Introduction
Autosomal dominant polycystic kidney disease (ADPKD)
is a hereditary kidney disease with a poor renal outcome.
Pathogenesis involves cyst formation and growth, which leads to an
increase in kidney volume and a decline in glomerular filtration
rate (1). Approximately 50% of ADPKD
patients finally need renal replacement therapy (1). In addition, ADPKD is sometimes
complicated with hypertension (1).
Epidemiologically, recent prevalence of ADPKD in
Japan remains unclear. However, Higashihara et al (2) reported a prevalence of ADPKD of 117 in
1,000,000 in Japan at the end of 1994. In Western countries, ADPKD
affects both sexes and all ethnicities, with an estimated frequency
of between 1 in 400 to 1 in 1,000 live births (1).
Primary aldosteronism (PA) is an endocrine disease
that causes secondary hypertension and increases the risk of
cardiovascular disease. Among cases of hypertension, Utsumi et
al (3) reported that 6–11% are
secondary to PA, although prevalence varies worldwide. The
pathogenesis of PA is excess secretion of aldosterone from
bilateral or unilateral adrenal gland or tumors (3). The early discovery and treatment of PA
is a crucial clinical issue to prevent progression of
atherosclerosis. The classical symptoms of PA are refractory
hypertension and hypokalemia. However, those symptoms do not always
occur, which makes early discovery difficult (3). Adrenalectomy is widely performed to
treat adrenal tumors; however, there is increasing focus on
attenuating the progression of renal dysfunction following
adrenalectomy to treat PA (3–5), the
mechanism of which has not been fully elucidated.
The primary aims of treating ADPKD and PA are
controlling hypertension and preserving kidney function. However,
only a few cases of the two diseases occurring together have been
reported (6,7). These reports have focused on diagnosing
these disorders, however to the best of our knowledge, the effect
of adrenalectomy on renal function in patients with ADPKD and PA
has not been fully elucidated.
The present report documents the case of a patient
with ADPKD who experienced a decline in estimated glomerular
filtration rate (eGFR) following adrenalectomy to treat PA.
Case report
In July 2010, a 49-year-old woman was referred to
the Department of Nephrology, Hypertension, Diabetology,
Endocrinology, and Metabolism, Fukushima Medical University
Hospital (Fukushima, Japan) for evaluation of poorly controlled
hypertension. The patient had a 9-year history of hypertension.
Hypertension was defined as systolic blood pressure >140 mmHg or
diastolic blood pressure >90 mmHg in a sitting position. In the
preceding 3 years, the patient had undergone treatment with
nifedipine (40 mg/day), imidapril (5 mg/day), and bisoprolol (5
mg/day). The patient's resting blood pressure at home had been
120–140/70–80 mmHg (normal range: Systolic blood pressure <135
mmHg and diastolic blood pressure <85 mmHg). The patient's
measured period of blood pressure was ~3 years.
However, 2 months before referral, the patient's
blood pressure increased to 160–170/80–90 mmHg. Upon admittance,
the patient underwent a physical examination that revealed a height
of 156.5 cm; a weight of 71.2 kg; a body mass index of 29.1
kg/m2; a temperature of 36.4°C; a resting blood pressure
of 157/83 mmHg and a regular pulse of 50 beats/min. Fundus
examination revealed hypertensive changes (H3S2 in the left eye and
H2S2 in the right eye by Scheie's classification) (8). Therefore, the patient had at least
moderate hypertensive and atherogenic fundus changes. The patient's
family history was significant, due to the patient's mother having
polycystic kidney disease. Laboratory examinations revealed
hypokalemia and anemia due to iron deficiency (Table I).
 | Table I.Laboratory data on first visit. |
Table I.
Laboratory data on first visit.
| Parameter | Result | Normal range |
|---|
| WBC (cells/µl) | 6,000 | 2,800–8,800 |
| RBC (cells
×104/µl) | 389 | 366–478 |
| Hb (g/dl) | 6.9 | 11.6–14.0 |
| Hct (%) | 25.1 | 34.1–41.7 |
| Plt (Plt
×104/µl) | 26.3 | 14.7–34.1 |
| MCV (fl) | 64.5 | 81.8–97.2 |
| MCH (%) | 17.7 | 27.1–33.5 |
| TP (g/dl) | 7.2 | 6.7–8.3 |
| Alb (g/dl) | 4.1 | 3.9–4.9 |
| AST (IU/l) | 14 | 13–33 |
| ALT (IU/l) | 10 | 6–27 |
| γGT (IU/l) | 17 | 10–47 |
| T-Bil (mg/dl) | 1.0 | 0.2–1.2 |
| CRP (mg/dl) | 0.67 | 0–0.3 |
| Fe (µg/dl) | 13 | 43–172 |
| UIBC (µg/dl) | 336 | 137–325 |
| Ferritin
(ng/ml) | 8 | 12–60 |
| FPG (mg/dl) | 98 | 70–109 |
| Na (mEq/l) | 143 | 138–146 |
| K (mEq/l) | 2.4 | 3.6–4.9 |
| Cl (mEq/l) | 98 | 99–109 |
| BUN (mg/dl) | 7 | 8–22 |
| Cre (mg/dl) | 0.51 | 0.4–0.7 |
| eGFR (ml/min/1.73
m2) | 143 | ≥60 |
| UA (mg/dl) | 6.1 | 2.3–7.0 |
| U-Glucose | (−) | (−) |
| U-Protein | (1+) | (−) |
| U-Blood | (−) | (−) |
| U-Na (mEq/l) | 9 | 40–90 |
| U-K (mEq/l) | 24 | 20–60 |
| U-Cl (mEq/l) | 227 | 40–120 |
| U-Cre (mg/dl) | 98 | 70–109 |
| PRA (ng/ml/h) | ≤0.1 | 0.1–0.3 |
| Aldosterone
(pg/ml) | 149 | 29.9–159 |
Plasma renin activity (PRA) was ≤0.1 ng/ml/h (normal
range, 0.1–0.3 ng/ml/h), thus bisoprolol was discontinued to
exclude a false low result. Oral iron supplements (100 mg/day) were
administered, resulting in gradual improvement of anemia.
Thereafter, a captopril (50 mg) stimulation test was conducted
producing a low renin response (Table
II; normal range, renin response >1.0 ng/ml/h,
aldosterone-to-renin ratio (ARR) of 60 or 90 min <200).
Abdominal computed tomography and magnetic resonance imaging
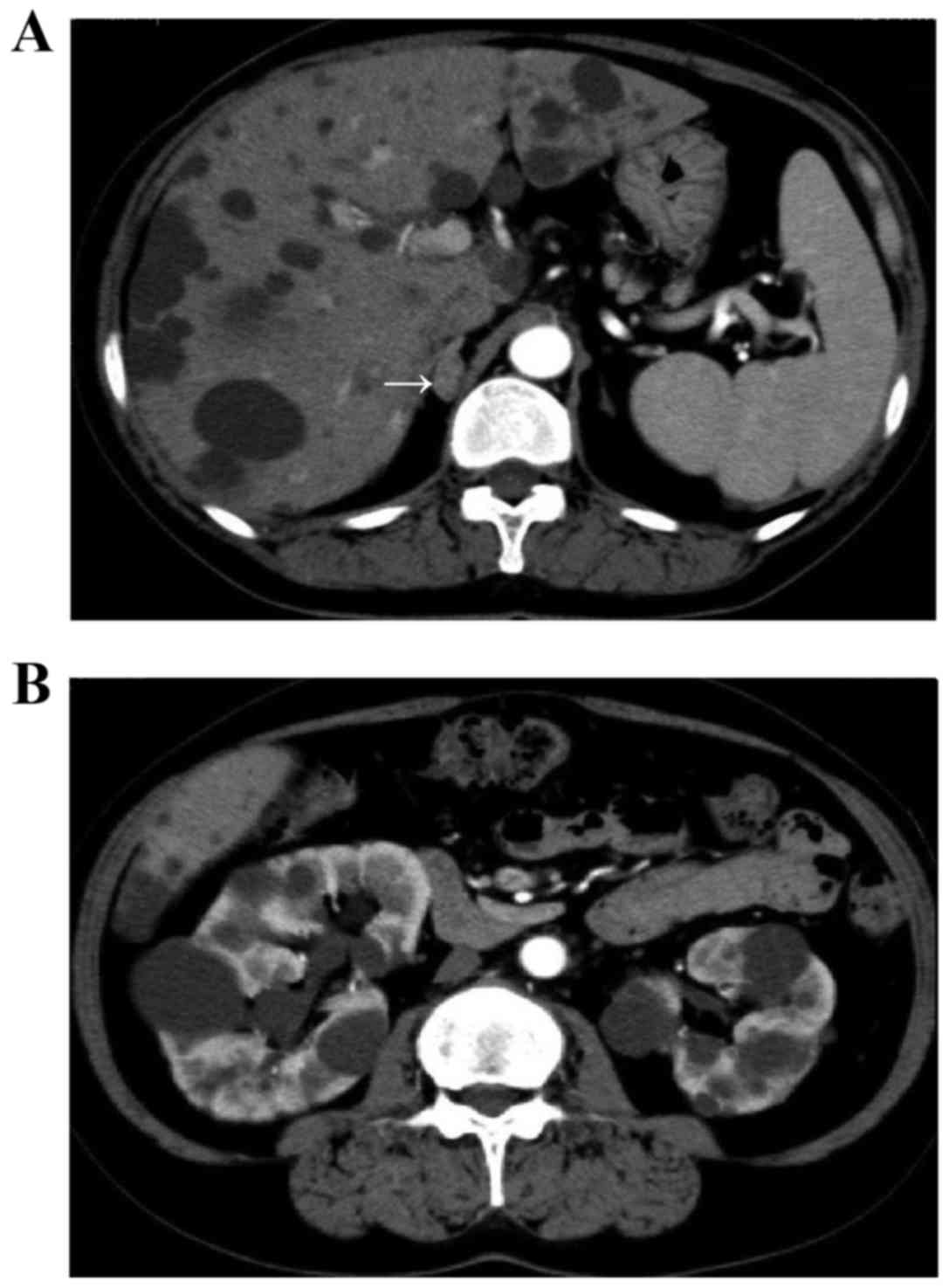
revealed a right adrenal tumor ~1.0 cm in size and multiple cysts
in the liver and kidneys (Fig. 1).
The estimated volume of the bilateral kidneys was calculated using
the volumetric method and the following formula: Volume = π/6x
length × width × depth (9,10) and was determined to be ~705.4 ml.
 | Table II.Captopril stimulation test. |
Table II.
Captopril stimulation test.
|
| Time (min) |
|
|---|
|
|
|
|
|---|
| Parameter | 0 | 60 | 90 | Normal range |
|---|
| Renin
(ng/ml/h) | 0.3 | 0.3 | 0.3 | 0.1–0.3 |
| Aldosterone
(pg/ml) | 129 | 194 | 228 | 29.9–159 |
| Aldosterone/renin
ratio | 430 | 646.7 | 760 | ≤200 |
To evaluate the adrenal tumor further, adrenal vein
sampling was conducted. A catheter was inserted into the bilateral
adrenal vein via the femoral vein. Blood samples were collected
from the inferior vena cava, left adrenal vein and right adrenal
vein. A 0.25 mg dose of tetracosactide acetate (adrenocorticotripic
hormone; Daiichi Sankyo Co., Ltd., Tokyo, Japan) was administered
intravenously, and after 30 min, blood samples were collected from
the same locations again. The serum aldosterone level was high on
the side of the tumor (Table III).
The patient's results fulfilled the diagnostic criteria for ADPKD
(11) and PA (12,13).
 | Table III.Adrenal vein sampling. |
Table III.
Adrenal vein sampling.
| Vein | Aldosterone
(pg/ml) | Cortisol
(µg/dl) |
Aldosterone/cortisol |
|---|
| Inferior vena
cava | 66.1 | 4.0 | 16.5 |
| Left adrenal
vein | 88.3 | 13.9 | 6.4 |
| Right adrenal
vein | 2,010 | 15.5 | 129.7 |
| Inferior vena
cavaa |
260 | 15 | 17.3 |
| Left adrenal
veina | 2,100 | 630 | 3.3 |
| Right adrenal
veina | 67,300 | 1,100 | 61.2 |
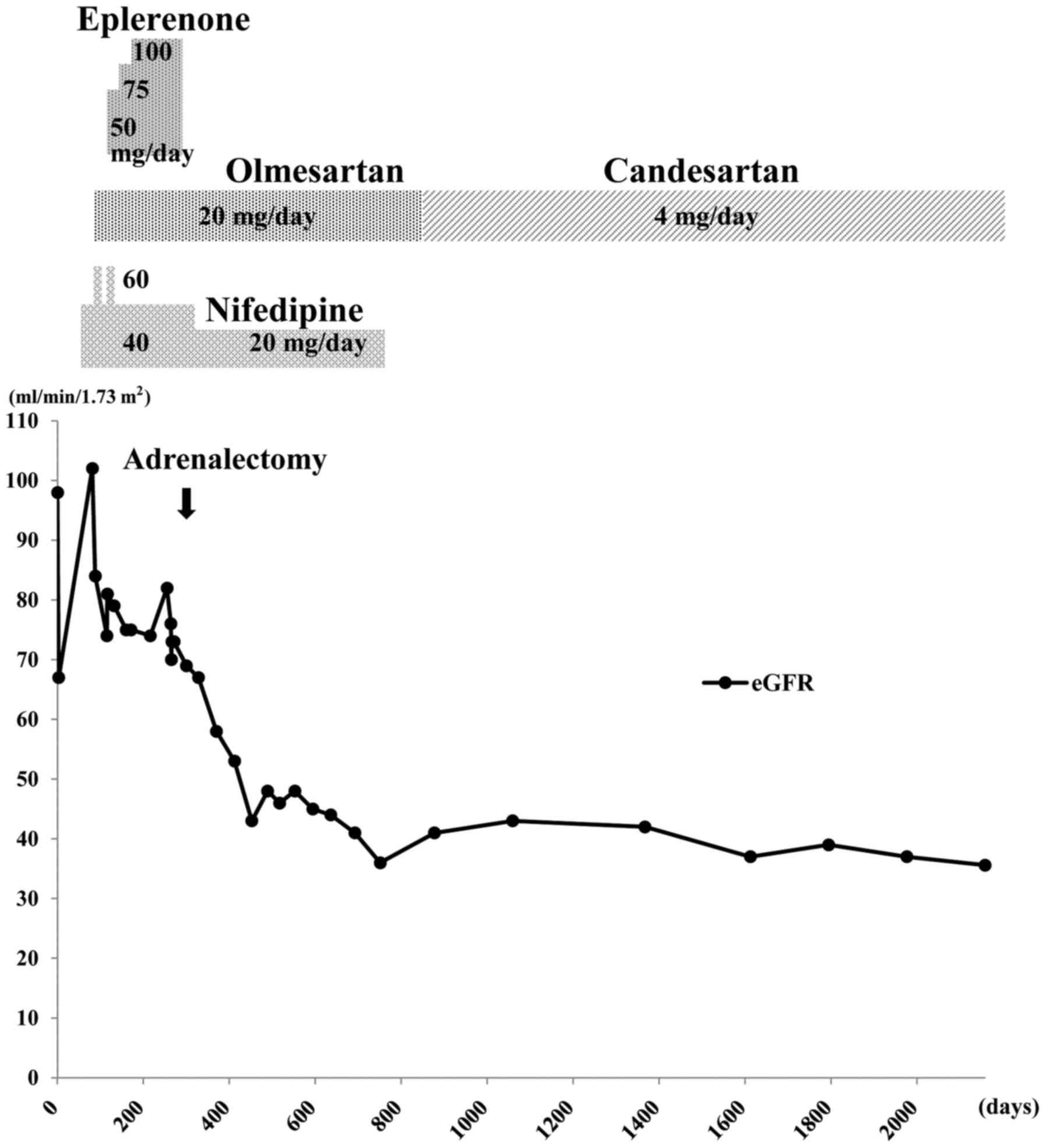
Eplerenone (selective aldosterone antagonist; Pfizer
Japan, Inc., Tokyo, Japan) was administered, beginning at 50 mg/day
(once after breakfast) for 15 days, thereafter, 75 mg/day (once
after breakfast) for 30 days and finally increasing to 100 mg/day
(50 mg twice a day, after breakfast and dinner) for ~15 weeks,
until adrenalectomy. This resulted in a reduction of blood pressure
to ~130/80 mmHg, which was within normal blood pressure range of
<135/85 mmHg. In April 2011, right adrenalectomy was
successfully performed. Hemodynamic disturbances during the surgery
or in the immediate post-surgical course were not evident. Weiss
criteria (14,15) were used to distinguish between
adrenal adenoma or malignant tumor. For pathological evaluation,
the specimen was fixed with 10% formalin fixation for 3 days at
room temperature. The thickness of sections was approximately 5 µm.
The paraffin embedded tissue sections were de-paraffinized by
xylene, and then stained with hematoxylin-eosin. Sections were
examined under an Olympus BX51 microscope at magnification ×200.
Pathologically, there were no evidence of necrosis, mitosis or
atypical mitosis. From the above pathological findings, the tumor
was diagnosed as adenoma.
Postoperatively, potassium levels normalized.
However, kidney function worsened following adrenalectomy, despite
good control of her hypertension. Fig.
2 summarizes the clinical course of the patient over ~5 years.
The eGFR (calculated as 194x creatinine−1.094x
age−0.287x0.739) (16)
decreased by 36.8% over the 7 months immediately following
adrenalectomy, from 76 to 48 ml/min/1.73 m2 (normal
range, >90 ml/min/1.73 m2). The serum creatinine
level increased from 0.64 to 0.97 mg/dl. Home blood pressure
measurements during this time remained 110–120/60–80 mmHg.
After the initial decline in eGFR over the 7 months
following adrenalectomy, it stabilized to some extent, with a much
slower rate of decline (Fig. 2),
suggesting that this was the natural level of the patient's renal
function due to ADPKD. Follow-up abdominal computed tomography ~9
months after surgery indicated no marked changes in the renal
cysts. The estimated bilateral kidney volume was ~707.7 ml;
virtually unchanged from the first examination. The patient
continued to take 4 mg/day candesartan (angiotensin II receptor
antagonist; Takeda Pharmaceutical Co., Ltd., Osaka, Japan), once
after breakfast, which effectively controlled blood pressure.
Discussion
The present report highlights three important
issues. Firstly, PA may co-exist with ADPKD. Secondly, as in this
case, PA might mask ADPKD kidney dysfunction, which only manifests
following adrenalectomy. Therefore, clinicians should remain
vigilant for the unmasking of kidney dysfunction following
adrenalectomy in patients with ADPKD. Finally, following
adrenalectomy, it appears that renal function may decrease to
levels expected over the natural clinical course of ADPKD.
ADPKD is attributed to mutations in the PKD1
or PKD2 genes (17) that
produce the polycystin-1 and polycystin-2 proteins, respectively.
Loss of function of either protein ultimately results in the
formation of cysts in the kidney. Growth and expansion of cysts is
a critical factor in progressive ADPKD. In individuals harboring a
mutation, cysts are almost always detectable by early adulthood
(18). The cysts are thought to
induce focal areas of renal ischemia and enhanced renin release,
increasing renin-aldosterone system activity, with the result that
extracellular volume expansion is often present in the early stages
of ADPKD (19). It is not unusual
for young adults with ADPKD to exhibit hypertension before they
experience evident renal dysfunction (20,21). The
mechanism of hypertension in ADPKD is thought to be complex. A
number of potential factors have been proposed, including the
renin-aldosterone system, endothelial dysfunction, increased
sympathetic activity, increased endothelin-l levels and arterial
stiffness (22).
However, the role of the renin-aldosterone system in
ADPKD is considered to be particularly important (1). One of the reasons is that
hyperaldosteronism may not only to increase cardiovascular risk and
secondary hypertension but may also to contribute to the growth of
cysts, with hypokalemia implicated as a direct growth factor for
cysts (22). Therefore, the early
diagnosis and correction of underlying PA is critical in patients
with ADPKD. However, as ADPKD is often complicated by hypertension
in the early stages, the coexistence of PA may not be considered.
In this regard, Kao et al (7)
reported that hypokalemia is an important feature that may enable
the diagnosis of the co-occurrence of ADPKD and PA. In addition, a
high aldosterone-renin ratio (ARR) may be a useful diagnostic tool
because as in PA cases, if the only symptom is hypertension, early
discovery of ADPKD and PA co-occurrence is difficult. In the
present case report, hypokalemia and a high ARR were present
initially (K, 2.4 mEq/l; PRA, ≤0.1 ng/ml/h and aldosterone, 149
pg/ml), suggesting that the patient had PA.
So far, there have been 12 reported cases of
combined ADPKD and PA (6,7,23–27).
Adrenalectomies were performed in 8 patients (6,7,23–26) and
anti-aldosterone drugs were administered to the other 4 cases
(22,23,27).
Worsening of kidney function following adrenalectomy for PA has
been reported (23); the cause may
be the correction of glomerular hyperfiltration once aldosterone
levels have been stabilized (3–5).
Therefore, overestimated kidney function due to glomerular
hyperfiltration was canceled out, which led to an accurate kidney
function. As a result, decline of eGFR may have occurred.
Hyperaldosteronism leads to sodium and water retention, for which
the kidney may compensate with hyperfiltration to excrete the
excess sodium and water. Following adrenalectomy, normalization of
aldosterone levels results in the return of filtration to normal
levels. Kidney dysfunction that was previously masked by
aldosterone-related hyperfiltration may then be revealed (3–5).
There is controversy regarding the length of time
take for eGFR to return to normal levels following adrenalectomy.
According to some reports, eGFR decreases following adrenalectomy
for ~1 month, after which it stabilizes, presumably indicating
underlying levels of renal function (3–5).
However, other authors have reported more variable rates of decline
in eGFR following adrenalectomy. Utsumi et al (3) reviewed the records of 78 Japanese
patients who underwent unilateral adrenalectomy to treat PA. The
eGFR decreased by 16.6–13.2 ml/min/1.73 m2 (16.3–13.0%)
in patients with a preoperative eGFR ≥90 ml/min/1.73 m2
(n=20); by 11.1–10.6 ml/min/1.73 m2 (14.7–14.4%) in
those with initial values of 60–89 ml/min/1.73 m2
(n=46); and by 4.8–8.7 ml/min/1.73 m2 (10.6–18.8%) in
those with a preoperative eGFR <60 ml/min/1.73 m2
(n=12). These values were all measured within 1 month
postoperation. The differences between pre- and postoperative
values in the three groups were statistically significant (3). In the current case report, the rate and
duration of the decline of eGFR following adrenalectomy likely
differed from that observed in patients with PA alone. What is
notable in the current case is that the kidney volume remained
essentially unchanged pre- and postoperatively and blood pressure
remained well controlled post-surgery. Therefore, a rapid
postoperative decline in eGFR was most likely due to correction of
hyperaldosteronism.
According to the Consortium for Radiologic Imaging
Studies of Polycystic Kidney Disease (CRISP) dataset (www.niddkrepository.org/studies/crisp), Chapman et
al (28) reported that eGFR
could be calculated as follows: (y=−0.0136 x+112.63, R=−0.37),
(y=eGFR, ml/min/1.73 m2, × = the volume of kidney, ml).
If the current case is applied, the volume of each kidney was 705.4
ml and eGFR is calculated as 103.04 ml/min/1.73 m2.
Therefore, the unmasked level of kidney dysfunction in the current
case may be more severe at the given level of total kidney volume.
The reasons for the poor kidney function of the patient cannot be
determined with certainty. One potential explanation is the
coexistence of progressive ADPKD masked by hyperfiltration, as well
as a history of long-term hypertension. Although predicting a
decrease in eGFR in patients with PA following adrenalectomy is
difficult, it is a critical clinical issue. Tanase-Nakao et
al (5) reported that a
preoperative eGFR ≤76.9 and ARR ≥305 were significant predictive
factors for postoperative chronic kidney disease in patients with
PA. In the current case, preoperative eGFR and ARR almost fulfilled
those criteria, although it is unclear if other patients similar to
ours would also have similar results. Identifying similar such
cases is required to clarify this issue. It was also observed that
the patient's eGFR stabilized ~7 months following adrenalectomy and
exhibited only a slow decline thereafter, indicating that eGFR is
associated with the natural clinical course of ADPKD. Torres and
Harris (29) reported that, once
renal dysfunction appears in ADPKD, there is a decline in eGFR of
~5 ml/min/year. Given the age of the patient, this rate of decline
is not inconsistent with eGFR values immediately following
postoperative decline.
The patient's blood pressure was well controlled
following adrenalectomy. However, the target for blood pressure
control in patients with ADPKD remains controversial. Sarnak et
al (30) reported that low
targets for blood pressure slowed the progression of nondiabetic
kidney disease in patients with moderately-to-severely decreased
GFRs and recommended a target of <130/80 mmHg (28). In this regard, the recent HALT-PKD
[Study A] (31) determined that in
early ADPKD, strict blood pressure control (i.e., blood pressure
<110/75 mm Hg) was associated with better outcomes, including a
slower increase in total kidney volume, decrease in
left-ventricular-mass index and proteinuria, compared with standard
blood pressure control, although there was no difference in the
eGFR. In the current case report, proteinuria was only detected on
the first visit and microalbuminuria was undetectable
thereafter.
In conclusion, the current study reports the case of
a patient with ADPKD whose kidney dysfunction was masked until
adrenalectomy for PA caused by adrenal adenoma. This case suggests
that, when patients undergo adrenalectomy to treat PA, the
clinician must be vigilant for a postoperative decline in eGFR,
particularly in patients with possible underlying renal
disease.
Acknowledgements
The authors would like to thank Dr Osamu Suzuki for
advising on pathological methods. This study was supported in part
by a Grant-in-Aid for Scientific Research (C) from the Ministry of
Education, Culture, Sports, Science and Technology of Japan (grant
no. 16K09363) awarded to Dr Hiroaki Satoh.
Glossary
Abbreviations
Abbreviations:
|
ADPKD
|
autosomal dominant polycystic kidney
disease
|
|
PA
|
primary aldosteronism
|
|
eGFR
|
estimated glomerular filtration
rate
|
References
|
1
|
Ramanathan G, Elumalai R, Periyasamy S and
Lakkakula B: Role of renin-angiotensin-aldosterone system gene
polymorphisms and hypertension-induced end-stage renal disease in
autosomal dominant polycystic kidney disease. Iran J Kidney Dis.
8:265–277. 2014.PubMed/NCBI
|
|
2
|
Higashihara E, Nutahara K, Kojima M,
Tamakoshi A, Yoshiyuki O, Sakai H and Kurokawa K: Prevalence and
renal prognosis of diagnosed autosomal dominant polycystic kidney
disease in Japan. Nephron. 80:421–427. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Utsumi T, Kawamura K, Imamoto T, Nagano H,
Tanaka T, Kamiya N, Nihei N, Naya Y, Suzuki H and Ichikawa T:
Preoperative masked renal damage in Japanese patients with primary
aldosteronism: Identification of predictors for chronic kidney
disease manifested after adrenalectomy. Int J Urol. 20:685–691.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwakura Y, Morimoto R, Kudo M, Ono Y,
Takase K, Seiji K, Arai Y, Nakamura Y, Sasano H, Ito S and Satoh F:
Predictors of decreasing glomerular filtration rate and prevalence
of chronic kidney disease after treatment of primary aldosteronism:
Renal outcome of 213 cases. J Clin Endocrinol Metab. 99:1593–1598.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanase-Nakao K, Naruse M, Nanba K, Tsuiki
M, Tagami T, Usui T, Okuno H, Shimatsu A, Hashimoto S, Katabami T,
et al: Chronic kidney disease score for predicting postoperative
masked renal insufficiency in patients with primary aldosteronism.
Clin Endocrinol (Oxf). 81:665–670. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gejyo F, Ishida K and Arakawa M: Autosomal
dominant polycystic kidney disease complicated by primary
aldosteronism. Case report and review of the literature. Am J
Nephrol. 14:236–268. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kao CC, Wu VC, Kuo CC, Lin YH, Hu YH, Tsai
YC, Wu CH and Wu KD; TAIPAI study group, : Delayed diagnosis of
primary aldosteronism in patients with autosomal dominant
polycystic kidney diseases. J Renin Angiotensin Aldosterone Syst.
14:167–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scheie HG: Evalution of ophthalmoscopic
changes of hypertension and arteriolar sclerosis. AMA Arch
Ophthalmol. 49:117–138. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neill WC, Robbin ML, Bae KT, Grantham
JJ, Chapman AB, Guay-Woodford LM, Torres VE, King BF, Wetzel LH,
Thompson PA and Miller JP: Sonographic assessment of the severity
and progression of autosomal dominant polycystic kidney disease:
The consortium of renal imaging studies in polycystic kidney
disease (CRISP). Am J Kidney Dis. 46:1058–1064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cadnapaphornchai MA, McFann K, Strain JD,
Masoumi A and Schrier RW: Prospective change in renal volume and
function in children with ADPKD. Clin J Am Soc Nephrol. 4:820–829.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ravine D, Gibson RN, Walker RG, Sheffield
LJ, Kincaid-Smith P and Danks DM: Evaluation of ultrasonographic
diagnostic criteria for autosomal dominant polycystic kidney
disease 1. Lancet. 343:824–827. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Omura M, Sasano H, Saito J, Yamaguchi K,
Kakuta Y and Nishikawa T: Clinical characteristics of
aldosterone-producing microadenoma, macroadenoma, and idiopathic
hyperaldosteronism in 93 patients with primary aldosteronism.
Hypertens Res. 29:883–889. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Satoh F, Abe T, Tanemoto M, Nakamura M,
Abe M, Uruno A, Morimoto R, Sato A, Takase K, Ishidoya S, et al:
Localization of aldosterone-producing adrenocortical adenomas:
Significance of adrenal venous sampling. Hypertens Res.
30:1083–1095. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss LM: Comparative histologic study of
43 metastazing and nonmetastasizing adrenocortical tumors. Am J
Surg Pathol. 8:163–169. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weiss LM, Medeiros LJ and Vickery AL Jr:
Pathologic features of prognostic significance in adrenocortical
carcinoma. Am J Surg Pathol. 13:202–206. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A:
Collaborators developing the Japanese equation for estimated GFR
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:982–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Torres VE, Harris PC and Pirson Y:
Autosomal dominant polycystic kidney disease. Lancet.
369:1287–1301. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parfrey PS, Bear JC, Morgan J, Cramer BC,
McManamon PJ, Gault MH, Churchill DN, Singh M, Hewitt R, Somlo S,
et al: The diagnosis and prognosis of autosomal dominant polycystic
kidney disease. N Engl J Med. 323:1085–1090. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Barrett BJ, Foley R, Morgan J, Hefferton D
and Parfrey P: Differences in hormonal and renal vascular responses
between normotensive patients with autosomal dominant polycystic
kidney disease and unaffected family members. Kidney Int.
46:1118–1123. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bell PE, Hossack KF, Gabow PA, Durr JA,
Johnson AM and Schrier RW: Hypertension in autosomal dominant
polycystic kidney disease. Kidney Int. 34:683–690. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chapman AB, Johnson A, Gabow PA and
Schrier RW: The renin-angiotensin-aldosterone system and autosomal
dominant polycystic kidney disease. N Engl J Med. 323:1091–1096.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peixoto AJ: A young patient with a family
history of hypertension. Clin J Am Soc Nephrol. 9:2164–2172. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bobrie G, Sirieix ME, Day M, Landais P,
Lacombe M and Grunfeld JP: Autosomal dominant polycystic kidney
disease with primary hyperaldosteronism. Nephrol Dial Transplant.
7:647–650. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saeki S, Ogihara T, Masugi F, Mikami H,
Tabuchi Y, Seto T and Kumahara Y: A case of primary aldosteronism
with polycystic kidney disease. Nihon Naika Gakkai Zasshi.
75:28–32. 1986.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chee TS Rajasoorya and Ng BK: Hypertension
in disguise - a trap for the unwary. Eur J Endocrinol. 133:93–66.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liou HH, Tsai SC, Chen WJ, Huang TP, Huang
WJ and Chen KK: The association of aldosterone-producing adrenal
adenoma in a patient with autosomal dominant polycystic kidney
disease. Am J Kidney Dis. 23:739–742. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoorn EJ, Hesselink DA, Kho MM, Roodnat
JI, Weimar W, van Saase JL, van den Meiracker AH and Zietse R: A
case of primary aldosteronism revealed after renal transplantation.
Nat Rev Nephrol. 7:55–60. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chapman AB, Guay-Woodford LM, Grantham JJ,
Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF Jr, Glockner
JF, Wetzel LH, et al: Renal structure in early autosomal-dominant
polycystic kidney disease (ADPKD): The consortium for radiologic
imaging studies of polycystic kidney disease (CRISP) cohort. Kidney
Int. 64:1035–1045. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Torres VE and Harris PC: Autosomal
dominant polycystic kidney disease: The last 3 years. Kidney Int.
76:149–168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarnak MJ, Greene T, Wang X, Beck G, Kusek
JW, Collins AJ and Levey AS: The effect of a lower target blood
pressure on the progression of kidney disease: Long-term follow-up
of the modification of diet in renal disease study. Ann Intern Med.
142:342–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schrier RW, Abebe KZ, Perrone RD, Torres
VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki
PG, Hogan MC, et al: Blood pressure in early autosomal dominant
polycystic kidney disease. N Engl J Med. 371:2255–2266. 2014.
View Article : Google Scholar : PubMed/NCBI
|