Introduction
Stroke is a major cause of mortality and permanent
disability in adults worldwide, particularly in low- and
middle-income countries (1,2). Approximately 87% of stroke cases are
due to ischemia (3). In the acute
stages of the disease, neurons in the ischemic lesion rapidly die
and other neuronal populations in the ischemic penumbra are
vulnerable to secondary injury (4).
Despite improvements in interventional techniques and
pharmacological agents, including thrombolytic therapy, only a
minority of stroke patients receive thrombolytic agents due to
their side effects and narrow time window of administration
(5). It is therefore critical to
develop safe and effective treatments with a longer treatment time
window.
Electroacupuncture (EA) was reported to have
beneficial effects in stroke patients (6,7), and the
Quchi (LI11) and Zusanli (ST36) acupoints are the most commonly
used acupoints to treat stroke in the clinic in China. Preliminary
studies by our group have demonstrated that EA on these two
acupoints had a significant neuroprotective effect via the
stimulation of cerebral cell proliferation (8–10).
Neuroprotection and neural regeneration are two major therapeutic
strategies to treat ischemic stroke (4). Previous studies have demonstrated that
axonal regeneration has a central role in neural plasticity and may
offer novel treatment approaches for stroke (11,12).
However, whether EA mediates axonal regeneration of injured neurons
in rats and the possible underlying mechanism remains to be
elucidated.
Nogo-A is a well-known myelin-associated axonal
growth inhibitory protein, which has been shown to inhibit
migration and spreading of nerve cells and has important roles in
blocking axonal regeneration and reconnection following stroke
(13,14). Nogo-A inhibits axonal outgrowth by
binding to Nogo receptor (NgR) via its functional component to
activate RhoA and its effector Rho-associated protein kinase
(ROCK), leading to axonal repulsion and growth cone collapse
(15). Studies have indicated that
anti-Nogo-A therapy improves neurological deficits and enhances
neuronal plasticity, suggesting that therapeutics targeting Nogo-A
hold promise for regenerative treatment following stroke (16,17).
Therefore, the purpose of the present study was to test the
hypothesis that the downregulation of Nogo-A contributes to the
axonal regeneration effects of EA at the Zusanli and Quchi
acupoints, evaluate the therapeutic efficacy of EA against ischemic
stroke and investigate whether its effect is associated with
downregulation of the Nogo-A signaling pathway.
Materials and methods
Materials and reagents
TRIzol reagent was obtained from Invitrogen (Thermo
Fisher Scientific, Waltham, MA, USA). Nogo-A (no. ab62024), NgR
(no. ab26291) and RhoA (no. ab54835) antibodies were obtained from
Abcam (Cambridge, MA, USA). ROCK, growth-associated protein 43
(GAP-43, no. 5307), β-actin (no. 3700) antibodies and anti-rabbit
immunoglobulin (Ig) G (H+L), biotinylated secondary antibodies (no.
14708) were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). Mouse proliferating cell nuclear antigen
immunohistochemical (IHC) kits were purchased from Beijing Golden
Bridge Biotechnology Co., Ltd. (Beijing, China). All other reagents
used, unless otherwise stated, were obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany).
Animals
The experimental animal protocol of the present
study was approved by the Ethics Committee of Fujian University of
Traditional Chinese Medicine (Fuzhou, China; no. 2014028). All
experiments were performed in strict accordance with the Guide for
the Care and Use of Laboratory Animals published by the US.
National Institutes of Health (18).
A total of 108 male Sprague Dawley rats (weighing 220–250 g and
aged 2.21±0.15 months) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China) and kept in a 23±2°C
temperature- and 50±5% humidity-controlled room under a 12-h
light/dark cycle. Rats had ad libitum access to food and
water.
Establishment of the focal cerebral
ischemia rat model and animal grouping
Rats (n=108) were randomly divided into three groups
prior to middle cerebral artery occlusion (MCAO) surgery: The sham
operation control group (SC; n=36), the ischemia control group (IC;
n=36) and the EA group (EA; n=36). To establish the focal cerebral
ischemia model, rats were subjected to MCAO, as described
previously by Longa et al (19). Following fasting for 24 h with access
to water, rats were anesthetized by intraperitoneal injection with
10% chloral hydrate (300 mg/kg, Beijing Golden Bridge Biotechnology
Co., Ltd.). Throughout the surgical procedures, the rectal
temperature of the rats was maintained at 37°C. In brief, a midline
cervical incision was performed to expose and isolate the left
common carotid artery (CCA), the left external carotid artery (ECA)
and the internal carotid artery (ICA). An embolus was advanced
through the ICA to the MCA (20±2 mm) until a mild resistance was
encountered, thereby occluding the origin of the MCA. Subsequently,
the cervical incision was closed with a silk suture. Rats in the
sham group were treated similarly, however no ligations or
occlusions were performed. Animals in each group were randomly
assigned to three subgroups to be sacrificed at days 3, 7 or 14
after surgery.
EA stimulation
In the EA group, EA treatment was performed at the
Zusanli (ST36) and Quchi (LI11) acupoints on the right paralyzed
limb using an EA stimulator instrument (Model G6805; Shanghai Huayi
Co., Shanghai, China) following recovery from MCAO surgery. Two
30-gauge (0.3 mm diameter) stainless acupuncture needles were
inserted at a depth of 2–3 mm into the aforementioned acupuncture
points. The stimulation parameters were a dense disperse wave of 1
and 20 Hz (adjusted to the muscle twitch threshold), 30 min each
time, once a day. Treatment was started the day after the operation
and continued until the animals were sacrificed.
Neurological assessment
Neurological deficits were evaluated to confirm
successful establishment of the rat model or cerebral ischemia
injury. The severity of neurological deficits in the model animals
was assessed at 2 and 24 h after surgery according to the
four-point scoring system developed by Longa et al (19) and Bederson et al (20): 0, no apparent deficits; 1, failure to
fully extend the right forepaw; 2, circling to the right; 3,
falling to the right when walking; 4, loss of ability to walk. MCAO
rats with neurological deficit scores of 1–3 were used for the
experiments. Neurological deficits in each group of rats were
re-evaluated at each time-point (day 3, 7 and 14 after surgery)
prior to sacrifice.
Evaluation of infarct volumes
Following cerebral ischemia injury for 72 h, the
rats were sacrificed under deep anesthesia using 10% chloral
hydrate (300 mg/kg) and transcardially perfused with 0.9% NaCl. The
brains of all rats were frozen at −80°C in a freezer for 20 min and
were cut into five coronal slices at a thickness of 2 mm/section.
The fresh slices were placed in a 2% solution of
2,3,5-triphenyl-tetrazolium chloride (TTC) in phosphate-buffered
saline (PBS; Hyclone; GE Healthcare, Logan, UT, USA) at 37°C for 20
min. The normal area of the brain was stained dark red based on
intact mitochondrial function, whereas the infarct area remained
unstained. Images of the stained slices were captured using a
high-resolution digital camera (Canon SX20; Canon, Inc., Tokyo,
Japan). A computerized image analysis system (Motic Med 6.0 System;
Motic, Hong Kong, China) was used for quantification of the
percentage of the total infarcted brain volume.
Immunohistochemistry (IHC)
The rats in each group (n=6) were humanely
sacrificed at 3, 7 and 14 days after MCAO surgery. The tissue was
subjected to routine paraffin embedding, sectioning at 5 µm
thickness and antigen retrieval, followed by rinsing with 0.01 M
PBS. Nogo-A, ROCK and GAP-43 levels were analyzed using an IHC
assay kit (DAB kit-0017; MaixinBiotech, Co. Ltd., Fujian, China)
according to the manufacturer's instructions. To block non-specific
protein activity, the sections were incubated in 3% hydrogen
peroxide and 10% normal goat serum (DAB kit-0017; MaixinBiotech,
Co. Ltd., Fujian, China) for 10 min at 37°C. Subsequently, the
sections were incubated overnight at 4°C with primary antibodies
against Nogo-A (1:100 dilution), ROCK (1:100 dilution) and GAP-43
(1:200 dilution), followed by incubation with secondary
biotinylated rabbit anti-mouse antibodies (1:100 dilution; DAB
kit-0017; MaixinBiotech, Co. Ltd., Fujian, China). Following
incubation with diaminobenzidine for 1 min, sections were washed
with distilled water and dehydrated in a graded series of alcohol,
cleared in xylene and mounted using xylene-based mounting medium.
Images were captured on a microscope (Leica Microsystems, Wetzlar,
Germany) and cells with positive staining were counted in four
randomly selected microscopic fields at ×400 magnification. The
positive expression rate was determined as the ratio of cells with
brown staining and Image-Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, US) was used to count the number of positive
cells.
Western blot analysis
The rats were sacrificed to obtain the left
peri-infarct cerebral cortex from each group at each time-point (3,
7 and 14 days post-surgery; n=6 per group). Tissues were
homogenized in RIPA Lysis Buffer (No. P0013, Beyotime Institute of
Biotechnology, Haimen, China) and centrifuged at 12,000 × g
for 15 min at 4°C. The supernatants were collected and frozen at
−80°C prior to immunoblotting. The protein concentration of each
homogenate was determined using a Bicinchoninic Acid Protein assay
kit (No. CW0014, Beijing Comwin Biotech Co., Ltd., Beijing, China).
A total of 50 µg protein per lane was separated by 10% SDS-PAGE.
Subsequently, blots were transferred onto polyvinylidene difluoride
membranes (Shanghai Bioscience Co., Shanghai, China) in a
Tris-glycine transfer buffer and were blocked for 2 h with 5%
non-fat dry milk at room temperature. Membranes were incubated with
primary antibodies against Nogo-A (1:1,000 dilution), NgR (1:10,00
dilution), RhoA (1:1,000 dilution), ROCK (1:1,000 dilution), GAP-43
(1:5,000 dilution) and β-actin (1:5,000 dilution) overnight at 4°C,
followed by incubation with the anti-rabbit IgG (H+L), biotinylated
secondary antibodies (1:5,000 dilution) for 1 h at 37°C. The bands
were visualized with the ECL Pico Western Blotting Substrate kit
(Beijing Golden Bridge Biotechnology Co., Beijing, China), images
were captured using a Bio-Rad ChemiDoc XRS+ (Bio-Rad Laboratories,
Hercules, CA, USA) and densitometric quantification was performed
using Quantity OneV4.62 (Bio-Rad Laboratories). The ratio of gray
scale values of the target protein to the internal control was used
to measure the relative amount of Raldh1 and Raldh2.
RNA extraction and
reverse-transcription polymerase chain reaction (RT-PCR)
Rats were sacrificed to obtain the left peri-infarct
cerebral cortex from each group each time-point (3, 7 and 14 days;
n=6 per group). Total RNA was isolated with the TRIzol reagent
according to the manufacturer's instructions. Primed RNA (1 µg) was
reverse-transcribed with RevertAid™ First Strand cDNA Synthesis kit
(5X Mix included Buffer, dNTP, HiScript® Reverse Transcriptase and
RNase inhibitor; Fermentas; Thermo Fisher Scientific Inc.)
according to the manufacturer's instructions. The reaction
conditions for reverse-transcription were 50°C for 15 min, followed
by 85°C for 2 min. cDNA was then amplified by PCR to determine the
amount of Nogo-A, NgR, RhoA, ROCK and GAP-43 mRNA. β-actin was
selected as a housekeeping gene. Primers were purchased from
Shanghai BioSun Sci&Tech Co., Ltd. (Shanghai, China). Primer
sequences were as follows: Nogo-A forward,
5′-AGGGATGTGCTGGCTGCTAG-3′ and reverse, 5′-GGTGCTTTCGGTTGCTGAGG-3′;
NgR, forward, 5′-GGGCAACCTCACGCATCTCT-3′ and reverse,
5′-TCATGAGTCGGCCAAGGTCC-3′; RhoA forward,
5′-ACCAGTTCCCAGAGGTTTAT-3′ and reverse, 5′-TTTGGTCTTTGCTGAACACT-3′,
ROCK forward, 5′-GATCCCCTGCAAAGTTTATT-3′ and reverse,
5′-AGCTTTTCCAAAAATGCAAA-3′; GAP-43 forward,
5′-TGCTGTGCTGTATGAGAAGAACC-3′ and reverse,
5′-GGCAACGTGGAAAGCCGTTT3′; β-actin forward,
5′-ACTGGCATTGTGATGGACTC-3′ and reverse, 5′-CAGCACTGTGTTGGCATAGA-3′.
Following the addition of 2x AceTaq® Master Mix (no. P411, Vazyme
biotech Co., Ltd.), which includes Buffer, dNTP and AceTaq® DNA
Polymerase, a MasterCycler nexus PCR (No. 5331, Eppendorf Ltd.,
Hamburg, Germany) was used for the PCR reaction. The thermocycling
conditions for real-time PCR were 95°C for 5 min, followed by 35
cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 60 sec.
Following 35 cycles, elongation was performed at 72°C for 7 min.
PCR products were analyzed by gel electrophoresis (1.5% agarose)
and a Gel Documentation system (Model Gel Doc 2000; Bio-Rad
Laboratories, Inc.). Quantity One (Bio-Rad Laboratories) was used
for quantification.
Statistical analysis
SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA)
was used for statistical analysis. Values are expressed as the mean
± standard deviation. Differences between the two groups were
compared using the independent-samples Student's t-test. For
multiple comparisons of quantitative data, one-way analysis of
variance was used. If the data fulfilled the criteria of
homogeneity of variance, Fisher's least significant difference
method was applied; otherwise, Tamhane's method was applied.
P<0.05 was considered to indicate a statistically significant
difference.
Results
EA treatment improves neurological
deficits following cerebral ischemia injury
The effect of EA on neurological function following
cerebral ischemic injury was examined by neurological deficit
scoring. As presented in Table I,
there was no manifestation of neurological deficits observed in the
SC group at each time-point, however rats in the IC and EA groups
exhibited marked neurological deficits. The results indicated that
successful cerebral ischemic models were established and rats in
the SC group did not exhibit any indication of cerebral injury.
There were no significant differences between the neurological
deficit scores of the IC and EA groups at 2 and 24 h following
cerebral ischemic injury (P>0.05). However, the scores in the EA
group were significantly lower than those in the IC group at 3, 7
and 14 days (P<0.01 or P<0.05; Table I) and gradually decreased over time.
These results suggest that EA effectively improved the neurological
dysfunction caused by cerebral ischemia and promoted the functional
recovery of nerves.
 | Table I.Neurological deficit score of rats at
different time-points following injury. |
Table I.
Neurological deficit score of rats at
different time-points following injury.
| Group | 2 h | 24 h | 3 d | 7 d | 14 d |
|---|
| SC | 0 | 0 | 0 | 0 | 0 |
| IC | 2.25±0.62 | 2.17±0.39 | 2.08±0.52 | 1.92±0.52 | 1.83±0.58 |
| EA | 2.33±0.65 | 2.17±0.58 |
1.67±0.49a |
1.50±0.52a |
1.33±0.49b |
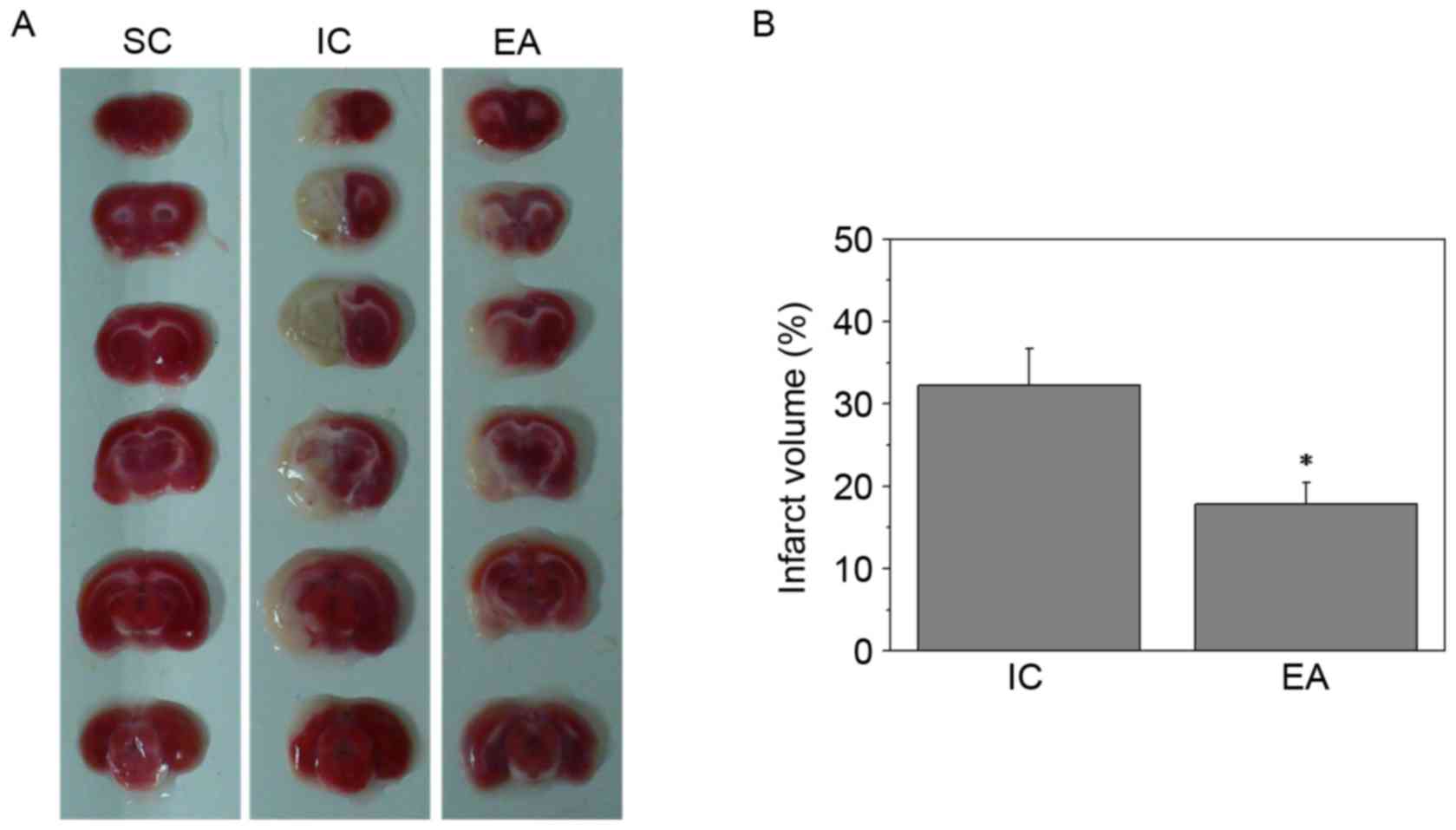
EA reduces cerebral infarct
volumes
To further investigate the therapeutic efficacy of
EA treatment against cerebral ischemia injury, cerebral infarct
volumes were evaluated using TTC staining. Sections of the SC group
were stained red, whereas the unstained infarct brain area was
visible on the left side in the IC and EA groups (Fig. 1A). Furthermore, EA treatment at the
Quchi and Zusanli acupoints significantly reduced cerebral infarct
volumes (P<0.05; Fig. 1B).
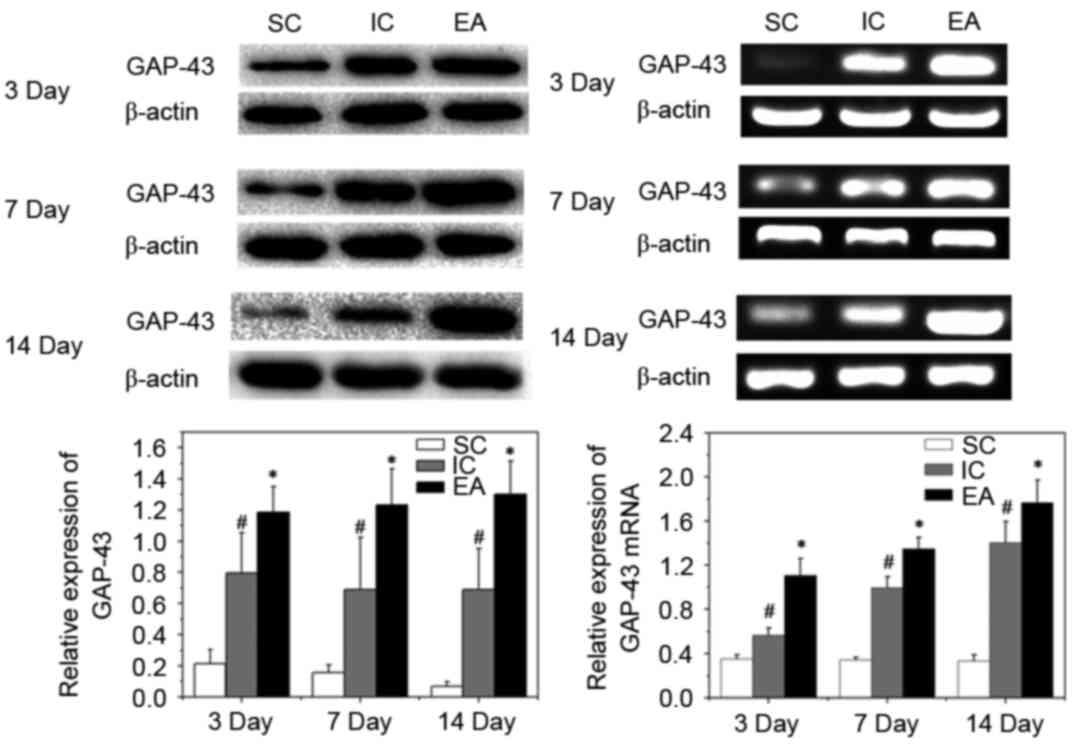
Impact of EA treatment on axonal
regeneration
Axonal regeneration can be identified by the
elevated expression of GAP-43. GAP-43 is a major protein of axonal
growth cones and has been implicated in the mechanism of axonal
regeneration (21). To identify
whether the expression of various axonal and myelin markers in the
peri-infarct cortex were affected by EA treatment, western
blotting, RT-qPCR and IHC analyses of GAP-43 in the peri-infarct
cortex were performed. Compared with the SC group, expression of
GAP-43 mRNA and protein increased following cerebral ischemia in
the IC and EA groups (P<0.05). Furthermore, EA treatment
significantly enhanced the expression of GAP-43 protein and mRNA on
days 3, 7 and 14 post-ischemia, compared with the IC group
(P<0.05; Fig. 2).
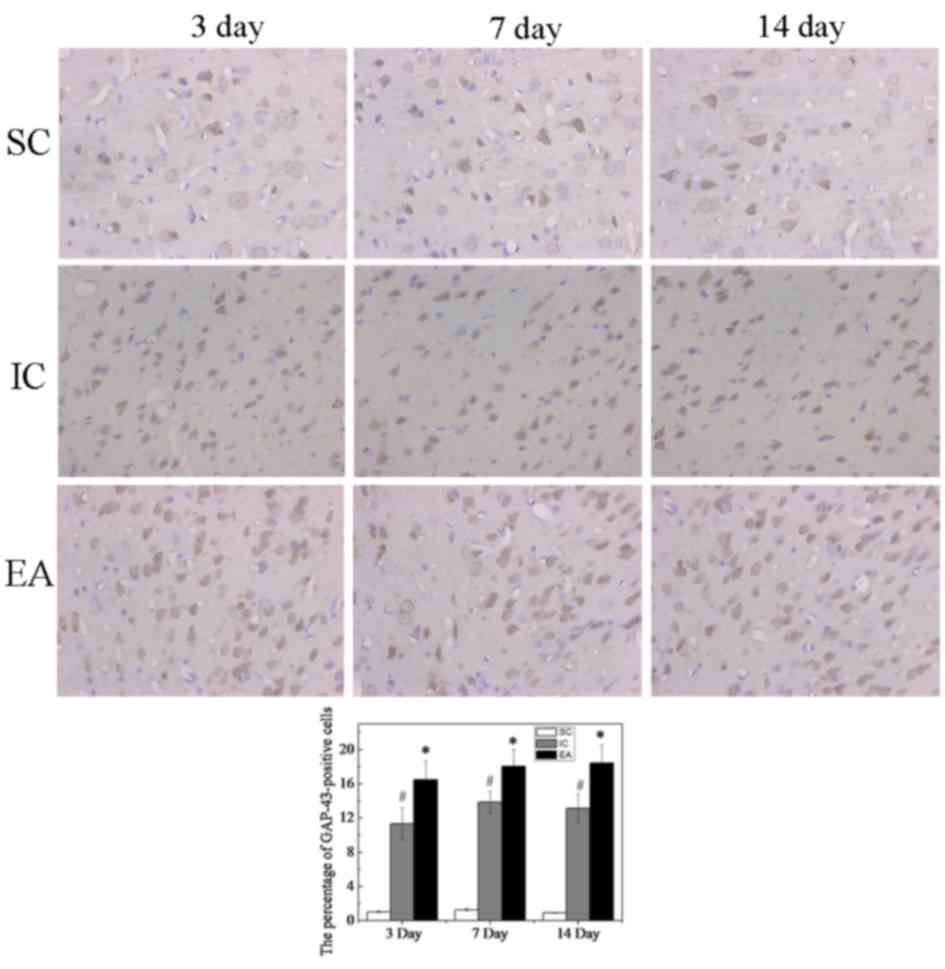
Immunohistochemical staining indicated that only few
GAP-43-positive cells were detected in the SC group, whereas the
number of GAP-43-positive cells was increased in the IC and EA
groups (Fig. 3). The percentage of
GAP-43-positive cells in the SC group at day 3 was 1.04±0.07%,
while those in the IC and EA groups were significantly higher
(P<0.05), at 11.32±1.85 and 16.51±2.17%, respectively. This was
also the case at days 7 and 14 (P<0.05). Furthermore, compared
with the IC group, the number of GAP-43-positive cells was
significantly increased in the EA group at days 3, 7 and 14
(P<0.05, Fig. 3).
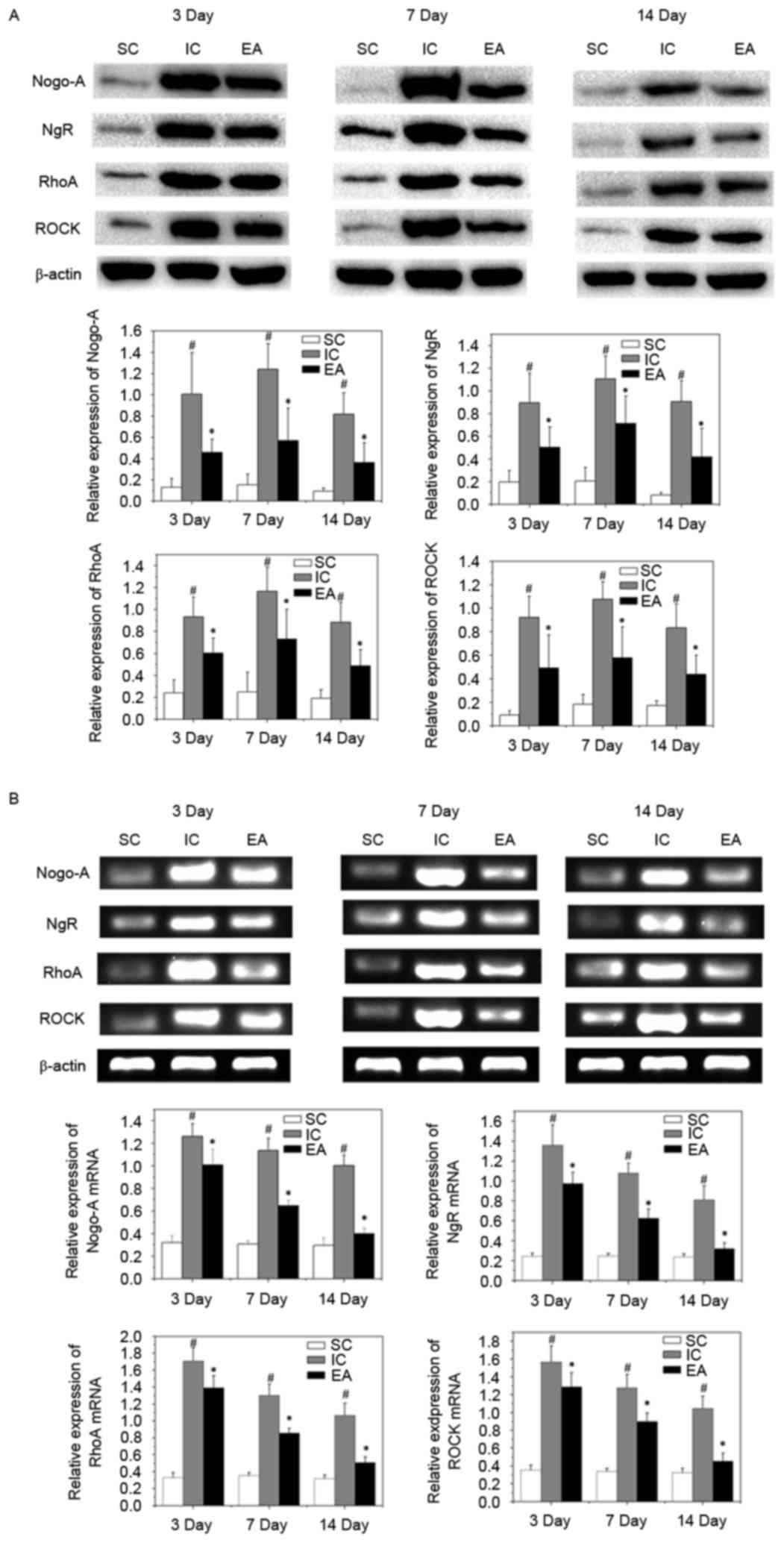
Effect of EA treatment on activation
of Nogo-A/NgR/RhoA/ROCK signaling
To investigate the mechanism of axonal regeneration
with EA treatment, the expression of the vital target genes of the
Nogo-A signaling pathway, Nogo-A, NgR, RhoA and ROCK, was
investigated. The expression of Nogo-A, NgR, RhoA and ROCK mRNA and
protein was significantly increased in the IC group compared with
the SC group at on days 3, 7 and 14 (all P<0.05). However,
expression of Nogo-A, NgR, RhoA and ROCK mRNA and protein were
significantly inhibited compared with the IC group, following EA
treatment (all P<0.05; Fig. 4).
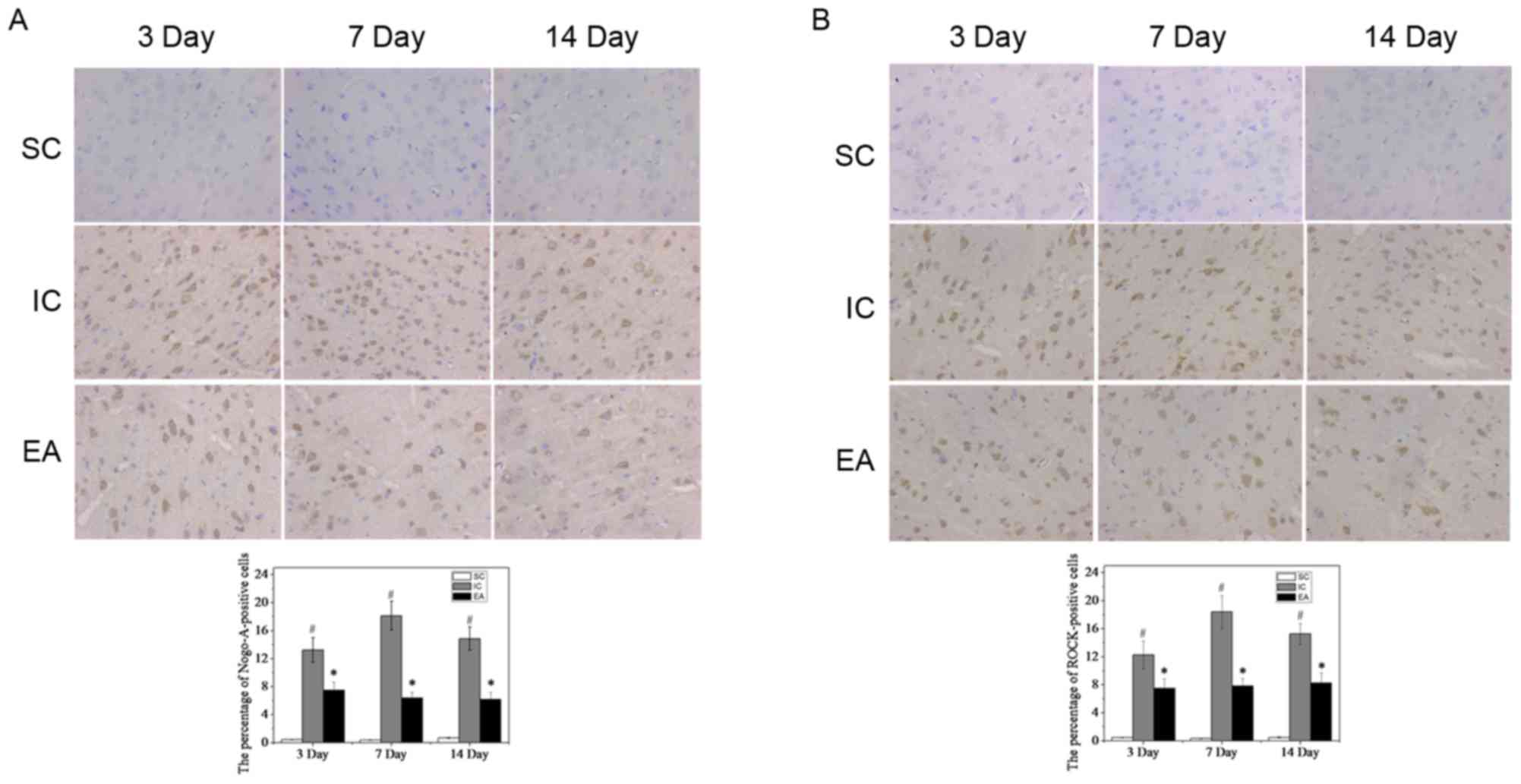
To confirm these results, Nogo-A and ROCK were detected in the
peri-infarct cortex tissue by immunohistochemistry. As expected,
few Nogo-A- and ROCK-positive cells were observed in the SC group
(Fig. 5). By contrast, in the IC
group, Nogo-A and ROCK expression significantly increased on day 3
(13.24±1.74 and 12.27±1.98%, respectively; P<0.05), peaked on
day 7 (18.13±2.06 and 18.41±2.31%, respectively; P<0.05), and
then decreased slightly but was still maintained at a high level on
day 14 (14.84±1.67 and 15.27±1.50%, respectively; P<0.05;
Fig. 5). However, compared with the
IC group, Nogo-A and ROCK expression significantly decreased in the
EA group (P<0.05; Fig. 5),
suggesting that EA treatment weakened the inhibition of axonal
growth following cerebral ischemia.
Discussion
Ischemic stroke has complex pathogenic mechanisms
and its consequences are devastating. Various studies have
identified that pathological alterations in neuronal cells within
the gray matter occur following stroke. However, the importance of
white matter integrity in long-term recovery from these conditions
has also been highlighted (22–24).
Brain white matter injury largely includes the injury of
myelin-ensheathed axons, which, due to limited capacity of the
adult brain for axonal regeneration, is strongly associated with
long-term deficits in neurological function following stroke
(25).
The molecular signaling in the brain following
cerebral infarction is complex and includes inhibitors that reduce
axonal regeneration and neurotrophic factors that promote synaptic
regeneration. Nogo-A, as the pivotal myelin-associated axonal
growth inhibitory protein, is a major impediment to axonal
regeneration following ischemic stroke (26). Previous studies have shown that
downregulation or inhibition of the Nogo-A signaling pathway
promoted axonal regeneration and functional recovery of stroke
(27–29). Nogo-A, via the NgR complex, activates
RhoA and ROCK, ultimately resulting in the inhibition of axonal
regeneration and growth cone collapse (15). Axonal regeneration is identified by
the elevated expression of GAP-43. GAP-43 is a major protein of
axonal growth cones and has been implicated in mechanisms of axonal
regeneration (21). The present
study demonstrated that EA treatment reduces the mitigating effect
of cerebral ischemia on axonal growth inhibitors by downregulating
the expression of Nogo-A/NgR/RhoA/ROCK signaling and upregulating
the expression of Gap-43.
EA treatment, engrafted electric stimulation, is
accepted as a complementary and alternative intervention for stroke
as well as in post-stroke rehabilitation (30). A number of studies have demonstrated
that EA treatment exerts a neuroprotective effect following
cerebral ischemia stroke (31–33). It
is thought that the neuroprotective mechanisms of EA include the
promotion of neural cell proliferation (34), enhancement of endogenous neurogenesis
(35), inhibition of neuronal
apoptosis (36) and reduction of the
inflammatory response (37).
In the present study, days 3, 7 and 14 after
cerebral ischemia were selected as the time-points of observation,
as they cover the key events of anti-apoptotic and neuroprotective
mechanisms following cerebral ischemia: Day 3 lies within the
trigger and onset period with minimal pathological changes and
minimal edema; at day 7, the progression of injury is maintained
with prominent fiber degeneration and endoneurial edema; and from
day 14, the period of regeneration is maintained with abundant
small regenerating fiber clusters and minimal edema. In the IC
group, significantly higher expression of the Nogo-A signaling
genes was observed on day 3 with a peak on day 7 and a slight
decrease on day 14, whereas this tendency was not observed in the
EA group. Possible explanations may include the time-dependent
treatment effects of EA stimulation as well as spontaneous
recovery. However, the present finding that the EA group exhibited
decreased expression of the Nogo-A signaling pathway compared with
the IC group suggested that EA treatment weakened the inhibition of
axonal growth following cerebral ischemia.
In conclusion, the present study determined that EA
weakened the inhibition of axonal regrowth through the
downregulation of Nogo-A/NgR/Rho-A/ROCK signaling following focal
cerebral ischemia stroke, thus potentially contributing to the
recovery of nerve function. Therefore, it may serve a role in
protecting the brain following stroke. To the best of our
knowledge, the present study was the first to demonstrate that EA
at the Zusanli (ST36) and Quchi (LI11) acupoints on the paralyzed
limb provided a less inhibitory environment for axonal regrowth
following ischemic stroke. Furthermore, these results suggested
that EA may have marked effects on white matter plasticity and
regulation of the microenvironment of axonal regeneration around
the infarction area. The present study also provided a molecular
mechanism for the therapeutic effects of EA to promote stroke
recovery.
Acknowledgements
The present study was financially supported by the
Natural Science Foundation of Fujian Province, China (grant no.
2014J01345) and Innovative Medicine Subject of Fujian Provincial
Health and Family Planning Commission, China (grant no.
2016-CX-47). The authors would like to thank the Fujian Provincial
Rehabilitation industrial institution, Fujian University of
Traditional Chinese Medicine, China for assistance.
Glossary
Abbreviations
Abbreviations:
|
EA
|
electroacupuncture
|
|
GAP-43
|
growth-associated protein 43
|
|
IHC
|
immunohistochemical
|
|
MCAO
|
middle cerebral artery occlusion
|
|
CCA
|
common carotid artery
|
|
ECA
|
external carotid artery
|
|
ICA
|
internal carotid artery
|
|
DAB
|
diaminobenzidine
|
|
SC
|
sham operation control
|
|
IC
|
ischemia control
|
References
|
1
|
Berkowitz AL: Stroke and the
noncommunicable diseases: A global burden in need of global
advocacy. Neurology. 84:2183–2184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Corbyn Z: Statistics: A growing global
burden. Nature. 510:S2–S3. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lloyd-Jones D, Adams R, Carnethon M, De
Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund
K, et al: heart disease and stroke statistics-2009 update: A report
from the American heart association statistics committee and stroke
statistics subcommittee. Circulation. 119:e21–e181. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Merson TD and Bourne JA: Endogenous
neurogenesis following ischaemic brain injury: Insights for
therapeutic strategies. Int J Biochem Cell Biol. 56:4–19. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Go AS, Mozaffarian D, Roger VL, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al:
Heart disease and stroke statistics-2013 update: A report from the
American heart association. Circulation. 127:e6–e245. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan F, Wang X, Li HQ, Lu L, Li M, Li JH,
Fang M, Meng D and Zheng GQ: A randomized controlled pilot study of
the triple stimulation technique in the assessment of
electroacupuncture for motor function recovery in patients with
acute ischemic stroke. Evid Based Complement Alternat Med.
2013:4319862013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang Z, Ning J, Xiong C and Shulin Y:
Effects of electroacupuncture at head points on the function of
cerebral motor areas in stroke patients: A pet study. Evid Based
Complement Alternat Med. 2012:9024132012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tao J, Chen B, Gao Y, Yang S, Huang J,
Jiang X, Wu Y, Peng J, Hong Z and Chen L: Electroacupuncture
enhances hippocampal NSCs proliferation in cerebral
ischemia-reperfusion injured rats via activation of notch signaling
pathway. Int J Neurosci. 124:204–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie G, Yang S, Chen A, Lan L, Lin Z, Gao
Y, Huang J, Lin J, Peng J, Tao J and Chen L: Electroacupuncture at
Quchi and Zusanli treats cerebral ischemia-reperfusion injury
through activation of ERK signaling. Exp Ther Med. 5:1593–1597.
2013.PubMed/NCBI
|
|
10
|
Chen A, Lin Z, Lan L, Xie G, Huang J, Lin
J, Peng J, Tao J and Chen L: Electroacupuncture at the Quchi and
Zusanli acupoints exerts neuroprotective role in cerebral
ischemia-reperfusion injured rats via activation of the PI3K/AKT
pathway. Int J Mol Med. 30:791–796. 2012.PubMed/NCBI
|
|
11
|
Hinman JD: The back and forth of axonal
injury and repair after stroke. Curr Opin Nrueol. 27:615–623.
2014.
|
|
12
|
Benowitz LI and Carmichael ST: Promoting
axonal rewiring to improve outcome after stroke. Neurobiol Dis.
37:259–266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwab ME and Strittmatter SM: Nogo limits
neural plasticity and recovery from injury. Curr Opin Neurobiol.
27:53–60. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wälchli T, Pernet V, Weinmann O, Shiu JY,
Guzik-Kornacka A, Decrey G, Yüksel D, Schneider H, Vogel J, Ingber
DE, et al: Nogo-A is a negative regulator of CNS angiogenesis. Proc
Natl Acad Sci USA. 110:pp. E1943–E1952. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zagrebelsky M and Korte M: Maintaining
stable memory engrams: New roles for Nogo-A in the CNS.
Neuroscience. 283:17–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lindau NT, Bänninger BJ, Gullo M, Good NA,
Bachmann LC, Starkey ML and Schwab ME: Rewiring of the
corticospinal tract in the adult rat after unilateral stroke and
anti-Nogo-A therapy. Brain. 137:739–756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsai SY, Papadopoulos CM, Schwab ME and
Kartje GL: Delayed anti-Nogo-a therapy improves function after
chronic stroke in adult rats. Steoke. 42:186–190. 2011.
|
|
18
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. National
Academies Press (US); Washington (DC): pp. 85–23. 1996
|
|
19
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Steoke. 20:84–91. 1989.
|
|
20
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Steoke. 17:472–476. 1986.
|
|
21
|
Benowitz LI and Routtenberg A: GAP-43: An
intrinsic determinant of neuronal development and plasticity.
Trends Neurosci. 20:84–91. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosenzweig S and Carmichael ST: The
axon-glia unit in white matter stroke: Mechanisms of damage and
recovery. Brain Res. 1623:123–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suenaga J, Hu X, Pu H, Shi Y, Hassan SH,
Xu M, Leak RK, Stetler RA, Gao Y and Chen J: White matter injury
and microglia/macrophage polarization are strongly linked with
age-related long-term deficits in neurological function after
stroke. Exp Neurol. 272:109–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matute C, Domercq M, Pérez-Samartin A and
Ransom BR: Protecting white matter from stroke injury. Stroke.
44:1204–1211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi H, Hu X, Leak RK, Shi Y, An C, Suenaga
J, Chen J and Gao Y: Demyelination as a rational therapeutic target
for ischemic or traumatic brain injury. Exp Neurol. 272:17–25.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar P and Moon LD: Therapeutics
targeting Nogo-A hold promise for stroke restoration. CNS Neurol
Disord Drug Targets. 12:200–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li C, Wen H, Wang Q, Zhang C, Jiang L, Dou
Z, Luo X and Zeng J: Exercise training inhibits the
Nogo-A/NgR1/Rho-A signals in the cortical peri-infarct area in
hypertensive stroke rats. Am J Phys Med Rehabil. 94:1083–1094.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu L, Zhu L, Zou Y, Liu W, Zhang X, Wei
X, Hu B and Chen J: Panax notoginseng saponins promotes stroke
recovery by influencing expression of Nogo-A, NgR and p75NGF, in
vitro and in vivo. Biol Pharm Bull. 37:560–568. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Wang N, Liu Y, Liu Y, Zhang T, Zhu
L, Wang Y, Wu C and Yang J: Yonkenafil: A novel phosphodiesterase
type 5 inhibitor induces neuronal network potentiation by a
cGMP-dependent Nogo-R axis in acute experimental stroke. Exp
Neurol. 261:267–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu P, Mills E, Moher D and Seely D:
Acupuncture in poststroke rehabilitation: A systematic review and
meta-analysis of randomized trials. Stroke. 41:e171–e179. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Deng B, Li Y, Zhou L, Yang L, Gou
X, Wang Q, Chen G, Xu H and Xu L: Electroacupuncture pretreatment
attenuates cerebral ischemic injury via notch pathway-mediated
up-regulation of hypoxia inducible factor-1α in rats. Cell Mol
Neurobiol. 35:1093–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen C, Zhang W, Lou BD, Pan J, Cao Y,
Zhong F, Zhou WJ and Wu J: Effect of Electroacupuncture stimulation
of acupoints of the Pericardium Meridian on serum NGF and Nogo-A
contents and cerebral NGF and Nogo-A expression in cerebral
ischemia rats. Zhen Ci Yan Jiu. 40:94–98. 2015.(In Chinese).
PubMed/NCBI
|
|
33
|
Cheng CY, Lin JG, Su SY, Tang NY, Kao ST
and Hsieh CL: Electroacupuncture-like stimulation at Baihui and
Dazhui acupoints exerts neuroprotective effects through activation
of the brain-derived neurotrophic factor-mediated
MEK1/2/ERK1/2/p90RSK/bad signaling pathway in mild transient focal
cerebral ischemia in rats. BMC Complement Altern Med. 14:922014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang J, Ye X, You Y, Liu W, Gao Y, Yang
S, Peng J, Hong Z, Tao J and Chen L: Electroacupuncture promotes
neural cell proliferation in vivo through activation of the ERK1/2
signaling pathway. Int J Mol Med. 33:1547–1553. 2014.PubMed/NCBI
|
|
35
|
Kim YR, Kim HN, Ahn SM, Choi YH, Shin HK
and Choi BT: Electroacupuncture promotes post-stroke functional
recovery via enhancing endogenous neurogenesis in mouse focal
cerebral ischemia. PLoS One. 9:e900002014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xue X, You Y, Tao J, Ye X, Huang J, Yang
S, Lin Z, Hong Z, Peng J and Chen L: Electro-acupuncture at points
of Zusanli and Quchi exerts anti-apoptotic effect through the
modulation of PI3K/Akt signaling pathway. Neurosci Lett. 558:14–19.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lan L, Tao J, Chen A, Xie G, Huang J, Lin
J, Peng J and Chen L: Electroacupuncture exerts anti-inflammatory
effects in cerebral ischemia-reperfusion injured rats via
suppression of the TLR4/NF-κB pathway. Int J Mol Med. 31:75–80.
2013.PubMed/NCBI
|