Introduction
Acute myeloid leukemia (AML) remains difficult to
cure because of its resistance to treatment. Although conventional
chemotherapy induces initial remission in ~60% of patients with AML
and is the standard induction therapy for children and adults with
AML, the majority of patients relapse after remission (1). The graft-versus-leukemia effects
observed following hematopoietic stem cell transplantation (HSCT)
in patients with AML indicate that inducing antitumor immunity
would enable the eradication of AML and prevent relapse. However,
because HSCT has high rates of treatment-associated mortality and
morbidity, the indications for performing HSCT in the first
remission remain controversial (2).
Immunotherapy, which has advanced rapidly in recent
years, is a potent tool that has potential therapeutic applications
in numerous cancer types (3).
Immunotherapy enables the killing tumor cells through boosting a
patient's endogenous immune responses, while not harming normal
cells (3). This property gives
immunotherapy an advantage compared with conventional chemotherapy
or radiotherapy, which frequently have toxic side effects (3). Notably, numerous studies have
investigated the use of immunotherapy for hematological
malignancies (4–7). The present approaches for AML
immunotherapy include antibody-based therapy, natural killer (NK)
cell therapy and adoptive T cell immunotherapy. These techniques
have demonstrated potential in preclinical applications, but
require further testing. In addition, the underlying mechanisms of
the cytotoxic effects of immunotherapy on tumor cells remain
unclear.
Tumor antigen-T specific cells are almost always
inefficient or suppressed in patients with cancer (8). In addition, chemotherapy and
immunosuppressive cytokines secreted by tumor cells may suppress
antitumor immunity in patients with cancer (9). Therefore, the adoptive transfer of
tumor-specific T cells has been investigated in recent years
(10,11). Cytotoxic T lymphocytes (CTLs) exhibit
more potent tumor cell-killing ability compared with NK cells, and
may eradicate cancer stem cells, making CTL immunotherapy a
promising cancer treatment with curative intent (12). Leukemia-derived CTL cell lines have
been demonstrated to inhibit the proliferation of leukemic
progenitor cells in vitro, which would enable the successful
treatment of chronic myeloid leukemia in the accelerated phase
(13). It has been demonstrated that
adoptively-transferred T cell clones persist in vivo in
response to low-dose interleukin-2 treatment, preferentially
localize to tumor sites and mediate an antigen-specific immune
response, which is characterized by the elimination of
tumor-specific antigen-positive tumor cells (14).
Due the refractoriness and heterogeneity of cancer,
multidisciplinary comprehensive treatment should be recommended in
the first instance. However, how conventional cancer treatments and
immunotherapies will affect one another remains unclear. Advances
in tumor immunology have revealed key molecular mechanisms that
represent the basis of therapeutic synergy or antagonism (15). For instance, previous studies have
indicated that chemotherapy promoted tumor cells to be more
susceptible to the cytotoxic effect of CTLs through a dramatic
perforin-independent increase in permeability to GrzB released by
the CTLs and this effect is mediated via upregulation of
mannose-6-phosphate receptors on the surface of tumor cells
(16,17). Furthermore, some drugs of lower
concentrations are able to upregulate the ability of dendritic
cells (DCs) to present antigens to antigen-specific T cells and the
stimulation of DC function has been associated with the
upregulation of expression of antigen-processing machinery
components and costimulatory molecules on DCs, which was
interleukin (IL)-12-dependent and mediated by the autocrine or
paracrine mechanisms (18). The
present study aimed to investigate the effect of combined treatment
with AML-specific CTLs and cytarabine (also known as Ara-C) on AML
cell apoptosis. AML-specific CTLs exhibited the ability to
recognize and kill tumor cells, which was enhanced through the
addition of cytarabine. Furthermore, the results of the present
study indicated that the cytotoxic effect of this combined
treatment was attributable to the suppression of B-cell lymphoma 2
(Bcl-2) expression.
Materials and methods
Cell lines and reagents
The human AML cell line Kasumi-3 [cluster of
differentiation (CD) 33+] was purchased from the
American Type Culture Collection (Manassas, VA, USA). The Kasumi-3
cells were cultured in RPMI-1640 medium supplemented with 20% fetal
bovine serum (FBS) (both Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) in an incubator at 37°C with 5% CO2.
Monocyte-depleted peripheral blood lymphocytes (PBLs) were cultured
in immune cell-specialized culture medium (GT-T551 medium (TAKARA
Biotechnology Co., Ltd., Dalian, China) in an incubator at 37°C
with 5% CO2. Ficoll lymphocyte separation liquid was
purchased from GE Healthcare Life Sciences (Shanghai, China).
Antibodies for flow cytometry analysis were purchased from BD
Biosciences (Franklin Lakes, NJ, USA).
AML-CTL culture and
characterization
A total of 3 males (age, 22–56 years old) and 2
females (age, 30–48 years old) healthy donors were recruited. The
healthy volunteers gave their written informed consent and donated
their blood for the present study. PBLs from healthy donors were
separated and collected using density gradient centrifugation,
according to the instruction book of the Ficoll (19), in the month of May 2015 at the
Hematology Department, Cheng Du Military General Hospital of PLA
(Sichuan, China). A total of 2×105 Kasumi-3 cells were
treated with mitomycin C (Genia Biology, Beijing, China) for 30 min
at 37 °C and then co-cultured with the 1×105 PBLs for 4
days. Half of the medium was discarded, and the PBLs were collected
and re-added into a fresh culture with 2×105 Kasumi-3
cells for continuous stimulation. The culture medium was replaced
with immune cell-specialized medium on day 3. A total of 10 U/ml
human recombinant IL-2 (BioDee, Beijing, China) was added into the
medium on day 3. Every 3 days, half of the medium was changed with
fresh immune cell-specialized culture medium (GT-T551 medium) and
the IL-2 concentration was kept at 10 U/ml. The stimulation
procedure was repeated every week for 3 weeks. In this system, the
T cells that could recognize the cancer cells survived and
proliferated, and the T cells that could not gradually underwent
apoptosis. Immune phenotype characterization of the resulting
AML-CTLs was performed using fluorescein-conjugated antibodies
directed against human CD3 and CD8 (cat. no. 558261 and 560662; BD
Biosciences, Franklin Lakes, NJ, USA) with a flow cytometer,
according to the manufacturer's instructions and our previous study
(20).
CTL cytotoxicity assay
Target cells (Kasumi-3 cells; 1×106) were
co-cultured with 2.5, 5.0, 10.0 and 20.0 CTLs concentrations of
effector:target (E:T) cell ratio at cell densities of
3.5×106, 6.0×106, 11.0×106 and
21.0×106, respectively, per 6-well plate for 4 h at
37°C. Subsequently, MTT assays were performed to evaluate cells
viability. MTT solution (20 µl; 5 mg/ml) was added to the wells and
the plates were incubated for 1.5 h. The supernatant was carefully
discarded and the plates were washed with PBS 3 times.
Dimethylsulfoxide (150 µl) was added to each well and the plates
were put on shaker for 20 min until the formazan crystals dissolved
completely. The absorbance of the wells at 570 nm was then measured
using a microplate reader to determine cell viability.
Cell apoptosis assay
Target cells (1×106) were treated with
200 nM cytarabine, 20×106 CTLs, or cytarabine and CTLs
for 4 h at 37°C. Untreated cells were sued as the control group. A
total of 1×105/100 µl target cells were collected. The
cells were washed with PBS and then resuspended with 200 µl PBS for
flow cytometry apoptosis analysis. An Annexin V-FITC Apoptosis
assay kit (BD Pharmingen, San Diego, CA, USA) was used to detect
the apoptosis rate of target cells according to the manufacturer's
protocol. The total number of cells to be harvested was set at
1×105 and the speed of collection was set at 200–300
cells/sec. FlowJo software (version 7.6; Tree Star, Inc., Ashland,
OR, USA) was used for data analysis.
Western blotting
The cells were harvested after 4 h treatment with
200 nM cytarabine, 2×107CTLs, or cytarabine and CTLs at
37°C. Untreated cells were sued as the control group. RIPA buffer
was used to lyze the cells, and then all products were transferred
into Eppendorf tubes for centrifugation at 4°C for 15 min at
100,000 × g. The supernatant was collected and cell lysates
concentration was detected using the Bradford method (21). Sample concentration was modulated to
20 µg/20 µl and the same concentration β-actin protein was used as
an internal reference. The samples were added into 2X SDS buffer
solutions and boiled at 90°C for 5 min. Then, 20 µl samples were
separated using 20% SDS-PAGE, followed by electrotransfer onto
polyvinylidene difluoride membranes. The membranes were washed with
TBS for 5 min and blocked with skimmed milk for 1 h at room
temperature. After 3 washes with TBS/T, the membranes were
incubated with the following primary antibodies: human Bcl-2
monoclonal antibody (1:1,000 dilution; cat. no. ab694; Abcam,
Cambridge, UK) at 4°C overnight and then incubated with
peroxidase-conjugated Immunoglobulin G antibodies (1:400 dilution;
cat no. 330; Medical & Biological Laboratories Co., Ltd.,
Nagoya, Japan) for 1 h at 37°C. The membranes were washed 3 times
with TBS/T and 1 time with TBS. Protein bands were visualized using
enhanced chemiluminescence and imaged with a Gel Doc™ system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the means ± standard
deviation. The statistical significance of differences between
groups was compared with one-way analysis of variance. SPSS
software (version 16.0; SPSS, Inc., Chicago, IL, USA) was used for
all statistical analyses. P<0.05 was considered indicate a
statistically significant difference.
Results
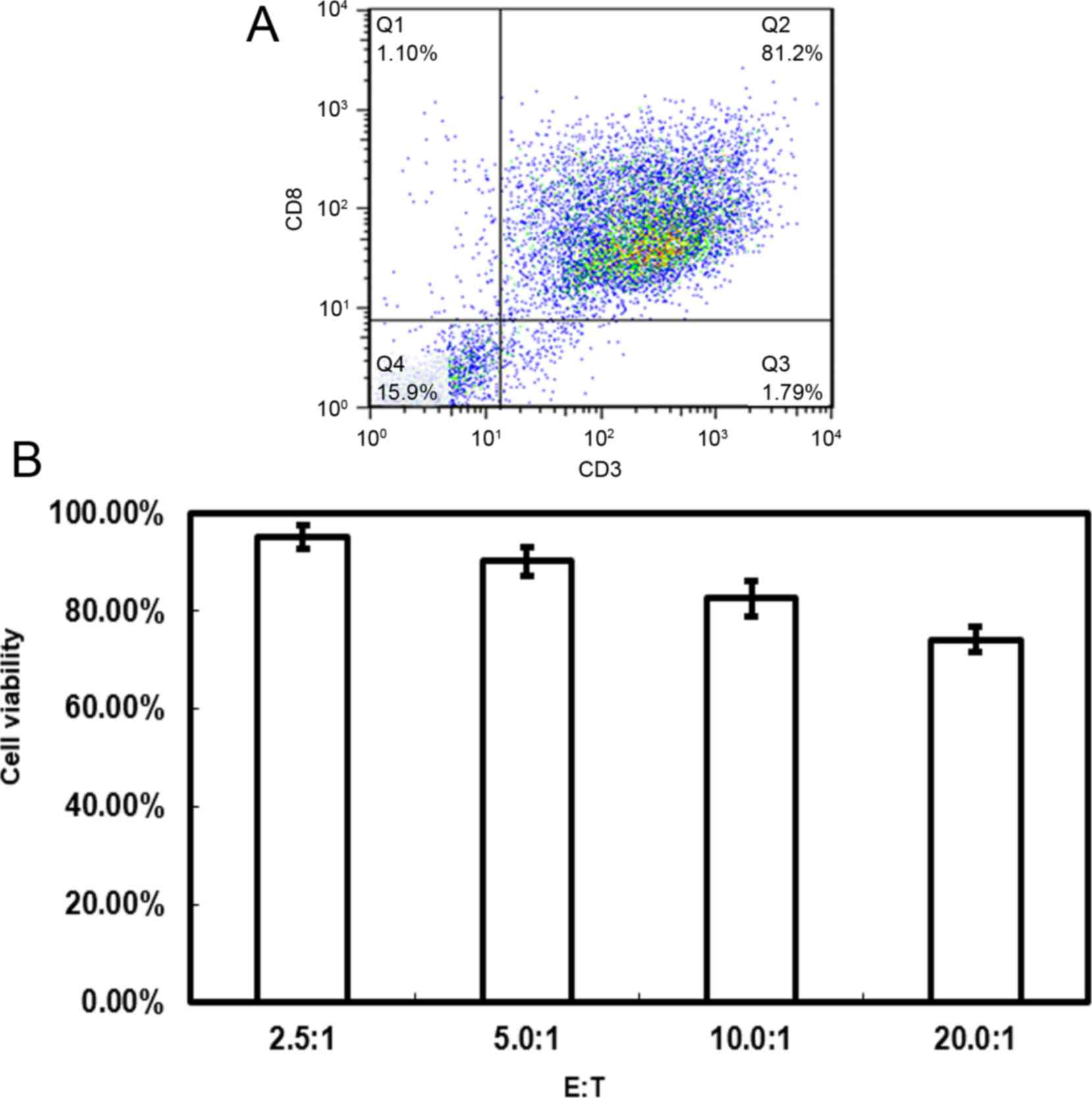
Cytotoxicity of the generated
AML-specific CTLs
CTLs are an important tool in cancer immunotherapy,
and the present study aimed to generate AML-specific CTLs in
vitro through immune cell stimulation. The results revealed
that >80% of the cells generated from the PBL samples were
CD3+CD8+, which are considered AML-specific
CTLs (Fig. 1A). The
CD3+CD8+ T cells generated displayed
cytotoxicity towards Kasumi-3 AML cells. MTT assays demonstrated
that the target cell viability rate differed with varying E:T
ratios (Fig. 1B). The cell viability
rate was 95.2±2.43%, 90.2±3.02%, 82.6±3.67% and 74.2±2.53% for E:T
ratios of 2.5:1, 5.0:1, 10.0:1 and 20.0:1, respectively.
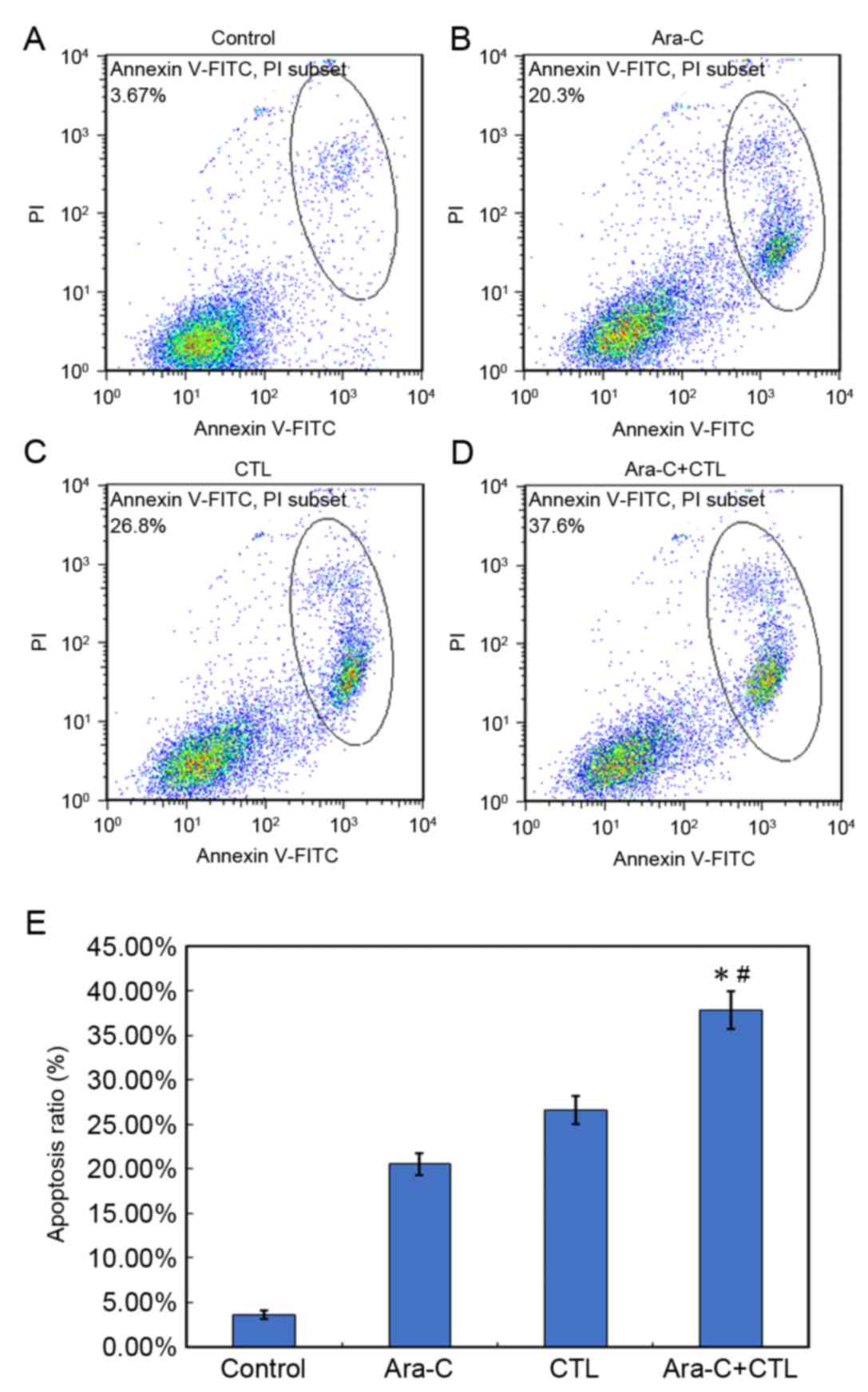
Cytarabine-induced AML cell apoptosis
is enhanced by CTL treatment
Cytarabine is an effective agent for the treatment
of AML, so the effect of combining CTL immunotherapy with
cytarabine on AML cell apoptosis was investigated (Fig. 2). After a 4 h treatment with 200 nM
cytarabine, >20% of Kasumi-3 AML cells underwent apoptosis.
Kasumi-3 cells treated with CTLs at a ratio of 20:1 underwent
apoptosis at a rate of 26.8±1.25%. Notably, the combination of
cytarabine and CTLs causes an AML cell apoptosis rate of
37.6±1.42%. Compared with either monotreatment, cytarabine and CTL
combined treatment significantly increased AML cell apoptosis
(P<0.05; Fig. 2E).
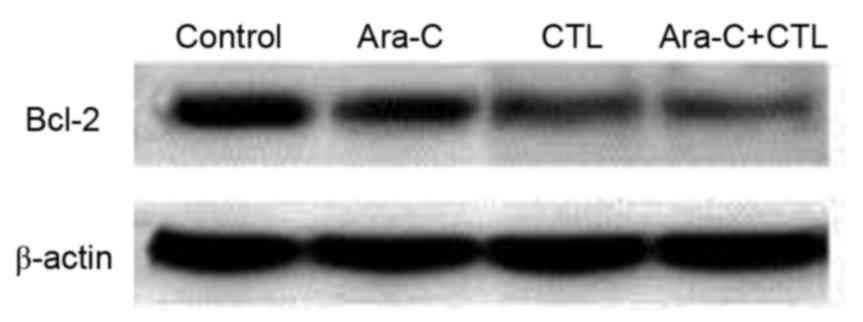
Cytarabine and CTL combined treatment
downregulates Bcl-2 expression in AML cells
The underlying molecular mechanisms of the
synergistic effect of cytarabine and CTL combined treatment on AML
cell apoptosis were investigated. Bcl-2 is an important protein in
apoptosis; therefore, Bcl-2 expression in the AML cells was
measured after cytarabine and/or CTL treatment (Fig. 3). Compared with the untreated control
group, Bcl-2 expression was inhibited in the treatment groups to
varying degrees. The most notable downregulation of Bcl-2 occurred
in the cytarabine and CTL combined treatment group, which indicates
that Bcl-2 downregulation mediates the synergistic effect of this
treatment in promoting AML cell apoptosis.
Discussion
The interactions between immune cells and
chemotherapeutics have been researched in depth. Previous reports
have demonstrated that docetaxel can modulate CD4+,
CD8+, CD19+, NK cell and T-regulatory (Treg)
cell populations in non-tumor bearing mice, and enhance
interferon-γ production by CD8+ T cells (22). In addition, the combination of
chemotherapy and radiation for the treatment of human head and neck
squamous cell carcinoma was identified to augment CTL-mediated cell
lysis (23). Another report revealed
that the exposure of lung tumor cells to cisplatin and vinorelbine
increased sensitivity to CTL-mediated cytolysis (24). Cisplatin may induce sub-myeloablative
leucopenia through modulating reconstitution of Treg vs. effector
T-cell subsets, and can be employed in combination with
immunotherapy to exploit the homeostatic peripheral expansion of T
cells (24). The enhanced antitumor
immune responses triggered by chemotherapeutics cause what is known
as immunogenic cell death. These results suggest that the
combination of immunotherapy with chemotherapy may improve the
clinical outcomes of cancer treatment. Indeed, small numbers of
CTLs can mediate potent antitumor effects when combined with
chemotherapy (17). The combination
of CD40 ligation immunotherapy with gemcitabine has been
demonstrated to induce solid tumor reduction and reverse drug
resistance (25). The activation of
CD40 on cervical carcinoma cells can facilitate CTL responses and
enhance chemotherapy-induced apoptosis (26). In addition, combining immunotherapy
and targeted therapies could improve clinical outcomes via cross
modulation (27).
Various mechanisms may explain the synergistic
effect of immunotherapy and chemotherapy. The direct effects of
chemotherapy on the tumor or tumor microenvironment, including
induction of tumor cell death, elimination of regulatory T cells
and enhancement of tumor cell sensitivity to lysis by CTLs may
account for enhancement of immunotherapy by chemotherapy.
Furthermore, the lymphopenia caused by chemotherapy has been
identified to increase the efficacy of adoptive lymphocyte infusion
in patients with cancer (28).
Immunotherapy may also directly regulate tumor chemosensitivity.
The present study investigated the effects of combined CTL and
cytarabine treatment on AML cells. The results revealed that,
compared with either treatment alone, the combination of CTLs with
cytarabine significantly increased AML cell apoptosis. Western blot
results also indicated that the inhibition of AML cell Bcl-2
expression may be the mechanism underlying to this effect.
There are several limitations to CTLs treatment,
including shortcomings in specificity and cytotoxic competence. The
specific targeting of immunotherapy could improve these issues; for
example, chimeric antigen receptor-modified T cell (CAR-T)
treatment. CART-123 cells, which target the CD123 antigen on tumor
cells, have achieved favorable results whereby CD123 CAR T cells
exhibited antileukemic activity in vivo against a xenogeneic
model of AML and significantly prolonged the models' survival
(29). CAR-T treatments are able to
reduce tumor burden prior to the application of alternative
intensive strategies, including HSCT (30). However, due to the potent cytotoxic
effects of CAR-T, serious side effects are currently associated
with treatment. Whether CAR-T therapy will improve upon CTL
immunotherapy remains unclear and further studies into these
treatments are warranted.
In conclusion, the results of the present study
demonstrated that the combination of CTL and cytarabine treatment
significantly increased AML cell apoptosis, and indicated that this
synergistic effect is due to the downregulation of Bcl-2.
Optimizing the specificity and potency of CTLs, and identifying
favorable combinations with other chemotherapeutic drug remain
important tasks for future work.
Acknowledgements
The present study was supported by Cheng Du Military
General Hospital Management Research Foundation (grant no.
2013YG-B045).
References
|
1
|
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC,
Bowman WP, Taub J, Pounds S, Razzouk BI, Lacayo NJ, Cao X, et al:
Minimal residual disease-directed therapy for childhood acute
myeloid leukaemia: Results of the AML02 multicentre trial. Lancet
Oncol. 11:543–552. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niewerth D, Creutzig U, Bierings MB and
Kaspers GJ: A review on allogeneic stem cell transplantation for
newly diagnosed pediatric acute myeloid leukemia. Blood.
116:2205–2214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Couzin-Frankel J: Breakthrough of the year
2013. Cancer immunotherapy. Science. 342:1432–1433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greiner J, Schmitt M, Li L, Giannopoulos
K, Bosch K, Schmitt A, Dohner K, Schlenk RF, Pollack JR, Dohner H
and Bullinger L: Expression of tumor-associated antigens in acute
myeloid leukemia: Implications for specific immunotherapeutic
approaches. Blood. 108:4109–4117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castella B, Vitale C, Coscia M and Massaia
M: Vγ9Vδ2 T cell-based immunotherapy in hematological malignancies:
From bench to bedside. Cell Mol Life Sci. 68:2419–2432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Driessche A, Berneman ZN and Van
Tendeloo VF: Active specific immunotherapy targeting the wilms'
tumor protein 1 (WT1) for patients with hematological malignancies
and solid tumors: Lessons from early clinical trials. Oncologist.
17:250–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brossart P, Schneider A, Dill P, Schammann
T, Grünebach F, Wirths S, Kanz L, Bühring HJ and Brugger W: The
epithelial tumor antigen MUC1 is expressed in hematological
malignancies and is recognized by MUC1-specific cytotoxic
T-lymphocytes. Cancer Res. 61:6846–6850. 2001.PubMed/NCBI
|
|
8
|
Theobald M, Biggs J, Hernández J,
Lustgarten J, Labadie C and Sherman LA: Tolerance to p53 by A2.
1-restricted cytotoxic T lymphocytes. J Exp Med. 185:833–841. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burstein HJ and Schwartz RS: Molecular
origins of cancer. N Engl J Med. 358:5272008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berger C, Turtle CJ, Jensen MC and Riddell
SR: Adoptive transfer of virus-specific and tumor-specific T cell
immunity. Curr Opin Immunol. 21:224–232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brimnes MK, Gang AO, Donia M, Straten P
Thor, Svane IM and Hadrup SR: Generation of autologous
tumor-specific T cells for adoptive transfer based on vaccination,
in vitro restimulation and CD3/CD28 dynabead-induced T cell
expansion. Cancer Immunol Immunother. 61:1221–1231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirohashi Y, Torigoe T, Inoda S, Morita R,
Kochin V and Sato N: Cytotoxic T lymphocytes: Sniping cancer stem
cells. Oncoimmunology. 1:123–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Falkenburg JF, Wafelman AR, Joosten P,
Smit WM, van Bergen CA, Bongaerts R, Lurvink E, van der Hoorn M,
Kluck P, Landegent JE, et al: Complete remission of accelerated
phase chronic myeloid leukemia by treatment with leukemia-reactive
cytotoxic T lymphocytes. Blood. 94:1201–1208. 1999.PubMed/NCBI
|
|
14
|
Yee C, Thompson J, Byrd D, Riddell SR,
Roche P, Celis E and Greenberg PD: Adoptive T cell therapy using
antigen-specific CD8+ T cell clones for the treatment of patients
with metastatic melanoma: In vivo persistence, migration, and
antitumor effect of transferred T cells. Proc Natl Acad Sci USA.
99:pp. 16168–16173. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bracci L, Schiavoni G, Sistigu A and
Belardelli F: Immune-based mechanisms of cytotoxic chemotherapy:
Implications for the design of novel and rationale-based combined
treatments against cancer. Cell Death Differ. 21:15–25. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ramakrishnan R, Huang C, Cho HI, Lloyd M,
Johnson J, Ren X, Altiok S, Sullivan D, Weber J, Celis E and
Gabrilovich DI: Autophagy induced by conventional chemotherapy
mediates tumor cell sensitivity to immunotherapy. Cancer Res.
72:5483–5493. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ramakrishnan R, Assudani D, Nagaraj S,
Hunter T, Cho HI, Antonia S, Altiok S, Celis E and Gabrilovich DI:
Chemotherapy enhances tumor cell susceptibility to CTL-mediated
killing during cancer immunotherapy in mice. J Clin Invest.
120:1111–1124. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shurin GV, Tourkova IL, Kaneno R and
Shurin MR: Chemotherapeutic agents in noncytotoxic concentrations
increase antigen presentation by dendritic cells via an
IL-12-dependent mechanism. J Immunol. 183:137–144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
English D and Andersen BR: Single-step
separation of red blood cells. Granulocytes and mononuclear
leukocytes on discontinuous density gradients of Ficoll-Hypaque. J
Immunol Methods. 5:249–252. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deng R, Wang SM, Yin T, Ye TH, Shen GB, Li
L, Zhao JY, Sang YX, Duan XG and Wei YQ: Inhibition of tumor growth
and alteration of associated macrophage cell type by an HO-1
inhibitor in breast carcinoma-bearing mice. Oncol Res. 20:473–482.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kruger NJ: The Bradford Method for Protein
Quantitation. Methods Mol Biol. 32:9–15. 1994.PubMed/NCBI
|
|
22
|
Garnett CT, Schlom J and Hodge JW:
Combination of docetaxel and recombinant vaccine enhances T-cell
responses and antitumor activity: Effects of docetaxel on immune
enhancement. Clin Cancer Res. 14:3536–3544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gelbard A, Garnett CT, Abrams SI, Patel V,
Gutkind JS, Palena C, Tsang KY, Schlom J and Hodge JW: Combination
chemotherapy and radiation of human squamous cell carcinoma of the
head and neck augments CTL-mediated lysis. Clin Cancer Res.
12:1897–1905. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gameiro SR, Caballero JA, Higgins JP,
Apelian D and Hodge JW: Exploitation of differential homeostatic
proliferation of T-cell subsets following chemotherapy to enhance
the efficacy of vaccine-mediated antitumor responses. Cancer
Immunol Immunother. 60:1227–1242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nowak AK, Robinson BW and Lake RA: Synergy
between chemotherapy and immunotherapy in the treatment of
established murine solid tumors. Cancer Res. 63:4490–4496.
2003.PubMed/NCBI
|
|
26
|
Hill SC, Youde SJ, Man S, Teale GR,
Baxendale AJ, Hislop A, Davies CC, Luesley DM, Blom AM, Rickinson
AB, et al: Activation of CD40 in cervical carcinoma cells
facilitates CTL responses and augments chemotherapy-induced
apoptosis. J Immunol. 174:41–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vanneman M and Dranoff G: Combining
immunotherapy and targeted therapies in cancer treatment. Nat Rev
Cancer. 12:237–251. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang T and Herlyn D: Combination of
active specific immunotherapy or adoptive antibody or lymphocyte
immunotherapy with chemotherapy in the treatment of cancer. Cancer
Immunol Immunother. 58:475–492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mardiros A, Dos Santos C, McDonald T,
Brown CE, Wang X, Budde LE, Hoffman L, Aguilar B, Chang WC,
Bretzlaff W, et al: T cells expressing CD123-specific chimeric
antigen receptors exhibit specific cytolytic effector functions and
antitumor effects against human acute myeloid leukemia. Blood.
122:3138–3148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo
B, Wang LL and Han WD: Treatment of CD33-directed chimeric antigen
receptor-modified T cells in one patient with relapsed and
refractory acute myeloid leukemia. Mol Ther. 23:184–191. 2015.
View Article : Google Scholar : PubMed/NCBI
|