 4 averages. Fused images obtained from
DWIBS/T2 were constructed with the Extended MR WorkSpace
workstation (Philips Medical Systems B.V.). In all patients, 5
stacks (46 slices/stack) were acquired consecutively to obtain
images from the head to the middle of the tibia. The total imaging
time was 13.31 min. An apparent diffusion coefficient (ADC) map was
produced from the recorded ADC values in order to eliminate the
possibility of T2 shine-through and to differentiate malignant
lesions from non-malignant causes of restricted diffusion (20).
 | Table I.MRI pulse sequences. |
Table I.
MRI pulse sequences.
| Parameter | T1-weighted
image | T2-weighted
image | DWI (DWIBS) |
|---|
| Sequences | GRE | Single-shot SE | EPI SE |
| TR, msec | Shortest | 1,000 | 11,250 |
| TE, msec | First: 2.3
(out-of-phase); second: 4.6 (in phase) | 90 | 83 |
| Flip angle, (°) | 75 | 90 | 90 |
| NSA | 1 | 1 | 4 |
| Slice thickness,
mm | 8 | 8 | 5 |
| Slice gap | 1 | 1 | 0 |
| Fat saturation | None | None | SPAIR |
| Phase encoding
direction |
Posterior-anterior |
Posterior-anterior |
Posterior-anterior |
Results
DWIBS/T2 detects clinical features of
acute cholecysitis
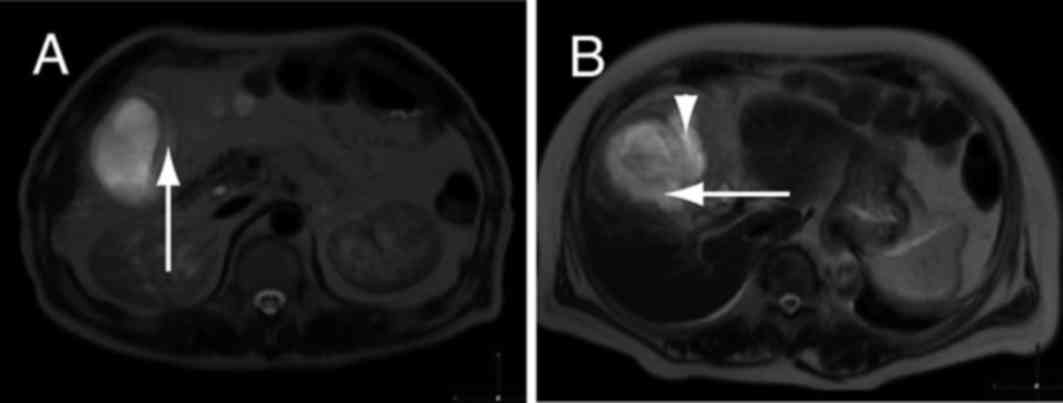
In one patient with acute cholecystitis, a thickened
gallbladder wall and high signal intensity were observed in images
obtained by DWIBS/T2 (Fig. 1A). The
high signal intensity in the pericholecystic space suggested
localized peritonitis (Fig. 1B).
These results indicate that wall thickening and high signal
intensity, as detected by DWIBS/T2, are typical features of acute
cholecystitis.
DWIBS/T2 has high sensitivity in the
detection of acute cholecystitis
The sensitivity of DWIBS/T2 in the diagnosis of
acute cholecystitis was determined. Images obtained by DWIBS/T2
from patients with acute cholecystitis (n=11) were evaluated to
determine the extent of wall thickening and signal intensity, both
positive indicators of cholecystitis. Out of the 11 patients, 10
exhibited a thickened wall and high signal intensity in the
DWIBS/T2 images (Table II). Thus,
positive DWIBS/T2 results were obtained in 10/11 patients (90.9%).
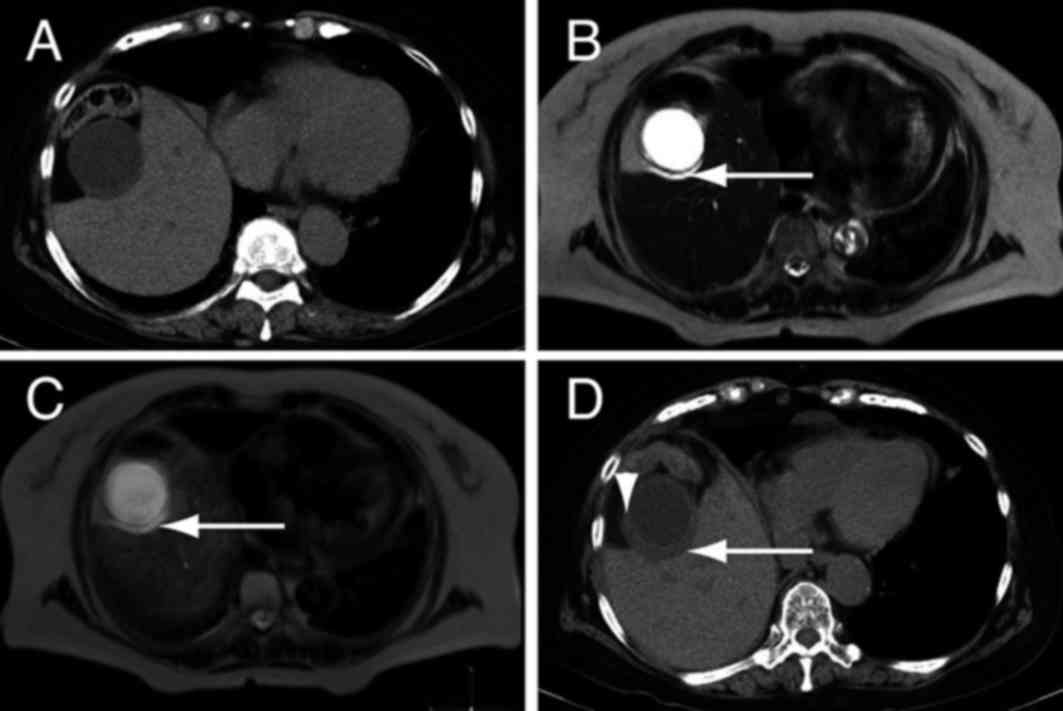
In the remaining patient, fluid was present between the liver and
gallbladder, indicating acute cholecystitis (Fig. 2A and B). The patient's DWIBS/T2
result was positive for cholecystitis (Fig. 2C), and a CT scan performed three days
later identified thickening of the gallbladder wall (Fig. 2D). Disparity between results of the
DWIBS/T2 and CT scans may be due to a lack of high signal intensity
in DWIBS/T2 in the absence of wall thickening. Alternatively,
DWIBS/T2 may infrequently yield a negative result for acute
cholecystitis. Nevertheless, these results suggest that high signal
intensity detected by DWIBS/T2 is associated with the severity of
gallbladder inflammation in acute cholecystitis.
 | Table II.Results of DWIBS/T2 in patients with
acute cholecystitis. |
Table II.
Results of DWIBS/T2 in patients with
acute cholecystitis.
| Patient number | Wall thickening | Wall high signal
intensity |
|---|
| 1 | (+) | (+) |
| 2 | (+) | (+) |
| 3 | (+) | (+) |
| 4 | (+) | (+) |
| 5 | (+) | (+) |
| 6 | (+) | (+) |
| 7 | (+) | (+) |
| 8 | (+) | (+) |
| 9 | (+) | (+) |
| 10 | (+) | (+) |
| 11 | (−) | (−) |
DWIBS/T2 detects improvements in acute
cholecystitis
The efficacy of DWIBS/T2 in evaluating improvements
in acute cholecystitis following clinical treatment was assessed.
Prior to treatment, acute cholecystitis detected by abdominal US
and CT examinations (Fig. 3A and B)
was also evident in DWIBS/T2 images as gallbladder wall thickening
coupled with high signal intensity (Fig.
3C). Following treatment with cefazolin sodium (3 g/day for 7
days; Nipro Corp., Osaka, Japan), improvements in acute
cholecystitis were detected by DWIBS/T2 as reductions in
gallbladder wall thickening and signal intensity (Fig. 3D).
Discussion
In acute cholecystitis, wall thickening is caused by
edema of the gallbladder wall, and correlates with the severity of
acute cholecystitis (21).
Therefore, measurements of wall thickness may be useful in aiding
the diagnosis of acute cholecystitis and in evaluating its severity
(22). In particular, the detection
of wall thickening and pericholecystic fluid by MRI may be a useful
diagnostic tool (23). In the
present study, the gallbladder wall of patients with acute
cholecystitis was thickened. DWIBS/T2 identified that the thickened
wall exhibited high signal intensity, which may correlate with the
extent of inflammation (24). These
data indicate that the detection of wall thickening by DWIBS/T2
make it an effective method in the evaluation and diagnosis of
acute cholecystitis.
The sensitivity of abdominal US in the diagnosis of
acute cholecystitis is 37.5–91% (25,26),
while the sensitivity of CT is 83% (25). In the current study, the sensitivity
of DWIBS/T2 in the diagnosis of acute cholecystitis was 90.9%.
Similarly, previous results have indicated that DWIBS/T2 is
superior to US and CT as a diagnostic tool (27,28),
possibly due to the distinct contrast between high signal intensity
regions and surrounding tissues detected by DWIBS/T2, which makes
diagnosis more accurate. Indeed, contrast agents are considered to
be useful in the diagnosis of biliary diseases (29). These results suggest that alterations
in the gallbladder wall and pericholecystic fluid are useful
diagnostic markers (29). DWIBS/T2
did not require the use of contrast agents, thus making it an
efficient method in the diagnosis of acute cholecystitis. In the
present study, DWIBS/T2 was able to detect reductions in wall
thickness and signal intensity following treatment of acute
cholecystitis (Fig. 3). These
results suggest that DWIBS/T2 may be useful for the evaluation of
acute cholecystitis severity.
The current study included limitations. Firstly,
only a small number of patients with acute cholecystitis were
enrolled. Secondly, the detection of gallbladder wall thickness and
high signal intensity by DWIBS/T2 were not compared with other
gallbladder conditions, including chronic cholecystitis and
gallbladder cancer, both of which exhibit gallbladder wall
thickening (30–32). As the treatments for acute
cholecystitis, chronic cholecystitis and gallbladder cancer differ,
distinguishing between these diseases is critical for successful
treatment (1).
In conclusion, DWIBS/T2 is able to indicate the
presence of acute cholecystitis through the detection of
gallbladder wall thickening and high signal intensity. Furthermore,
the present results indicate that DWIBS/T2 may be useful in
determining the severity of acute cholecystitis.
References
|
1
|
Knab LM, Boller AM and Mahvi DM:
Cholecystitis. Surg Clin North Am. 94:455–470. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinto A, Reginelli A, Cagini L, Coppolino
F, Ianora AA Stabile, Bracale R, Giganti M and Romano L: Accuracy
of ultrasonography in the diagnosis of acute calculous
cholecystitis: Review of the literature. Crit Ultrasound J. 5 Suppl
1:S112013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singal R, Mittal A, Gupta S, Singh B and
Jain P: Management of gall bladder perforation evaluation on
ultrasonography: Report of six rare cases with review of
literature. J Med Life. 4:364–371. 2011.PubMed/NCBI
|
|
4
|
Tang S and Wang Y and Wang Y:
Contrast-enhanced ultrasonography to diagnose gallbladder
perforation. Am J Emerg Med. 31:1240–1243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang YR, Ahn YJ, Jang JY, Kang MJ, Kwon
W, Jung WH and Kim SW: Percutaneous cholecystostomy for acute
cholecystitis in patients with high comorbidity and re-evaluation
of treatment efficacy. Surgery. 155:615–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Komatsu S, Tsukamoto T, Iwasaki T,
Toyokawa A, Hasegawa Y, Tsuchida S, Takahashi T, Takebe A, Wakahara
T, Watanabe A, et al: Role of percutaneous transhepatic gallbladder
aspiration in the early management of acute cholecystitis. J Dig
Dis. 15:669–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Na BG, Yoo YS, Mun SP, Kim SH, Lee HY and
Choi NK: The safety and efficacy of percutaneous transhepatic
gallbladder drainage in elderly patients with acute cholecystitis
before laparoscopic cholecystectomy. Ann Surg Treat Res. 89:68–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trowbridge RL, Rutkowski NK and Shojania
KG: Does this patient have acute cholecystitis? JAMA. 289:80–86.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sehy JV, Ackerman JJ and Neil JJ: Apparent
diffusion of water, ions, and small molecules in the Xenopus oocyte
is consistent with Brownian displacement. Magn Reson Med. 48:42–51.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahara T, Imai Y, Yamashita T, Yasuda S,
Nasu S and Van Cauteren M: Diffusion weighted whole body imaging
with background body signal suppression (DWIBS): Technical
improvement using free breathing, STIR and high resolution 3D
display. Radiat Med. 22:275–282. 2004.PubMed/NCBI
|
|
11
|
Ohno Y, Koyama H, Onishi Y, Takenaka D,
Nogami M, Yoshikawa T, Matsumoto S, Kotani Y and Sugimura K:
Non-small cell lung cancer: Whole-body MR examination for M-stage
assessment-utility for whole-body diffusion-weighted imaging
compared with integrated FDG PET/CT. Radiology. 248:643–654. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fischer MA, Nanz D, Hany T, Reiner CS,
Stolzmann P, Donati OF, Breitenstein S, Schneider P, Weishaupt D,
von Schulthess GK and Scheffel H: Diagnostic accuracy of whole-body
MRI/DWI image fusion for detection of malignant tumours: A
comparison with PET/CT. Eur Radiol. 21:246–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kwee TC, Takahara T, Ochiai R, Nievelstein
RA and Luijten PR: Diffusion-weighted whole-body imaging with
background body signal suppression (DWIBS): Features and potential
applications in oncology. Eur Radiol. 18:1937–1952. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sommer G, Wiese M, Winter L, Lenz C,
Klarhöfer M, Forrer F, Lardinois D and Bremerich J: Preoperative
staging of non-small-cell lung cancer: Comparison of whole-body
diffusion-weighted magnetic resonance imaging and
18F-fluorodeoxyglucose-positron emission tomography/computed
tomography. Eur Radiol. 22:2859–2867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nechifor-Boilă IA, Bancu S, Buruian M,
Charlot M, Decaussin-Petrucci M, Krauth JS, Nechifor-Boilă AC and
Borda A: Diffusion weighted imaging with background body signal
suppression/T2 image fusion in magnetic resonance mammography for
breast cancer diagnosis. Chirurgia (Bucur). 108:199–205.
2013.PubMed/NCBI
|
|
16
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Diffusion-weighted whole body
imaging with background body signal suppression/T2 image fusion is
negative for patients with intraductal papillary mucinous neoplasm.
Hepatogastroenterology. 62:463–465. 2015.PubMed/NCBI
|
|
17
|
Yokoe M, Takada T, Strasberg SM, Solomkin
JS, Mayumi T, Gomi H, Pitt HA, Gouma DJ, Garden OJ, Büchler MW, et
al: New diagnostic criteria and severity assessment of acute
cholecystitis in revised Tokyo guidelines. J Hepatobiliary Pancreat
Sci. 19:578–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirota M, Takada T, Kawarada Y, Nimura Y,
Miura F, Hirata K, Mayumi T, Yoshida M, Strasberg S, Pitt H, et al:
Diagnostic criteria and severity assessment of acute cholecystitis:
Tokyo guidelines. J Hepatobiliary Pancreat Surg. 14:78–82. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ralls PW, Halls J, Lapin SA, Quinn MF,
Morris UL and Boswell W: Prospective evaluation of the sonographic
Murphy sign in suspected acute cholecystitis. J Clin Ultrasound.
10:113–115. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Miller FH, Chen ZE, Merrick L,
Mortele KJ, Hoff FL, Hammond NA, Yaghmai V and Nikolaidis P:
Diffusion-weighted MR imaging of solid and cystic lesions of the
pancreas. Radiographics. 31:E47–E64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Borzellino G, Steccanella F, Mantovani W
and Genna M: Predictive factors for the diagnosis of severe acute
cholecystitis in an emergency setting. Surg Endosc. 27:3388–3395.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogawa T, Horaguchi J, Fujita N, Noda Y,
Kobayashi G, Ito K, Koshita S, Kanno Y, Masu K and Sugita R: High
b-value diffusion-weighted magnetic resonance imaging for
gallbladder lesions: Differentiation between benignity and
malignancy. J Gastroenterol. 47:1352–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tonolini M, Ravelli A, Villa C and Bianco
R: Urgent MRI with MR cholangiopancreatography (MRCP) of acute
cholecystitis and related complications: Diagnostic role and
spectrum of imaging findings. Emerg Radiol. 19:341–348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Padhani AR, Koh DM and Collins DJ:
Whole-body diffusion-weighted MR imaging in cancer: Current status
and research directions. Radiology. 261:700–718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Vargas Macciucca M, Lanciotti S, De
Cicco ML, Coniglio M and Gualdi GF: Ultrasonographic and spiral CT
evaluation of simple and complicated acute cholecystitis:
Diagnostic protocol assessment based on personal experience and
review of the literature. Radiol Med. 111:167–180. 2006.(In
English, Italian). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith EA, Dillman JR, Elsayes KM, Menias
CO and Bude RO: Cross-sectional imaging of acute and chronic
gallbladder inflammatory disease. AJR Am J Roentgenol. 192:188–196.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hakansson K, Leander P, Ekberg O and
Håkansson HO: MR imaging in clinically suspected acute
cholecystitis. A comparison with ultrasonography. Acta Radiol.
41:322–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kiewiet JJ, Leeuwenburgh MM, Bipat S,
Bossuyt PM, Stoker J and Boermeester MA: A systematic review and
meta-analysis of diagnostic performance of imaging in acute
cholecystitis. Radiology. 264:708–720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta RT: Evaluation of the biliary tree
and gallbladder with hepatocellular MR contrast agents. Curr Probl
Diagn Radiol. 42:67–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung SE, Lee JM, Lee K, Rha SE, Choi BG,
Kim EK and Hahn ST: Gallbladder wall thickening: MR imaging and
pathologic correlation with emphasis on layered pattern. Eur
Radiol. 15:694–701. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshioka M, Watanabe G, Uchinami H,
Miyazawa H, Abe Y, Ishiyama K, Hashimoto M, Nakamura A and Yamamoto
Y: Diffusion-weighted MRI for differential diagnosis in gallbladder
lesions with special reference to ADC cut-off values.
Hepatogastroenterology. 60:692–698. 2013.PubMed/NCBI
|
|
32
|
Lee NK, Kim S, Kim TU, Kim DU, Seo HI and
Jeon TY: Diffusion-weighted MRI for differentiation of benign from
malignant lesions in the gallbladder. Clin Radiol. 69:e78–e85.
2014. View Article : Google Scholar : PubMed/NCBI
|