Introduction
Cervical cancer is the third most common cancer, as
well as the fourth most frequent cause of cancer-related morality
in women worldwide (1). According to
recent global cancer statistics, there are ~530,000 new cervical
cancer cases annually, most of which occur in developing countries,
including China (1). Despite
improvements in surgery combined with radiotherapy and/or
chemotherapy, the prognosis for patients with cervical cancer
remains poor, primarily due to its recurrence and ability to
metastasize (2). Studies on the
molecular mechanism underlying cervical cancer growth and
metastasis are urgently required for the development of effective
therapeutic strategies for cervical cancer.
MicroRNA (miR) belong to a class of small non-coding
RNA and are critical regulators of gene expression at the
post-transcriptional level (3,4). MiR
directly bind to the 3′-untranslated region (UTR) of their target
mRNA, causing mRNA degradation or translation inhibition (3,4). Through
negatively mediating the protein expression of their targets, miR
are involved in a variety of biological processes, including cell
survival, proliferation, differentiation, apoptosis, migration,
autophagy, invasion, angiogenesis and tumorigenesis (3–7). In
recent decades, deregulations of various miR have been implicated
in the development and malignant progression of cervical cancer,
some of which have been suggested to be used as potential
diagnostic and therapeutic targets for cervical cancer (8–10). For
instance, downregulation of miR-143 in cervical cancer promotes
apoptosis and inhibits tumor formation by targeting B-cell
lymphoma-2 (11). Furthermore,
miR-214 is aberrantly expressed in cervical cancer and inhibits the
growth of HeLa cells (12).
Among these cancer-related miR, miR-92a has been
demonstrated to possess an oncogenic role in different cancer
types, and several targets have been identified (13–15). A
previous study indicated that miR-92a promoted the metastasis of
colorectal cancer cells through inhibition of phosphatase and
tensin homolog, leading to the upregulation of the phosphoinositide
3-kinase/protein kinase B pathway (13). Furthermore, miR-92a was also revealed
to promote pancreatic cancer cell proliferation through inhibiting
the protein expression of dual specificity phosphatase 10, which
enhanced the activation of the c-Jun N-terminal kinase signaling
pathway (16). Recently, miR-92a was
reported to be upregulated in cervical cancer and involved in
promoting cell proliferation and invasion by targeting F-Box and WD
repeat domain containing 7 (17). As
one miR has multiple target genes, whether alternative targets of
miR-92a exist in cervical cancer cells still requires further
investigation.
In the present study, the expression, clinical
significance and regulatory mechanism of miR-92a in cervical cancer
was explored. Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blotting were used to evaluate mRNA
and protein expression. MTT and wound healing assays were used to
determine the cell viability and migration. A luciferase reporter
gene assay was used to confirm the targeting relationship.
Materials and methods
Tissue collection
The present study was approved by the Ethics
Committee of General Hospital of Daqing Oil Field (Daqing, China).
A total of 65 primary cervical cancer tissues and their matched
adjacent non-tumor tissues were collected at the Department of
Obstetrics and Gynecology of General Hospital of Daqing Oil Field
between April 2012 and March 2014. All patients enrolled in the
study provided written informed consent. The patients with cervical
cancer involved in this study were all female, from 41 to 65 years
old, and were diagnosed by pathologists at Pathology Department of
General Hospital of Daqing Oil Field. Prior to surgical resection,
all patients did not receive radiation therapy or chemotherapy.
Tissues were immediately snap-frozen in liquid nitrogen after
surgical resection, and stored in liquid nitrogen prior to use. The
clinical information of patients involved in the present study is
summarized in Table I.
 | Table I.Association between miR-92a expression
and clinicopathological characteristics of patients with cervical
cancer. |
Table I.
Association between miR-92a expression
and clinicopathological characteristics of patients with cervical
cancer.
|
|
| miR-92a expression
level |
|
|---|
|
|
|
|
|
|---|
| Variables | n | Low | High | P-value |
|---|
| Age |
|
|
| 0.987 |
|
<55 | 24 | 10 | 14 |
|
| ≥55 | 41 | 17 | 24 |
|
| Tumor size, cm |
|
|
| 0.29 |
| ≤4 | 41 | 15 | 26 |
|
|
>4 | 24 | 12 | 12 |
|
| Differentiation |
|
|
| 0.031 |
|
Well-moderate | 46 | 23 | 23 |
|
|
Poor | 19 | 4 | 15 |
|
| Clinical stage |
|
|
| 0.011 |
|
I–II | 44 | 23 | 21 |
|
|
III–IV | 21 | 4 | 17 |
|
| Lymph node
metastasis |
|
|
| 0.014 |
| No | 39 | 21 | 18 |
|
|
Yes | 26 | 6 | 20 |
|
| Distant
metastasis |
|
|
| 0.205 |
| No | 56 | 25 | 31 |
|
|
Yes | 9 | 2 | 7 |
|
Cell culture
The human cervical cancer HeLa cell line was
purchased from Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). A total of 1×108 cells were
cultured in Dulbecco's modified Eagle medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere containing 5% CO2.
Cell transfection
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.)
was used for cell transfection, according to the manufacturer's
instructions. HeLa cells (1×107 cells) were transfected
with scramble miR mimic, which was miR-negative control (miR-NC),
miR-92a mimic, miR-92a inhibitor, NC inhibitor, or co-transfected
with miR-92a mimic (all purchased from Fulengen Co., Ltd.,
Guangzhou, China) and pcDNA3.1-Dickkopf-related protein 3 (DKK3)
expression plasmid (Yearthbio, Changsha, China), respectively.
After transfection for 48 h at 37°C, RT-qPCR or western blotting
was conducted to examine the mRNA and protein expression levels,
respectively, of miR-92a or DKK3.
RT-qPCR
Total RNA from tissues and cell lines was extracted
using TRIzol reagent (Thermo Fisher Scientific, Inc.) and converted
into cDNA using a PrimeScript 1st Strand cDNA Synthesis kit (Takara
Bio, Inc., Otsu, Japan), according to the manufacturer's
instructions. For mRNA expression detection, SYBR Green I Real-Time
PCR kit (Biomics Biotechnologies Co., Ltd., Nantong, China) was
used to perform qPCR on an ABI 7500 thermocycler (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
GAPDH was used as an internal control. For miR expression
detection, qPCR was performed using an miRNA Q-PCR Detection kit
(GeneCopoeia, Inc., Rockville, MD, USA) on an ABI 7500
thermocycler, according to the manufacturer's instructions. The U6
gene was used as an internal control. DKK3 forward primer:
5′-AGGACACGCAGCACAAATTG-3′; reverse primer:
5′-CCAGTCTGGTTGTTGGTTATCTT-3′. GAPDH forward primer:
5′-GGAGCGAGATCCCTCCAAAAT-3′; reverse primer:
5′-GGCTGTTGTCATACTTCTCATGG-3′. The reaction conditions were 95°C
for 3 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 15
sec. The relative expression was analyzed by the 2−ΔΔCq
method (18).
Western blotting
HeLa cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). The protein concentration was
determined using Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions, and
protein (50 µg) was separated with 10% SDS-PAGE and transferred
onto a polyvinylidene difluoride membrane (PVDF; Thermo Fisher
Scientific, Inc.). The PVDF membrane was incubated with
phosphate-buffered saline (PBS) containing 5% milk (Mengniu Dairy
Co., Ltd., Beijing, China) for 3 h at room temperature. After
washing three times with PBS (Thermo Fisher Scientific, Inc.), the
membrane was incubated with Rabbit monoclonal to Dkk3 primary
antibody (1:50, ab186409, Abcam, Cambridge, MA, USA) and rabbit
polyclonal to GAPDH primary antibody (1:50, ab9845, Abcam) at room
temperature for 3 h. Subsequently, the membrane was washed three
times with PBS and horseradish peroxidase-conjuncted goat
anti-rabbit secondary antibody (1:5,000, ab6721, Abcam) was added
and incubated at room temperature for 40 min. An enhanced
chemiluminescent kit (Thermo Fisher Scientific, Inc.) was then used
to perform chemiluminescent detection. The relative protein
expression levels were represented as the density ratio vs. GAPDH.
Protein expression was analyzed using Image-Pro Plus software 6.0
(Media Cybernetics, Inc., Rockville, MD, USA).
MTT assay
HeLa cell suspension (5×104 cells/well)
was plated in a 96-well plate and cultured for 0, 24, 48 or 72 h at
37°C. Subsequently, MTT (10 µl; 5 mg/ml) was added into each well
and then incubated at 37°C for 4 h. The supernatant was removed and
100 µl of dimethyl sulfoxide was added into each well. The
absorbance at 570 nm was determined using a Model 680 Microplate
Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Invasion assay
A Transwell assay was conducted to examine the cell
invasion capacity by using Transwell chambers (BD Biosciences,
Franklin Lakes, NJ, USA) pre-coated with Matrigel (BD Biosciences).
A HeLa cell suspension (1×106 cells/ml) was prepared in
serum-free DMEM, 300 µl of which was added into the upper chamber.
A total of 300 µl of DMEM supplemented with 10% FBS was added into
the lower chamber. After 24 h of culture at 37°C, cells that did
not invade through the membrane in the filter were lightly wiped
out by using a cotton-tipped swab. The filter was subsequently
fixed in 90% alcohol at room temperature for 10 min. Cells were
stained by 0.1% crystal violet at room temperature for 20 min
(Sigma-Aldrich; Merck KGaA, D armstadt, Germany). The invading
cells was observed under a light microscope (CX22, Olympus Corp.,
Tokyo, Japan) and images were captured (magnification, ×40).
Bioinformatics predication
Targetscan (targetscan.org) was used to predict the potential
targets of miR-92a, according to the manufacturer's
instructions.
Luciferase reporter assay
The mutant-type (MUT) DKK3 3′UTR lacking
complementarity with miR-92a seed sequence was generated using the
QuickChange Site-Directed Mutagenesis kit (Stratagene; Agilent
Technologies, Inc., Santa Clara, CA, USA), according to the
manufacturer's instructions. Wild-type (WT) or MUT of DKK3 3′UTR
was then cloned into the downstream of the firefly luciferase
coding region of pMIR-GLOTM Luciferase vector (Promega Corp.,
Madison, WI, USA), respectively. HeLa cells were co-transfected
with WT-DKK3-3′UTR or MUT-DKK3-3′UTR plasmid, and miR-92a mimic or
miR-NC, using Lipofectamine 2000. The control cells were only
transfected with WT-DKK3-3′UTR or MUT-DKK3-3′UTR plasmid. After
transfection for 48 h at 37°C, the luciferase activity was
determined using the dual-Luciferase Reporter Assay system (Promega
Corp.), according to the manufacturer's instructions. Expression
was normalized against Renilla luciferase activity.
Statistical analysis
Results were expressed as the mean ± standard
deviation of three independent experiments. Student's t-tests were
used to analyze the difference between two groups. One-way analysis
of variance followed by Tukey's post hoc test was used to analyze
the differences among more than two groups. SPSS v. 19.0 software
(IBM Corp., Armonk, NY, USA) was used to perform statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
MiR-92a is upregulated in cervical
cancer and is associated with its malignant progression
To study the role of miR-92a in cervical cancer,
RT-qPCR was performed to determine the miR-92a expression levels in
a total of 65 primary cervical cancer tissues and their matched
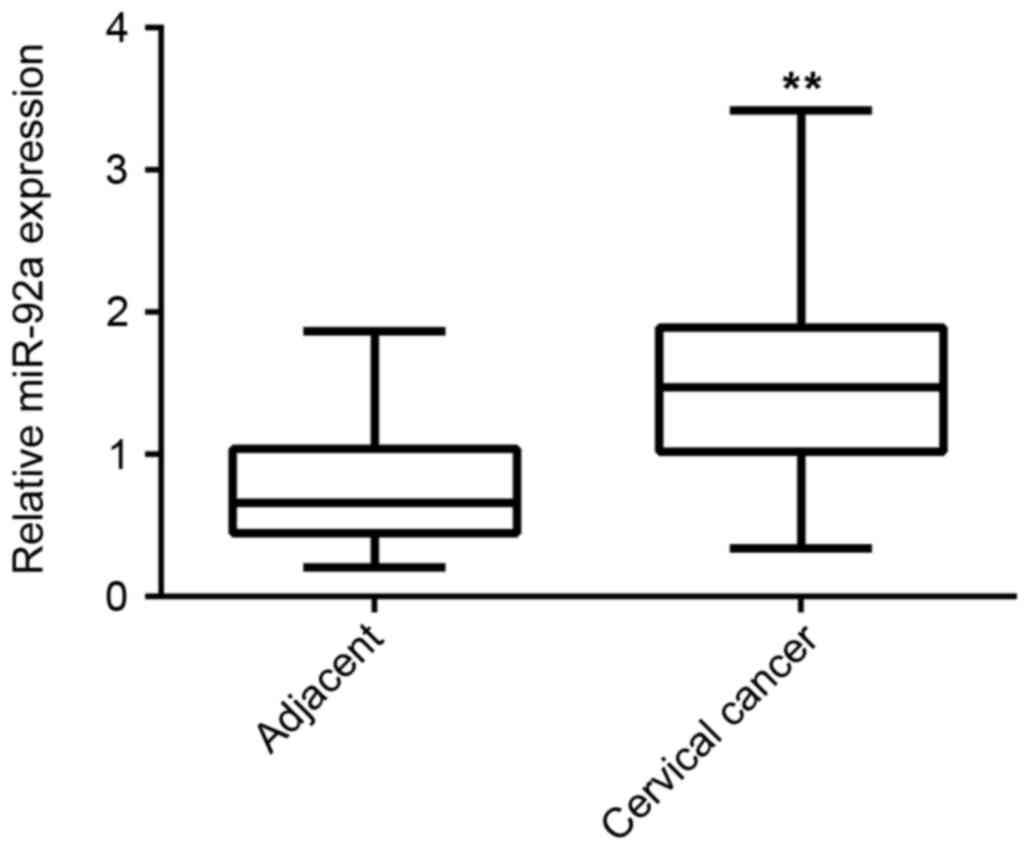
adjacent non-tumor tissues. As indicated in Fig. 1, the expression level of miR-92a was
significantly increased in cervical cancer tissues compared with
adjacent non-tumor tissues (P<0.01). The association between the
miR-92a expression levels and the clinical characteristics of
cervical cancer were further investigated. All 65 cases of cervical
cancer patients were further divided into the high miR-92a level
group and low miR-92a level group, according to the mean value of
the miR-92a level (1.48) as the cutoff point. As demonstrated in
Table I, 38 cases were in the high
miR-92a expression group and 27 cases were in the low miR-92a
expression group. Furthermore, the increased miR-92a expression was
significantly associated with poor differentiation (P=0.031),
advanced clinical stage (P=0.011) and lymph node metastasis
(P=0.014; Table I); however, no
statistically significant association of miR-92a expression was
found with age, tumor size and distant metastasis (Table I). These findings suggested that
upregulation of miR-92a may contribute to the malignant progression
of cervical cancer.
MiR-92a promotes HeLa cell viability
and invasion
The regulatory role of miR-92a in cervical cancer
cell viability and invasion was further studied. Cervical cancer
HeLa cells were transfected with miR-92a inhibitor or mimic, and NC
inhibitor and miR-NC were used as control groups, respectively.
After transfection, RT-qPCR was conducted to examine the miR-92a
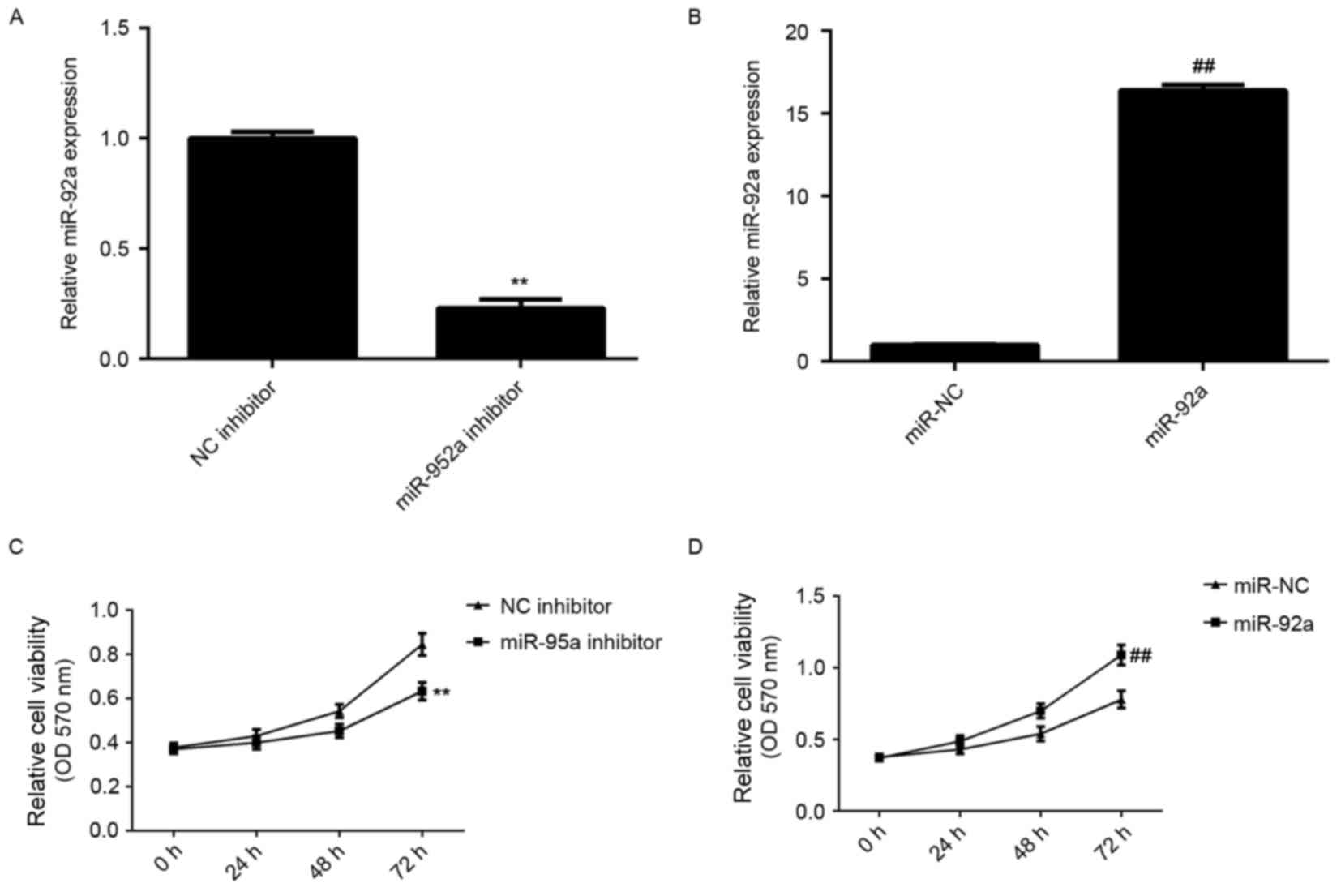
expression levels. As demonstrated in Fig. 2A and B, transfection with miR-92a
inhibitor significantly decreased the miR-92a expression level
compared with the NC inhibitor group, whereas transfection with
miR-92a mimic led to a significant increase in miR-92a expression
level compared with the miR-NC group (both P<0.01). MTT assay
was then used to examine cell viability. As demonstrated in
Fig. 2C and D, knockdown of miR-92a
significantly reduced HeLa cell viability, whereas overexpression
of miR-92a significantly enhanced HeLa cell viability (P<0.01).
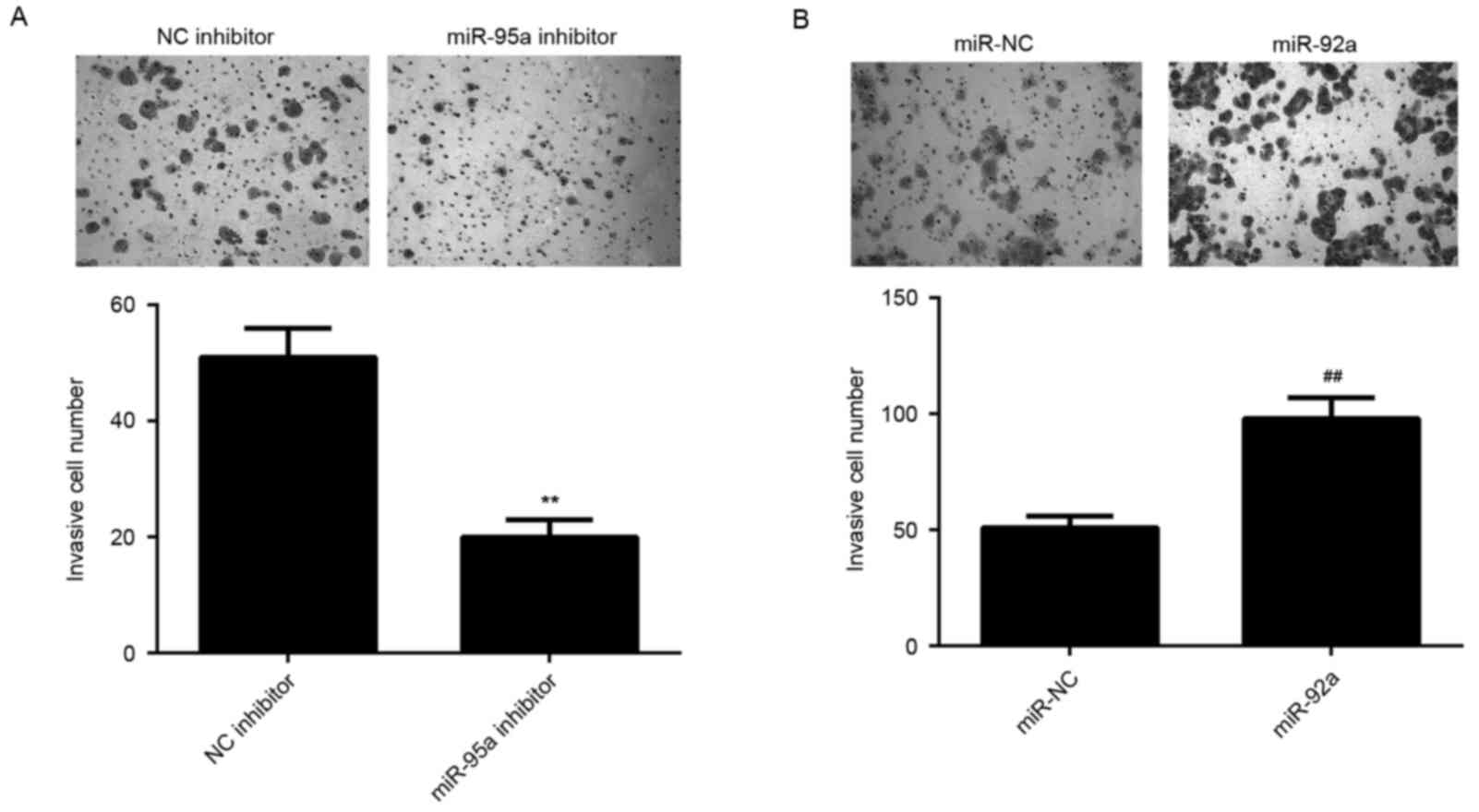
Similar findings were also observed in the invasion assay, which
indicated that miR-92a inhibition significantly reduced HeLa cell
invasion, whereas its upregulation significantly promoted HeLa cell
invasion (P<0.01; Fig. 3A and B).
Based on these data, the present results demonstrated that miR-92a
promotes HeLa cell viability and invasion.
DKK3 is a target gene of miR-92a in
HeLa cells
As miR function through the inhibition of their
target genes, bioinformatics analysis was performed to analyze the
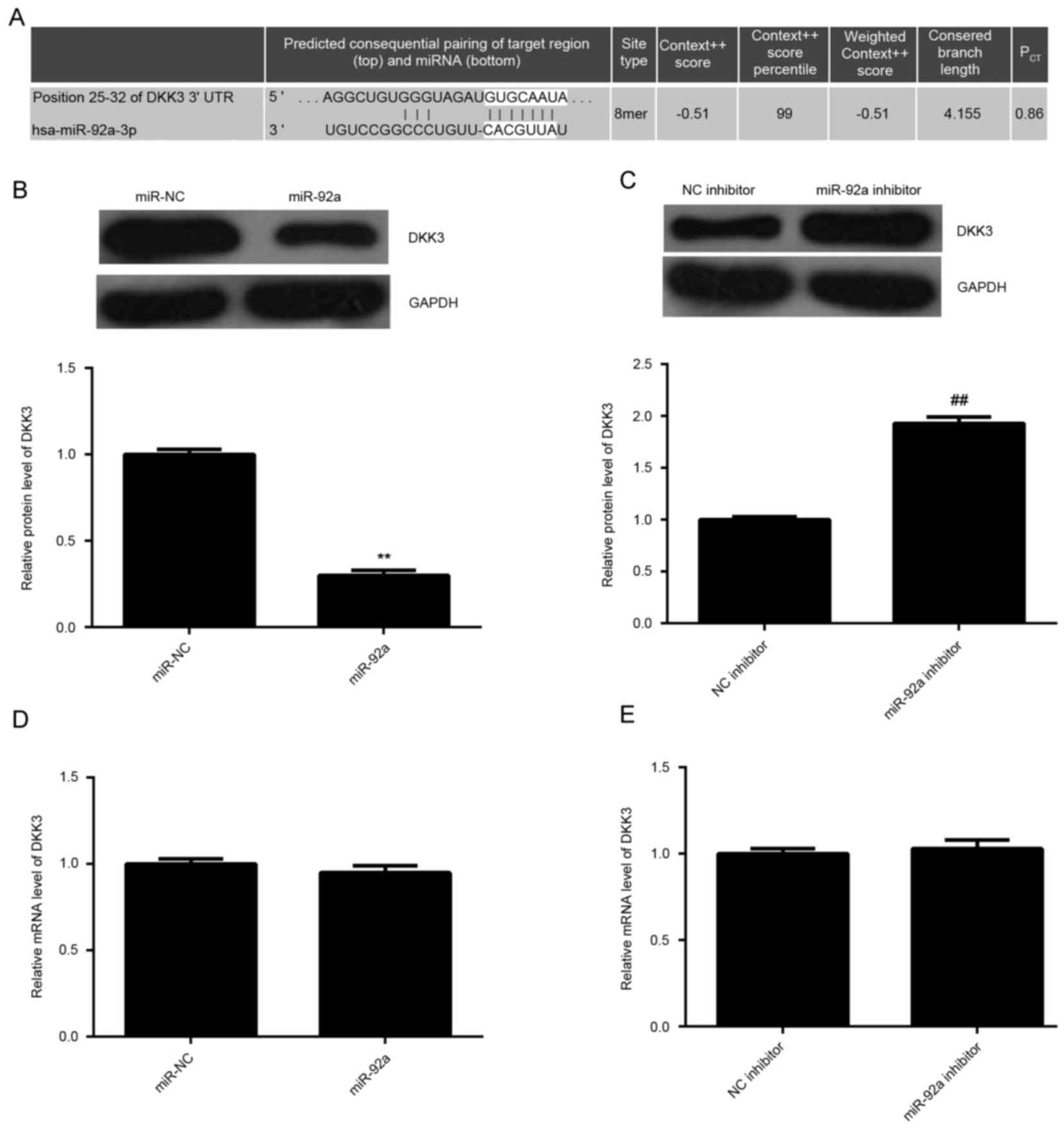
targets of miR-92a. As demonstrated in Fig. 4A, DKK3 was identified to be a
putative target of miR-92a. The expression levels of DKK3 in HeLa
cells in each group were examined. As demonstrated in Fig. 4B and C, overexpression of miR-92a
significantly decreased the protein expression levels of DKK3
compared with miR-NC, whereas downregulation of miR-92a
significantly enhanced the protein expression levels of DKK3
(P<0.01). However, there was no significant difference in the
mRNA expression levels of DKK3 among these groups (Fig. 4D and E). These findings suggest that
DKK3 may be a direct target gene of miR-92a.
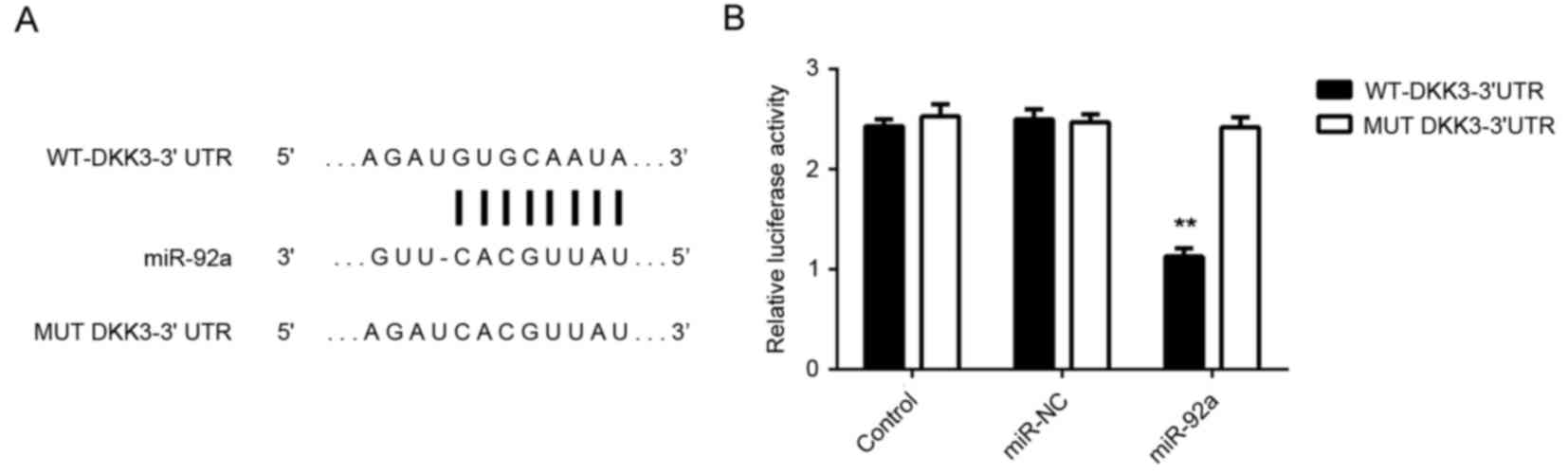
To further investigate these findings, HeLa cells
were co-transfected with WT-DKK3-3′UTR or MUT-DKK3-3′UTR luciferase
reporter plasmid (Fig. 5A), and
miR-92a mimic or miR-NC, respectively. Luciferase reporter assays
demonstrated that the luciferase activity was significantly
decreased in HeLa cells co-transfected with miR-92a mimic and
luciferase reporter vector containing the WT-DKK3-3′UTR compared
with the control group (P<0.01). However, no significant
difference in activity was observed in HeLa cells co-transfected
with miR-92a mimic and luciferase reporter vector containing the
MUT-DKK3-3′UTR (Fig. 5B). Therefore,
the present findings indicated that DKK3 is a target gene of
miR-92a in HeLa cells.
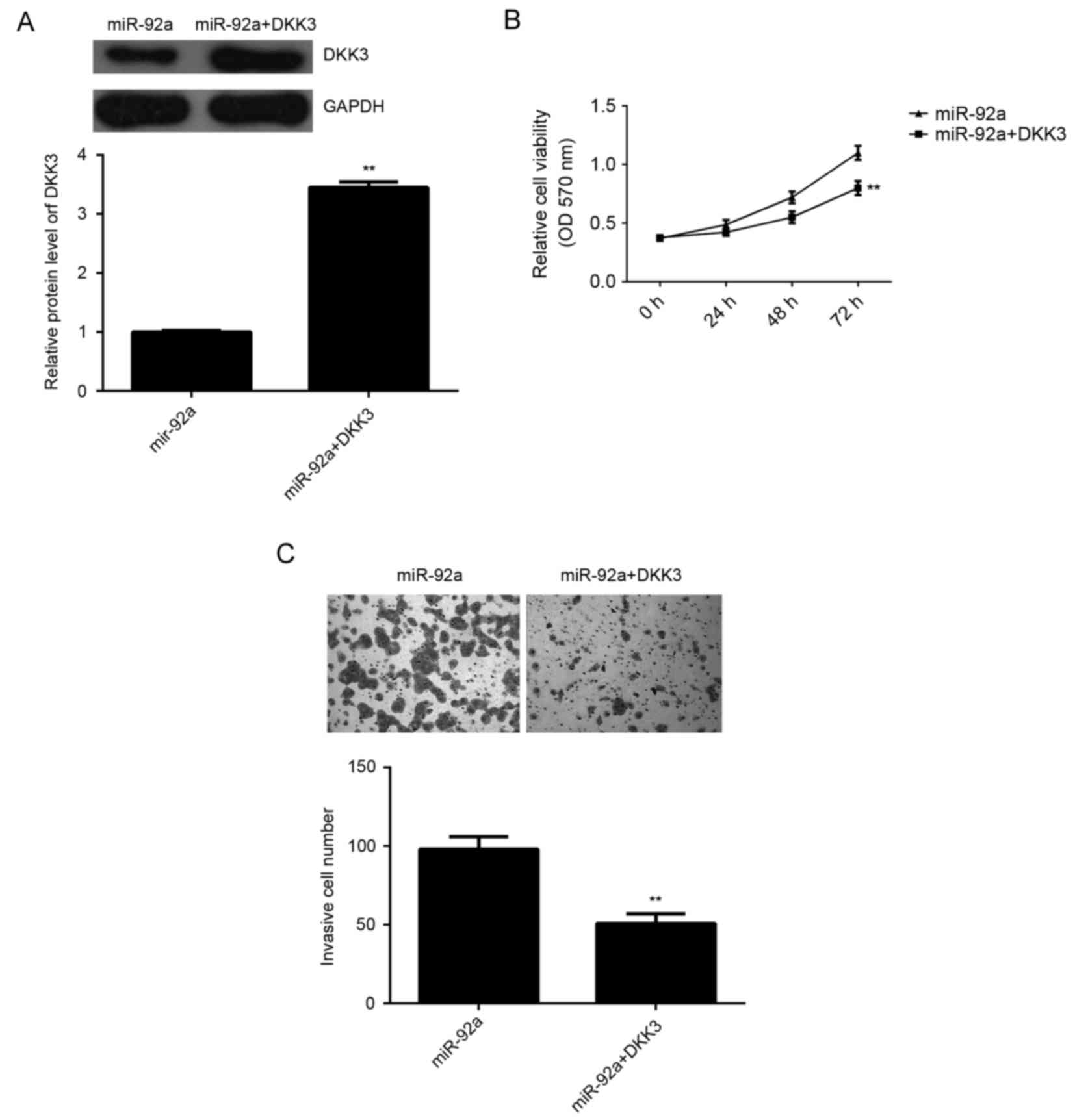
Overexpression of DKK3 attenuated the
stimulative effects of miR-92a on HeLa cell viability and
invasion
As DKK3 has been demonstrated to act as a tumor
suppressor in various types of human cancer such as pancreatic
cancer and breast cancer (19,20),
DKK3 may be involved in the miR-92a-induced invasion and cell
viability of HeLa cells. To explore this speculation,
miR-92a-overexpressing cervical cancer cells were transfected with
pcDNA3.1-DKK3 expression plasmid. As demonstrated in Fig. 6A, the protein expression levels of
DKK3 were significantly increased in the miR-92a + DKK3 group
compared with the miR-92a group (P<0.01). MTT assay and
Transwell assay were then used to determine the cell viability and
invasion capacities, respectively. The data indicated that the
viability and invasion of HeLa cells were significantly reduced in
the miR-92a + DKK3 group compared with those in the miR-92a group,
respectively (P<0.01; Fig. 6B and
C). Therefore, overexpression of DKK3 attenuated the
stimulative effects of miR-92a on HeLa cell viability and invasion.
These findings suggested that the stimulative effects of miR-92a on
HeLa cell viability and invasion may be through the inhibition of
DKK3 expression.
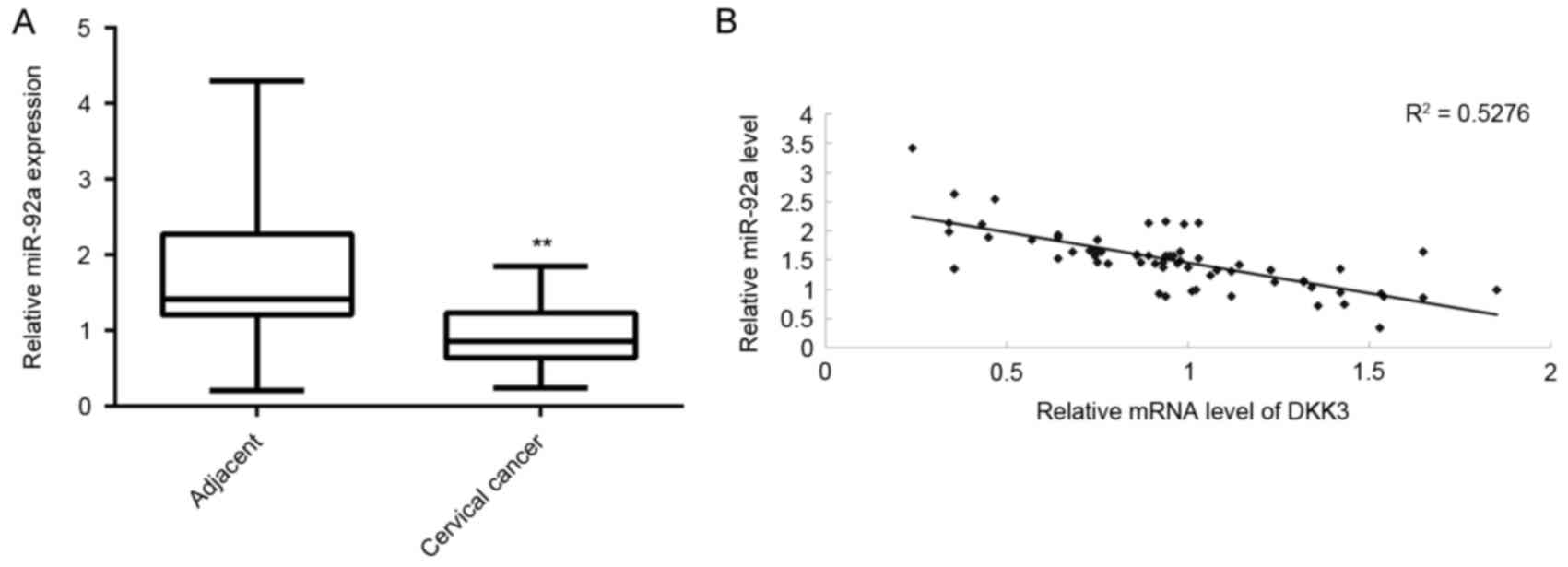
DKK3 is downregulated in cervical
cancer tissues and inversely correlated with miR-92a
expression
The expression of DKK3 in cervical cancer tissues
was determined. RT-qPCR data indicated that mRNA expression levels
of DKK3 were significantly reduced in cervical cancer tissues
compared with adjacent non-tumor tissues (P<0.01, Fig. 7A). Furthermore, an inverse
correlation was identified between miR-92a and DKK3 expression
levels in cervical cancer tissues (Fig.
7B), suggesting that the increased expression of DKK3 may be
due to the downregulation of miR-92a.
Discussion
The regulatory mechanisms of miR-92a on the
proliferation and invasion of cervical cancer cells remains largely
unknown. The present findings demonstrated that miR-92a was
significantly upregulated in cervical cancer tissues compared with
matched adjacent non-tumor tissues, and high miR-92a levels were
significantly associated with poor differentiation, advanced
clinical stage and lymph node metastasis. Further investigation
demonstrated that miR-92a promoted HeLa cell viability and
invasion. Furthermore, DKK3 was identified as a direct target of
miR-92a, and its protein expression was negatively regulated by
miR-92a in HeLa cells. Overexpression of DKK3 eliminated the
stimulative effects of miR-92a on HeLa cell viability and invasion.
Additionally, DKK3 was significantly downregulated in cervical
cancer tissues compared with adjacent non-tumor tissues and
inversely correlated to the miR-92a expression levels in cervical
cancer tissues.
Aberrant upregulation of miR-92a has been
demonstrated in some common human cancers such as rectal cancer and
gastric cancer, and generally has an oncogenic role (21,22).
MiR-92a was demonstrated to be significantly upregulated in gastric
cancer tissues compared with paracancerous normal tissue, and high
expression of miR-92a was a significant predictor of shorter
survival in stage II and stage III gastric cancer (22). Inhibition of miR-92a with locked
nucleic acid was able to prevent cell proliferation and induce cell
apoptosis in acute megakaryoblastic leukemia (23). A study by Hu et al (24) also demonstrated that butyrate
inhibited colon cancer cell proliferation and induced cell
apoptosis via downregulation of miR-92a. These findings suggest
that miR-92a may become a potential therapeutic target for cancer
treatment. Recently, miR-92a was reported to promote cervical
cancer. A study by Zhou et al (17) reported that miR-92a was significantly
upregulated in cervical cancer tissues and cell lines.
Overexpression of miR-92a significantly enhanced cervical cancer
cell proliferation by promoting cell cycle transition from G1 to S
phase and promoted cell invasion. A study by Wang et al
(25) suggested that upregulation of
miR-92a may be used as an important biomarker for oncogenic human
papillomavirus infections. In the present study, a significant
upregulation of miR-92a expression in cervical cancer tissues
compared to matched adjacent non-tumor tissues was also observed.
Furthermore, the increased miR-92a expression was significantly
associated with poor differentiation, advanced clinical stage and
lymph node metastasis, suggesting that its upregulation may
contribute to the malignant progression of cervical cancer. In
vitro study further identified that knockdown of miR-92a
significantly inhibited HeLa cell viability and invasion, whereas
overexpression of miR-92a enhanced these cellular events. Based on
these findings and others, we suggest that miR-92a may be a
potential therapeutic target for cervical cancer growth and
metastasis.
DKK3, a member of the dickkopf family, encodes a
secreted protein containing two cysteine-rich regions (26). Through interacting with the Wnt
signaling pathway, DKK3 has been identified to be involved in
various biological processes, including embryonic development and
tumorigenesis (27,28). The expression of DKK3 has been
indicated to be frequently decreased in various cancers such as
pancreatic cancer and breast cancer, and DKK3 has been suggested to
function as an important tumor suppressor (19,20). A
study by Ryu et al (29)
reported that downregulation of DKK3 was associated with adverse
clinical outcomes of cervical cancer. Furthermore, overexpression
of DKK3 was revealed to inhibit cell growth and colony formation of
cervical cancer cells (30). In the
present study, bioinformatics analysis and luciferase reporter
assay were conducted, which demonstrated that DKK3 is a direct
target gene of miR-92a. Furthermore DKK3 protein expression was
negatively mediated by miR-92a in HeLa cells. Additionally,
overexpression of DKK3 significantly attenuated the effects of
miR-92a upregulation on HeLa cell viability and invasion,
suggesting that miR-92a promotes cervical cancer cell viability and
invasion via directly targeting DKK3. In fact, the targeting
relationship between miR-92a and DKK3 has also been indicated in
neuroblastoma (31). Therefore, the
present study expands the understanding of miR-92a in the
regulation of DKK3 in human cancer.
In the present study, mRNA and protein expression
levels of DKK3 were significantly increased in cervical cancer
tissues compared with matched adjacent non-tumor tissues. These
findings were consistent with another study, Ryu et al
(29) also reported that DKK3 was
significantly downregulated in cervical cancer and demonstrated
that low expression of DKK3 was associated with advanced clinical
stage and poor 5-year disease-free survival rate of patients with
cervical cancer. Furthermore, the present data indicated an inverse
correlation between miR-92a and DKK3 expression levels in cervical
cancer tissues. As DKK3 is a direct target gene of miR-92a and its
expression was negatively regulated by miR-92a, we suggest that the
increased miR-92a expression may contribute to the downregulation
of DKK3 in cervical cancer.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that miR-92a promotes
cell viability and invasion of cervical cancer cells through
suppressing the protein expression of DKK3. These findings
highlight the importance of the miR-92a/DKK3 axis in the clinical
application for the treatment of cervical cancer in the future.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wright JD, Huang Y, Ananth CV, Tergas AI,
Duffy C, Deutsch I, Burke WM, Hou JY, Neugut AI and Hershman DL:
Influence of treatment center and hospital volume on survival for
locally advanced cervical cancer. Gynecol Oncol. 139:506–512. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moss EG: MicroRNAs: Hidden in the genome.
Curr Biol. 12:R138–R140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye JJ and Cao J: MicroRNAs in colorectal
cancer as markers and targets: Recent advances. World J
Gastroenterol. 20:4288–4299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yao J, Deng B, Zheng L, Dou L, Guo Y and
Guo K: miR-27b is upregulated in cervical carcinogenesis and
promotes cell growth and invasion by regulating CDH11 and
epithelial-mesenchymal transition. Oncol Rep. 35:1645–1651.
2016.PubMed/NCBI
|
|
9
|
Ribeiro J, Marinho-Dias J, Monteiro P,
Loureiro J, Baldaque I, Medeiros R and Sousa H: miR-34a and
miR-125b expression in HPV infection and cervical cancer
development. Biomed Res Int. 2015:3045842015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei Q, Li YX, Liu M, Li X and Tang H:
MiR-17-5p targets TP53INP1 and regulates cell proliferation and
apoptosis of cervical cancer cells. IUBMB Life. 64:697–704. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Yu X, Guo X, Tian Z, Su M, Long Y,
Huang C, Zhou F, Liu M, Wu X and Wang X: miR-143 is downregulated
in cervical cancer and promotes apoptosis and inhibits tumor
formation by targeting Bcl-2. Mol Med Rep. 5:753–760.
2012.PubMed/NCBI
|
|
12
|
Yang Z, Chen S, Luan X, Li Y, Liu M, Li X,
Liu T and Tang H: MicroRNA-214 is aberrantly expressed in cervical
cancers and inhibits the growth of HeLa cells. IUBMB Life.
61:1075–1082. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ke TW, Wei PL, Yeh KT, Chen WT and Cheng
YW: MiR-92a promotes cell metastasis of colorectal cancer through
PTEN-mediated PI3K/AKT pathway. Ann Surg Oncol. 22:2649–2655. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin HY, Chiang CH and Hung WC: STAT3
upregulates miR-92a to inhibit RECK expression and to promote
invasiveness of lung cancer cells. Br J Cancer. 109:731–738. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jikuzono T, Kawamoto M, Yoshitake H,
Kikuchi K, Akasu H, Ishikawa H, Hirokawa M, Miyauchi A, Tsuchiya S,
Shimizu K and Takizawa T: The miR-221/222 cluster, miR-10b and
miR-92a are highly upregulated in metastatic minimally invasive
follicular thyroid carcinoma. Int J Oncol. 42:1858–1868.
2013.PubMed/NCBI
|
|
16
|
He G, Zhang L, Li Q and Yang L:
miR-92a/DUSP10/JNK signalling axis promotes human pancreatic cancer
cells proliferation. Biomed Pharmacother. 68:25–30. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou C, Shen L, Mao L, Wang B, Li Y and Yu
H: miR-92a is upregulated in cervical cancer and promotes cell
proliferation and invasion by targeting FBXW7. Biochem Biophys Res
Commun. 458:63–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo Q and Qin W: DKK3 blocked
translocation of β-catenin/EMT induced by hypoxia and improved
gemcitabine therapeutic effect in pancreatic cancer Bxpc-3 cell. J
Cell Mol Med. 19:2832–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohammadpour H, Pourfathollah AA, Zarif M
Nikougoftar and Khalili S: Key role of Dkk3 protein in inhibition
of cancer cell proliferation: An in silico identification. J Theor
Biol. 393:98–104. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cristóbal I, Torrejón B, Madoz-Gúrpide J,
Rojo F and Garcia-Foncillas J: Deregulation of miR-92a in locally
advanced rectal cancer. Genes Chromosomes Cancer. 55:6122016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren C, Wang W, Han C, Chen H, Fu D, Luo Y,
Yao H, Wang D, Ma L, Zhou L, et al: Expression and prognostic value
of miR-92a in patients with gastric cancer. Tumour Biol.
37:9483–9491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharifi M and Salehi R: Blockage of
miR-92a-3p with locked nucleic acid induces apoptosis and prevents
cell proliferation in human acute megakaryoblastic leukemia. Cancer
Gene Ther. 23:29–35. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu S, Liu L, Chang EB, Wang JY and Raufman
JP: Butyrate inhibits pro-proliferative miR-92a by diminishing
c-Myc-induced miR-17-92a cluster transcription in human colon
cancer cells. Mol Cancer. 14:1802015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Wang HK, Li Y, Hafner M, Banerjee
NS, Tang S, Briskin D, Meyers C, Chow LT, Xie X, et al: microRNAs
are biomarkers of oncogenic human papillomavirus infections. Proc
Natl Acad Sci USA. 111:pp. 4262–4267. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fukusumi Y, Meier F, Götz S, Matheus F,
Irmler M, Beckervordersandforth R, Faus-Kessler T, Minina E, Rauser
B, Zhang J, et al: Dickkopf 3 promotes the differentiation of a
rostrolateral midbrain dopaminergic neuronal subset in vivo and
from pluripotent stem cells in vitro in the mouse. J Neurosci.
35:13385–13401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Z, Lin L, Thomas DG, Nadal E, Chang
AC, Beer DG and Lin J: The role of Dickkopf-3 overexpression in
esophageal adenocarcinoma. J Thorac Cardiovasc Surg.
150:377–385.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ryu SW, Kim JH, Kim MK, Lee YJ, Park JS,
Park HM, Kim DH, Lee SH and Lee EJ: Reduced expression of DKK3 is
associated with adverse clinical outcomes of uterine cervical
squamous cell carcinoma. Int J Gynecol Cancer. 23:134–140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park
J, Chae M, Zhang W and Lee JH: Dkk3, downregulated in cervical
cancer, functions as a negative regulator of beta-catenin. Int J
Cancer. 124:287–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haug BH, Henriksen JR, Buechner J, Geerts
D, Tømte E, Kogner P, Martinsson T, Flægstad T, Sveinbjørnsson B
and Einvik C: MYCN-regulated miRNA-92 inhibits secretion of the
tumor suppressor DICKKOPF-3 (DKK3) in neuroblastoma.
Carcinogenesis. 32:1005–1012. 2011. View Article : Google Scholar : PubMed/NCBI
|