Introduction
Prolonged exposure of human skin to ultraviolet B
(UVB) radiation (290–320 nm) induces clinical and histological
alterations due to the onset of simultaneous destruction and repair
(1). Environmental factors, such as
solar light, may lead to extrinsic aging, which occurs due to
dyspigmentation, telangiectasia and collagen degradation (2,3). The
pathogenesis of skin photoaging induced by UVB involves reactive
oxygen species (ROS)-mediated inflammation (4–6),
keratinocyte apoptosis (7,8) and the degradation of matrix
macromolecules by matrix metalloproteinases (MMPs) (9). Consequently, wrinkle formation,
epidermal thickening (10),
extracellular matrix (ECM) degradation-related water loss and skin
dryness (11) all increase. Thus, it
is important to reduce UVB-induced skin aging, as the maintenance
of skin health against UVB exposure is directly associated with
whole body health (10,12).
ROS may be generated by UV irradiation and damage
cellular lipids, proteins and DNA, resulting in the alteration and
destruction of skin structures. This may result in the failure of
the skin to function normally (13).
UVB-induced ROS overproduction leads to an imbalance between ROS
and antioxidant enzymes, as well as a reduction in glutathione
(GSH) levels (14,15). Furthermore, UVB exposure triggers the
release of numerous cytokines that participate in the onset of
cutaneous inflammation (16,17). These molecules serve an important
role in edema, which occurs as a result of vasodilation, the
opening of interendothelial junctions and the separation of
endothelial cells, which increases microvascular protein and fluid
leakage into the interstitium (18).
It has been suggested that there is a link between
oxidative stress and levels of inflammatory cytokines. For example,
the potent pro-inflammatory cytokine interleukin (IL)-1β may
trigger nicotinamide adenine dinucleotide phosphate (NADPH)
oxidase, which can produce free radical including superoxide
anions. In turn, superoxide anion-induced nuclear factor-κB (NF-κB)
serves an important in the production of cytokines (19). In this context, antioxidants from
natural sources may provide novel possibilities for inhibiting
UVB-induced oxidative stress-mediated events (4).
Pomegranates contain fibre, pectin, sugar and
several tannins (20), as well as
flavonoids and anthocyanidins in their seed oil and juice (21). Kim et al (22) demonstrated that pomegranates possess
chemopreventative and adjuvant therapeutic effects against human
breast cancer cells. Due to such biological activities, the
consumption of pomegranate-containing foods is increasing (21).
In a previous study by our group, it was determined
that pomegranate juice concentrated solution (PCS) exhibited
protective effects against UVB-induced photoaging in in vivo
and in vitro model systems (23,24).
Additionally, dried pomegranate juice concentrated powder (PCP)
significantly inhibited melanin formation in B16F10 melanocytes
(25). Thus, it is hypothesized that
PCP may exert protective effects against photoaging.
The aim of the present study was to examine the
anti-aging effect of PCP in UVB-induced skin photoaging. An oral
dose of 2 ml/kg PCS was used as a reference, as it has been
demonstrated that PCS exerts protective effects against UVB-induced
skin photoaging (23,24).
Materials and methods
Animals and husbandry
A total of 66 6-week healthy female SKH-1 hairless
mice were obtained from Orient Bio, Inc. (Seongnam, Korea). The
experimental groups were divided into the following 6 groups (8
mice per group), based on the body weights 7 days after
accclimatization (23.32±0.93 g, range, 21.4–24.8 g/head): Intact
vehicle control-unexposed (mice received vehicle-control); UVB
control-UVB exposed (mice received vehicle control and UVB
exposure); PCS-UVB exposed (mice received PCS 2 ml/kg and UVB
exposure); PCP 100 mg/kg-UVB exposed (mice received PCP 100 mg/kg
and UVB exposure); PCP 200 mg/kg-UVB exposed (mice received PCP 200
mg/kg and UVB exposure); and PCP 400 mg/kg-UVB exposed (mice
received PCP 400 mg/kg and UVB exposure). During acclimatization
and experimental periods, animals were kept at a temperature of
20–25°C, a humidity of 50–55% and were exposed to a 12-h light/dark
cycle in polycarbonate cages (4 rats per cage). Mice had ad
libitum access to standard rodent chow (Samyang Corporation,
Seoul, Korea) and tap water. All procedures involving laboratory
animals followed the national regulations for the usage and welfare
of laboratory animals (26). The
protocol of the current study was approved by the Institutional
Animal Care and Use Committee of Daegu Haany University (Gyeongsan,
Korea) prior to the experiment (approval no. DHU2015-066).
UVB irradiation
UVB irradiation was performed three times a week for
15 weeks at 0.18 J/cm2 following the protocol of a
previous study (27). Peak emission
was measured at 312 nm using a UV Crosslinker system (Hoefer
Scientific Instruments, San Francisco, CA, USA). Unexposed intact
control mice were also exposed to the non-emitting Crosslinker
system for the same duration as the UVB-exposed hairless mice.
Preparation and administration of test
materials
PCS was purchased from ASYA Meyve Suyu ve Gida San.
A.Ş. (Ankara, Turkey). PCS contained 2.31 mg/g ellagic acid as an
active ingredient and consisted of 58.86% carbohydrate, 1.21% total
protein, 0.49% fat, 27.97% water, 1.47% ash and 10% unidentified
ingredients, as well as 28.03 mg/100 g sodium at proximate
analysis. Health-Love Co. (Anyang, Korea) purchased raw materials
of PCS and PCP from ASYA Meyve Suyu ve Gida San. A.Ş. and supplied
the processed products after ingredients analysis and quality
control. PCP (ASYA Meyve Suyu ve Gida San. A.Ş.) containing 1.15
mg/g ellagic acid and 0.22 mg/g punicalagin as active ingredients.
All test materials were stored at 4°C in a refrigerator to protect
from light and moisture. A PCP dose of 200 mg/kg was selected based
on the in vitro skin whitening effect (25) and the clinical dose in humans [mouse
dosage was 200 mg/kg, ~12-fold the human dosage; (1,000 mg/60 kg)
×12=200 mg/kg] and doses of 400 and 100 mg/kg were selected as the
highest and lowest doses, respectively, using a ratio of 2.
Similarly, a PCS dose of 2 ml/kg was selected based on a previous
efficacy study investigating UVB-induced photoaging (23,24) and
also based on the clinical dose in humans [mouse dosage was
~12-fold the human dosage; (10 ml/60 kg) ×12=2 ml/kg]. PCP was
dissolved in distilled water to obtain concentrations of 40, 20 and
10 mg/ml, and a volume of 10 ml/kg was orally administered using a
gastric gavage attached syringe, resulting in administration of
400, 200 and 100 mg/kg, respectively. PCS was diluted in distilled
water in 1:4 ratios (v/v) and a volume of 10 ml/kg was orally
administered, resulting in administration of 2 ml/kg of body
weight, respectively. The different doses of PCP 100, 200 and 400
mg/kg, and PCS 2 ml/kg were administered to 8 mice in each PCP
group once per day for 15 weeks beginning 1 h after initial UVB
exposure. Equal volumes of vehicle (distilled water) were orally
administered to all mice in the intact and UVB control groups.
Generation of replicas and image
analysis
After obtaining photos of the dorsal skin around the
gluteal region, animals were sacrificed by over exdanguination
through vena cava under anesthesia (3% isoflurane; Hana Pharm Co.,
Ltd., Hwasung, Korea) and dissected. Replicas of mouse dorsal skin
were obtained using the Repliflo Cartridge kit (CuDerm Corp.,
Dallas, TX, USA). Impression replicas were set on a horizontal
sample stand and wrinkle shadows were produced by illumination with
a fixed-intensity light at a 40° angle. Black and white images were
recorded using a CCD camera and analyzed using the Skin-Visiometer®
VL650 (Courage and Khazaka, Cologne, Germany). To analyze skin
wrinkles, the average length and average depth of wrinkles were
measured using a previously described method (10).
Edema evaluation
To assess the effects of PCP and PCS on UVB-induced
skin edema, changes in dorsal skin weight were examined using a
previously established method (4).
Dorsal skin was removed, a 6-mm diameter was delimited with the aid
of a punch and this area was then weighed. Changes were measured
following 15 weeks of continuous oral administration of PCP, PCS or
distilled water. The results were calculated by comparing the
weight of the skin between groups and expressed as g/6-mm diameter
of dorsal skin.
Skin water content measurement
The day after the final administration of test
substances, 6 mm-diameter of dorsal back skin samples were removed
and skin water contents (%) were measured using an automated
moisture analyzer balance (MB23; Ohaus Corporation, Parsippany, NJ,
USA), according to a previous study (28).
Detection of collagen type I (COL1)
contents in skin tissue
COL1 contents were measured following a previously
established method (29). Briefly,
parts of the dorsal back skin tissues were separated and tissue
homogenates were prepared using a homogenization system (Model
TacoTMPre; GeneResearch Biotechnology Corp., Taichung, Taiwan), an
ultrasonic cell disruptor (Model KS-750; Madell Technology Corp.,
Ontario, CA, USA) and radioimmunoprecipitation assay buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Following the
seperation of supernatants by centrifugation at 21,000 × g for 10
min at 4°C, the amount of pro-collagen type I was measured using a
pro-collagen type I C peptide assay kit (Takara Bio, Inc., Otsu,
Japan) according to the manufacturer's protocol and absorbance was
measured at 450 nm using a microplate reader (Tecan Group Ltd.,
Männedorf, Switzerland).
Hyaluronan contents in skin
tissue
Hyaluronan contents in the supernatant prepared from
skin tissue homogenates were measured using a previously described
method (30). Briefly, the fat in
the sampled dorsal back skins was removed using acetone. Samples
were then dried, weighed and boiled for 20 min in 50 mM Tris/HCl
(pH 7.8) buffer. Subsequently, samples underwent proteolytic
digestion with 1% w/v actinase E (Sigma-Aldrich; Merck KGaA) for 1
week at 40°C. Trichloroacetic acid (Sigma-Aldrich; Merck KGaA) was
added to the samples at a final concentration of 10% w/v to induce
deproteinization prior to centrifugation at 4,000 × g at 4°C for 20
min. The supernatants were subsequently neutralized using 10 N
NaOH. Hyaluronan levels were measured using the QnE hyaluronic acid
enzyme-linked immunosorbent assay (ELISA) kit (cat no. BTP-96200;
Biotech Trading Partners, Encinitas, CA, USA), according to the
manufacturer's protocol. Color intensity was measured at 450 nm
using a spectrophotometer (OPTIZEN POP, Mecasys, Daejeon,
Korea).
Measurement of myeloperoxidase (MPO)
activity
MPO activity was measured using the MPO
kinetic-colorimetric assay, according to a previously described
method (4). A total of 50 mM
K2HPO4 buffer (pH 6.0; Sigma-Aldrich; Merck
KGaA) containing 0.5% hexadecyltrimethylammonium bromide (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to
collect and homogenize skin samples in an ice bath for 15 sec.
Following centrifugation at 1,000 × g for 2 min at 4°C, the
supernatant was removed. A total of 30 µl supernatant sample was
mixed with 200 µl 0.05 M K2HPO4 buffer (pH
6.0), containing 0.167 mg/ml o-dianisidine dihydrochloride
(Sigma-Aldrich; Merck KGaA) and 0.05% hydrogen peroxide. After 5
min, the absorbance of the samples was measured using a
spectrophotometer at 450 nm. The MPO activity of samples was
compared with a standard curve of neutrophils and protein levels in
the skin homogenates was measured using the Lowry method (31). The results are presented as MPO
activity (number of total neutrophils/mg protein).
Detection of IL-1β and IL-10 in skin
tissue
The dorsal back skin tissue from the area around
gluteal region was collected. Collected tissue was homogenized and
processed as described by Botelho et al (32). IL-10 and IL-1β concentrations were
determined using the enzyme-linked immunosorbent assay (ELISA) kit
(cat no. ab108870 for IL-10; cat no. ab100705 for IL-1β; Abcam,
Cambridge, UK), according to the manufacturer's instructions.
Absorbance was measured at 490 nm using a microplate reader.
GSH assay
Following skin homogenization (1:3, w/w dilution) in
100 mM NaH2PO4 (pH 8.0; Sigma-Aldrich; Merck
KGaA) containing 5 mM EDTA, 30% trichloroacetic acid
(Sigma-Aldrich; Merck KGaA) was added to homogenates. Mixtures were
then centrifuged twice (once at 1,940 × g for 6 min at 4°C and once
at 485 × g for 10 min at 4°C) and the fluorescence of the resulting
supernatant was measured using a fluorescence spectrophotometer
(RF-5301PC; Shimadzu Corporation, Tokyo, Japan). Supernatant (100
µl) was mixed with 1 ml buffer 1 and 100 µl o-phthalaldehyde (1
mg/ml in methanol; Sigma-Aldrich; Merck KGaA). After 15 min, the
absorbance was measured at 420 nm. A standard curve containing
0.0–75.0 µM GSH was prepared. Protein levels in the skin
homogenates were measured using the Lowry method (31) and results are presented as µM GSH/mg
protein. GSH levels were measured as reported previously (4).
Lipid peroxidation
Protein content of the homogenate (10 mg/ml in 1.15%
KCl) was measured using the Lowry method (31). To determine lipid peroxidation,
levels of thiobarbituric acid reactive substances were measured
following a previously described method (33). Briefly, 10% trichloroacetic acid
(Sigma-Aldrich; Merck KgaA) was mixed with the homogenate and this
mixture was then centrifuged at 1,000 × g at 4°C for 3 min to
obtain protein-free samples. Thiobarbituric acid (0.67%) was
treated and the mixture was left to stand at 100°C for 15 min.
Levels of malondialdehyde (MDA), an intermediate product of
lipoperoxidation, were measured by determining the difference
between the absorbance at 535 and 572 nm using a microplate
spectrophotometer reader. The results were reported as nM/mg of
protein (34).
Superoxide anion production
A nitroblue tetrazolium (NBT) assay was used to
determine the production of superoxide anion in dorsal back skin
tissue homogenates (10 mg/ml in 1.15% KCl), following a previously
described protocol (35). Briefly,
NBT (1 mg/ml; Sigma-Aldrich; Merck KGaA) was added to 50 µl
homogenate at 37°C for 1 h. The supernatant was then removed and
reduced formazan was solubilized following addition of 120 µl 2 M
KOH and 140 µl dimethyl sulfoxide. NBT reduction was measured at
600 nm using a microplate spectrophotometer reader and data were
normalized to the protein content.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in the individual dorsal back skins,
sampled by skin puncher 24 h following the final administration of
PCP or PCS, was extracted using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to a previously described method
(30). RNA concentration and quality
was determined using a CFX96™ Real-Time system using iTaq™
SYBR-Green (both from Bio-Rad Laboratories, Inc., Hercules, CA,
USA). To remove contaminating DNA, samples were treated with
recombinant DNase I (DNA-free DNA Removal kit; Ambion; Thermo
Fisher Scientific, Inc.). RNA was reverse-transcribed using the
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. The PCR cycling conditions were as follows: Initial
pre-denaturation of 95°C for 1 min, denaturation for 15 sec,
annealing of 55–65°C for 20 sec and extension of 72°C for 30 sec. A
total of 50 cycles were performed. The β-actin mRNA level was used
as a control for tissue integrity in all samples. Primer sequences
for Has 1, 2 and 3, COL1A1 and 2, MMP-1, 9 and 13, Nox2, GSH
reductase and β-actin are presented in Table I. For quantitative analysis, the
intact control skin tissue was used as the control, and the
relative expression of Has 1, 2 and 3, COL1A1 and 2, MMP-1, 9 and
13, Nox2, GSH reductase was calculated using the 2−ΔΔCq
method (36).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Target | Primer sequence
(5′-3′) |
|---|
| Has 1 |
|
|
Forward |
GCATGGGCTATGCTACCAAGTAT |
|
Reverse |
AGGAGGGCGTCTCCGAGTA |
| Has 2 |
|
|
Forward |
GACCCTATGGTTGGAGGTGTTG |
|
Reverse |
ACGCTGCTGAGGAAGGAGATC |
| Has 3 |
|
|
Forward |
AGACCGAGCTAGCCTTCCTAGT |
|
Reverse |
TAATGGCCAGATACAGCATGAG |
| COL1A1 |
|
|
Forward |
GCGGTAACGATGGTGCTGTT |
|
Reverse |
CTTCACCCTTAGCACCAAC |
| COL1A2 |
|
|
Forward |
ATTGTCGCCAGTGAG |
|
Reverse | CTGGTCCTGCTGGT |
| MMP-1 |
|
|
Forward |
AAGGTTAGCTTACTGTCACACGCTT |
|
Reverse |
CGACTCTAGAAACACAAGAGCAAGA |
| MMP-9 |
|
|
Forward |
CCCGGACCAAGGATACAG |
|
Reverse |
GGCTTTCTCTCGGTACTG |
| MMP-13 |
|
|
Forward |
CATCCATCCCGTGACCTTAT |
|
Reverse |
GCATGACTCTCACAATGCGA |
| GSH reductase |
|
|
Forward |
TGCGTGAATGTTGGATGTGTACCC |
|
Reverse |
CCGGCATTCTCCAGTTCCTCG |
| Nox2 |
|
|
Forward |
AGCTATGAGGTGGTGATGTTAGTGG |
|
Reverse |
CACAATATTTGTACCAGACAGACTTGAG |
| β-actin |
|
|
Forward |
AGCTGCGTTTTACACCCTTT |
|
Reverse |
AAGCCATGCCAATGTTGTCT |
Histopathology
Samples from dorsal back skins were separated, fixed
in 10% neutral buffered formalin at room temperature for 24 h,
embedded in paraffin wax, cut into sections 3–4 µm thick and
stained with hematoxylin and eosin (H&E) for general
histopathology or Masson's trichrome (MT) to detect collagen fiber,
according to previously established methods (37). The histopathological profiles of each
sample were observed under a light microscope (Model 80i; Nikon
Corporation, Tokyo, Japan). To quantify changes in further detail,
the number of microfolds formed on the surface of epithelium
(folds/mm epithelium), mean epithelial thickness (µm/epithelium)
and mean numbers of inflammatory cells infiltrated in the dermis
(cells/mm2 of dermis) were determined in general
histomorphometrical analysis using image analysis software
(iSolution FL ver 9.1; IMT i-solution Inc., Vancouver, Canada)
under H&E staining and in collagen fiber-occupied regions of
the dermis (%/mm2 of dermis) under MT staining. The
histopathologist was blinded to the group distribution when
conducting the analysis.
Immunohistochemistry
Following dewaxing of the prepared skin histological
paraffin wax sections, citrate buffer antigen (epitope) retrieval
pretreatment was conducted (37).
Briefly, a water bath was pre-heated to 95–100°C with a staining
dish containing 10 mM citrate buffer (pH 6.0). Slides were immersed
in the staining dish for 20 min and the lid was placed loosely. The
staining dish was then kept at room temperature for 20 min to cool.
Sections were immunostained using avidin-biotin complex (ABC)
methods for caspase-3, cleaved poly(ADP-ribose) polymerase (PARP),
nitrotyrosine (NT), 4-hydroxynonenal (4-HNE), inducible nitrogen
oxide synthase (iNOS) and matrix metalloproteinase (MMP)-9
according to the results of a previous study (37). Briefly, endogenous peroxidase
activity was blocked by incubation in methanol and 0.3%
H2O2 at room temperature for 30 min and
non-specific binding was blocked with 1% normal horse serum
blocking solution (dilution, 1:100; Vector Laboratories,
Peterborough, UK) for 1 h in a humidity chamber. Sections were
incubated with primary antibodies (listed in Table II) overnight at 4°C in a humidity
chamber and then incubated with biotinylated universal secondary
antibody (dilution, 1:50; Vector Laboratories) using a Vectastain
Elite ABC kit (dilution, 1:50; Vector Laboratories; Table II) for 1 h at room temperature in a
humidity chamber acooriding to the manufacturer's instructions.
Finally, sections were incubated with a peroxidase substrate kit
(Vector Laboratories; Table II) for
3 min at room temperature according to the manufacturer's
instructions. Between steps, all sections were rinsed in 0.01 M
PBS. Cells or fibers comprising >30% immunoreactivity for each
antiserum compared with intact dermal keratinocytes or dermal
tissue, were regarded as positive. The mean numbers of caspase-3,
PARP, NT and 4-HNE-immunoreactive epithelial cells (%, cells/100
epithelial cells) were counted using an established automated image
analysis process (37).
Additionally, the percentage of the dermis occupied by MMP-9
immunoreactive fibers was calculated (%/mm2 dermis). The
histopathologist was blinded to the group distribution when
performing the analysis.
 | Table II.Primary antiserum and detection kits
used in the present study. |
Table II.
Primary antiserum and detection kits
used in the present study.
| Product | Catalog no. | Supplier | Dilution |
|---|
| Primary
antiserums |
|
|
|
|
Anti-cleaved caspase-3
(Asp175) polyclonal antibody | 9661 | Cell Signaling
Technology Inc., Danvers, MA, USA | 1:400 |
|
Anti-cleaved poly(ADP-ribose)
polymerase (Asp214) specific antibody | 9545 | Cell Signaling
Technology Inc., Danvers, MA, USA | 1:100 |
|
Anti-4-Hydroxynonenal
polyclonal antibody | Ab46545 | Abcam, Cambridge,
UK | 1:100 |
|
Anti-Nitrotyrosine polyclonal
antibody | 06–284 | EMD Millipore,
Billerica, MA, USA | 1:200 |
|
Anti-Matrix metalloprotease-9
mouse antibody | Ab38898 | Abcam, Cambridge,
UK | 1:100 |
| Detection kits |
|
|
|
|
Vectastain Elite ABC kit | PK-6200 | Vector
Laboratories, Inc., Burlingame, CA, USA | 1:50 |
|
Peroxidase substrate kit | SK-4100 | Vector
Laboratories, Inc., Burlingame, CA, USA | 1:50 |
Statistical analyses
All data are presented as the mean ± standard
deviation of eight hairless mice. Multiple comparison tests of the
different groups were conducted. Variance homogeneity was examined
using the Levene test and if no significant deviation from variance
homogeneity was detected, the data were analyzed by a one-way
analysis of variance followed by a Least Significant Difference
multi-comparison test to determine whether differences between
pairs of groups were significant. If significant deviations from
variance homogeneity were detected by the Levene test, the
non-parametric Kruskal-Wallis H comparison test was conducted. When
a significant difference was observed in the Kruskal-Wallis H test,
the Mann-Whitney U test with Bonferroni correction was used to
determine whether there were significant differences between
specific pairs of groups. Statistical analyses were performed using
SPSS software ver. 14.0 for Windows (SPSS, Inc., Chicago, IL, USA)
and P<0.05 was considered to indicate a significant
difference.
Results
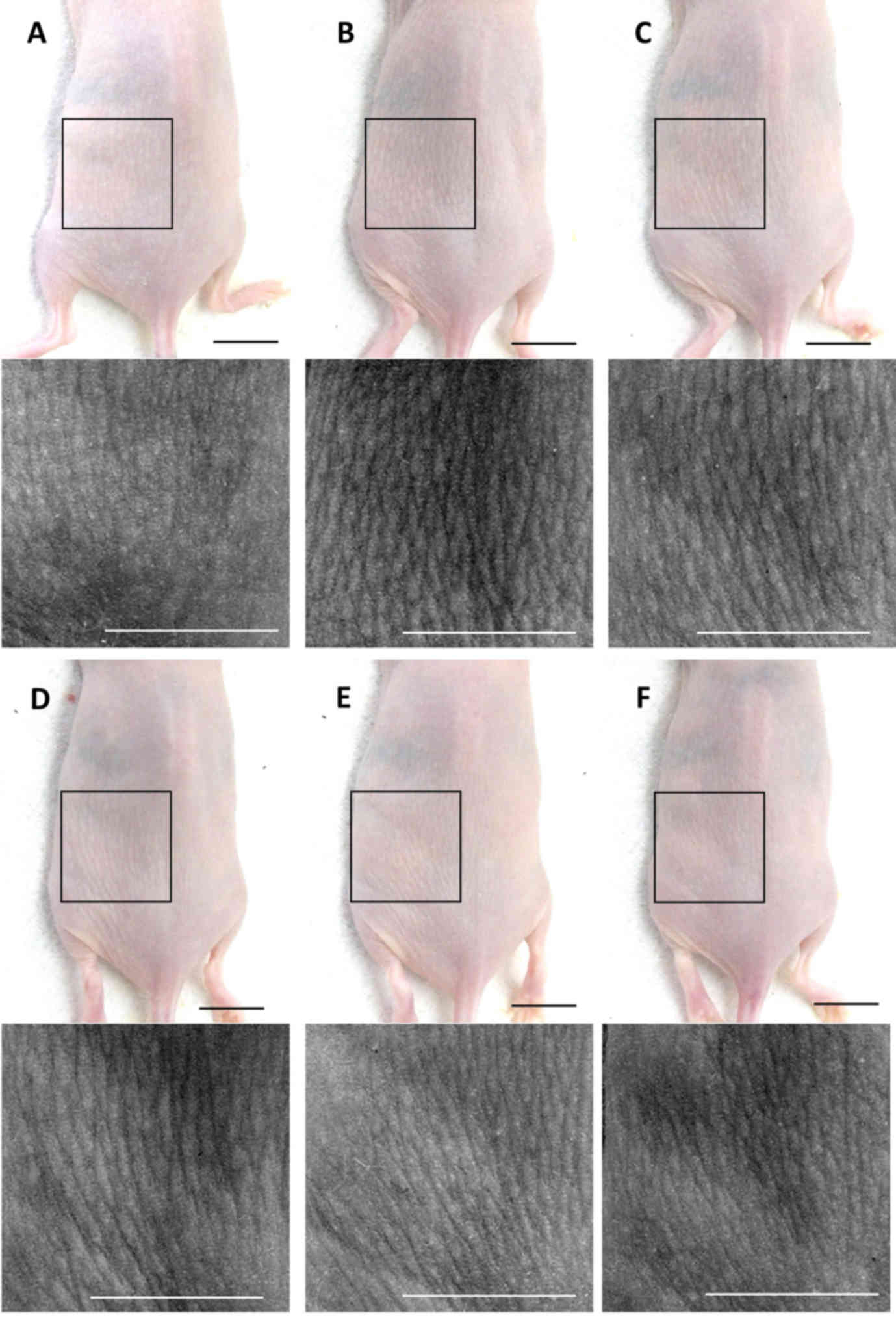
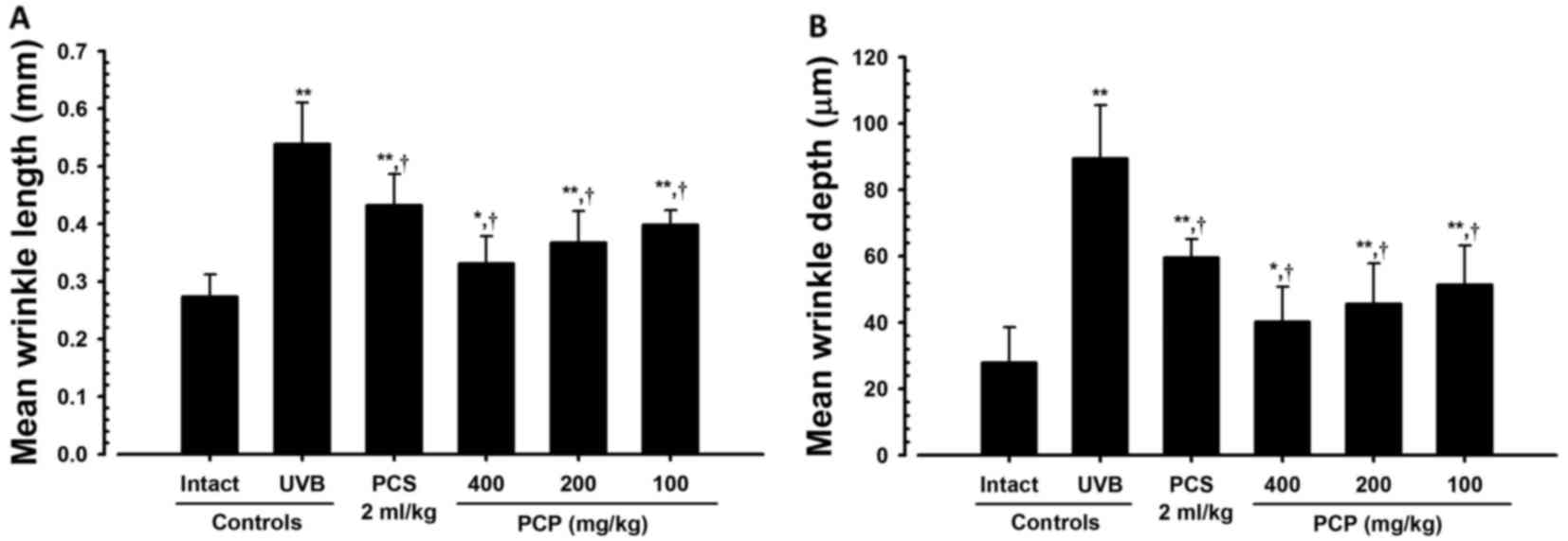
PCP decreases wrinkle formation in
UVB-exposed mice
To confirm the wrinkle alleviation effect of PCP,
the mean length and depth of the wrinkles were measured (Fig. 1). It was determined that the mean
length and depth of the wrinkles were significantly greater in the
skin of the UVB-exposed control mice compared with the intact
control (both P<0.01; Fig. 2).
However, these increases in wrinkle formations significantly
decreased following oral administration of PCP compared with the
UVB control (all P<0.05). These decreases occurred in a
dose-dependent manner (Fig. 2).
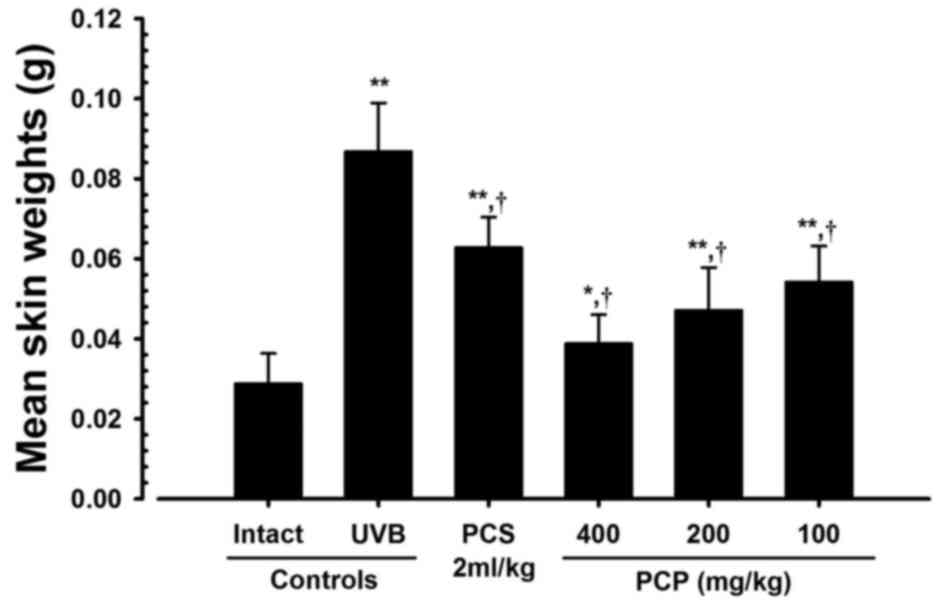
PCP decreases UVB-induced skin edema
in UVB-exposed mice
The effect of PCP against UVB-induced skin edema was
analyzed by measuring 6-mm-diameter skin weights (edema score). The
6-mm-diameter skin weights (edema score) in mice exposed to UVB
were significantly greater than those of intact controls
(P<0.01). However, in mice treated with 400, 200 and 100 mg/kg
PCP, mean skin weight significantly decreased (P<0.01) compared
with those of the UVB-exposed mice, by −55.33, −45.68 and −37.61%,
respectively (Fig. 3).
PCP increases skin water content in
UVB-exposed mice
To clarify the moisturizing effects of PCP, the skin
water content in 6-mm-diameter tissues was observed. Skin-water
content was significantly decreased following UVB treatment
(P<0.01). However, UVB-induced decreases in skin water contents
were significantly reversed (P<0.01) following treatment with
100, 200 and 400 mg/kg PCP, in a dose-dependent manner.
Additionally, mice administered 2 ml/kg PCS exhibited significant
increases in skin water content compared with UVB control mice
(P<0.01; Table III).
 | Table III.Changes in skin water, COL1 and
hyaluronan contents following 15 weeks continuous oral
administration of PCP or PCS in UVB-exposed mice. |
Table III.
Changes in skin water, COL1 and
hyaluronan contents following 15 weeks continuous oral
administration of PCP or PCS in UVB-exposed mice.
| Groups | Skin water contents
(%/6 mm-diameter skin) | Skin COL1 contents
(%) | Skin hyaluronan
contents (ng/ml) |
|---|
| Controls |
|
|
|
|
Intact | 37.14±3.47 | 98.61±14.32 | 2.51±0.54 |
|
UVB |
17.96±3.05a |
44.41±10.53a |
1.01±0.18a |
| Reference |
|
|
|
| PCS 2
ml/kg |
26.54±2.80a,b |
58.16±5.50a,b |
1.35±0.20a,b |
| PCP |
|
|
|
| 100
mg/kg |
26.74±2.42a,b |
58.82±7.44a,b |
1.42±0.14a,b |
| 200
mg/kg |
29.66±3.31a,b |
65.34±8.57a,b |
1.68±0.21a,b |
| 400
mg/kg |
32.18±2.86a,b |
73.83±6.30a,b |
1.82±0.21a,b |
PCP inhibits the decreases in skin
COL1 and hyaluronan content in UVB-exposed mice
To confirm the moisturizing effects of PCP, changes
in COL1 and hyaluronan contents were assessed. COL1 and hyaluronan
contents were significantly lower in 6-mm-diameter skin tissues
from UVB control mice compared with intact controls (P<0.01).
However, these decreases were significantly inhibited following
oral administration of all three doses of PCP (P<0.01; Table III).
PCP decreases MPO activity and IL-1β
levels while increasing IL-10 levels in UVB-exposed mice
To clarify the anti-inflammatory effects of PCP, MPO
activity, as well as IL-1β and IL-10 levels, were assessed. Skin
MPO activity and IL-1β levels were significantly higher in
UVB-exposed control mice than intact control mice (P<0.01). MPO
activity and IL-1β levels were significantly inhibited following
oral administration of PCS (P<0.01) and all doses of PCP
(P<0.01) in a dose-dependent manner, compared with the UVB
control mice (Table IV). IL-10
levels in UVB-exposed control mice were significantly decreased
compared with those in unexposed intact control mice (P<0.01).
However, significant (P<0.05) and dose-dependent increases in
skin IL-10 levels were observed following administration of all
three doses of PCP compared with untreated UVB-exposed mice
(Table IV).
 | Table IV.Changes in skin MPO activity, IL-1β
and IL-10 levels following 15 weeks continuous oral administration
of PCP or PCS in UVB-exposed mice. |
Table IV.
Changes in skin MPO activity, IL-1β
and IL-10 levels following 15 weeks continuous oral administration
of PCP or PCS in UVB-exposed mice.
| Groups | MPO (Numbers of
neutrophils ×105/mg protein) | IL-1β (pg/100 mg
protein) | IL-10 (pg/100 mg
protein) |
|---|
| Controls |
|
|
|
|
Intact | 1.41±0.42 | 20.43±3.28 | 318.88±72.13 |
|
UVB |
11.14±2.38a |
62.10±11.63a |
146.63±30.91a |
| Reference |
|
|
|
| PCS 2
ml/kg |
7.77±1.72a,b |
42.63±5.49a,b |
192.63±8.85a,b |
| PCP |
|
|
|
| 100
mg/kg |
7.38±1.60a,b |
41.53±11.02a,b |
211.00±44.98a,c |
| 200
mg/kg |
4.84±1.40a,b |
37.31±12.16a,b |
229.63±33.41a,d |
| 400
mg/kg |
4.41±1.19a,b |
31.21±10.85a,d |
238.00±24.37a,d |
PCP increases GSH content and
decreases MDA levels in UVB-exposed mice
To determine the anti-oxidative effects of PCP, GSH
contents and MDA levels were analyzed. GSH contents were
significantly lower in the UVB-exposed mice compared with the
intact control (P<0.01; Table V).
Furthermore, significant increases in skin MDA levels, indicating
increased lipid peroxidation and superoxide anion production, were
detected in UVB-exposed mice compared with unexposed control mice
(P<0.01; Table V).
 | Table V.Changes in the skin antioxidant
defense systems following 15 weeks continuous oral administration
of PCP or PCS in UVB-exposed mice. |
Table V.
Changes in the skin antioxidant
defense systems following 15 weeks continuous oral administration
of PCP or PCS in UVB-exposed mice.
| Groups | GSH (mg/mg
protein) | MDA (ng/mg
protein) | Superoxide anion
production (OD at 600 nm) |
|---|
| Controls |
|
|
|
|
Intact | 0.46±0.17 | 25.58±10.14 | 0.36±0.12 |
|
UVB |
0.11±0.03a |
132.59±22.30a |
1.04±0.20a |
| Reference |
|
|
|
| PCS 2
ml/kg |
0.17±0.04a,b |
84.49±18.89a,b |
0.74±0.09a,b |
| PCP |
|
|
|
| 100
mg/kg |
0.18±0.03b,b |
70.08±10.33a,b |
0.73±0.11a,b |
| 200
mg/kg |
0.24±0.03a,b |
59.63±8.53a,b |
0.63±0.17a,b |
| 400
mg/kg |
0.27±0.04b,c |
48.10±10.85a,b |
0.52±0.12b,c |
Skin GSH contents were significantly increased in
all PCP-administered hairless mice compared with UVB control mice
(P<0.01; Table V). This increase
occurred in a dose-dependent manner. Furthermore, skin MDA levels
and superoxide anion production were significantly decreased in
PCP-treated mice compared with UVB control mice (P<0.01;
Table V).
PCP decreases skin MMP-1, −9, and −13
and Nox2 mRNA expression in UVB-exposed mice
Although no significant changes in skin Has 1, 2,
and 3 or in COL1A1 and 2 mRNA levels (relative to the control) were
identified in UVB-exposed control mice compared with unexposed
intact control mice, significant increases (P<0.01) in skin Has
1, 2, and 3 and COL1A1 and 2 mRNA levels were detected in mice
receiving the three doses of PCP, compared with UVB control mice,
suggesting that PCP increases hyaluronan and COL1 synthesis
(Table VI).
 | Table VI.Changes in skin mRNA expression
following 15 weeks continuous oral administration of PCP or PCS in
UVB-exposed mice. |
Table VI.
Changes in skin mRNA expression
following 15 weeks continuous oral administration of PCP or PCS in
UVB-exposed mice.
|
| Control |
| PCP |
|---|
|
|
|
|
|
|---|
| Groups | Intact | UVB | Reference PCS 2
ml/kg | 100 mg/kg | 200 mg/kg | 400 mg/kg |
|---|
| Has 1 | 1.02±0.11 | 1.03±0.21 |
1.85±0.32a,b |
2.41±0.37a,b |
2.91±0.55a,b |
3.89±1.17a,b |
| Has 2 | 1.05±0.11 | 0.99±0.11 |
1.59±0.22a,b |
1.79±0.27a,b |
2.25±0.41a,b |
4.10±1.23a,b |
| Has 3 | 1.05±0.15 | 0.98±0.11 |
1.58±0.41a,b |
1.71±0.34a,b |
3.05±0.93a,b |
3.67±1.70a,b |
| COL1A1 | 1.00±0.12 | 0.97±0.20 |
2.60±0.41a,b |
3.03±0.56a,b |
3.75±0.99a,b |
4.76±1.62a,b |
| COL1A2 | 1.01±0.14 | 1.00±0.09 |
2.62±0.45a,b |
3.01±0.54a,b |
3.74±0.98a,b |
4.73±1.85a,b |
| MMP-1 | 1.01±0.08 |
2.05±0.21a |
1.71±0.24a,b |
1.63±0.19a,b |
1.51±0.24a,b |
1.33±0.26a,b |
| MMP-9 | 1.02±0.07 |
1.91±0.21a |
1.65±0.15a,b |
1.55±0.18a,b |
1.37±0.16a,b |
1.24±0.11a,b |
| MMP-13 | 0.95±0.08 |
2.57±0.47a |
1.95±0.21a,c |
1.86±0.22a,b |
1.65±0.19a,b |
1.40±0.11a,b |
| GSH reductase | 1.18±0.34 |
0.57±0.14a |
0.76±0.11a,b |
0.84±0.12b |
1.07±0.17b |
1.21±0.18b |
| Nox2 | 1.05±0.14 |
2.02±0.27a |
1.52±0.19a,b |
1.49±0.16a,b |
1.41±0.14a,b |
1.27±0.17b,b |
Significant increases in skin MMP-1, −9 and −13 and
Nox2 mRNA levels were detected in UVB-exposed control mice compared
with unexposed intact mice (P<0.01), indicating increased MMP
activity and ROS-dependent inflammation in UVB-exposed control
mice. However, levels of skin MMP and Nox2 mRNA were significantly
inhibited following administration of all three doses of PCP
compared with UVB control mice (P<0.01; Table VI).
Levels of skin GSH reductase mRNA were significantly
decreased in UVB-exposed mice compared with intact control mice
(P<0.01). However, significant dose-dependent increases in skin
GSH reductase mRNA expression were detected in mice receiving 100,
200 and 400 mg/kg PCP compared with UVB control mice (P<0.01;
Table VI).
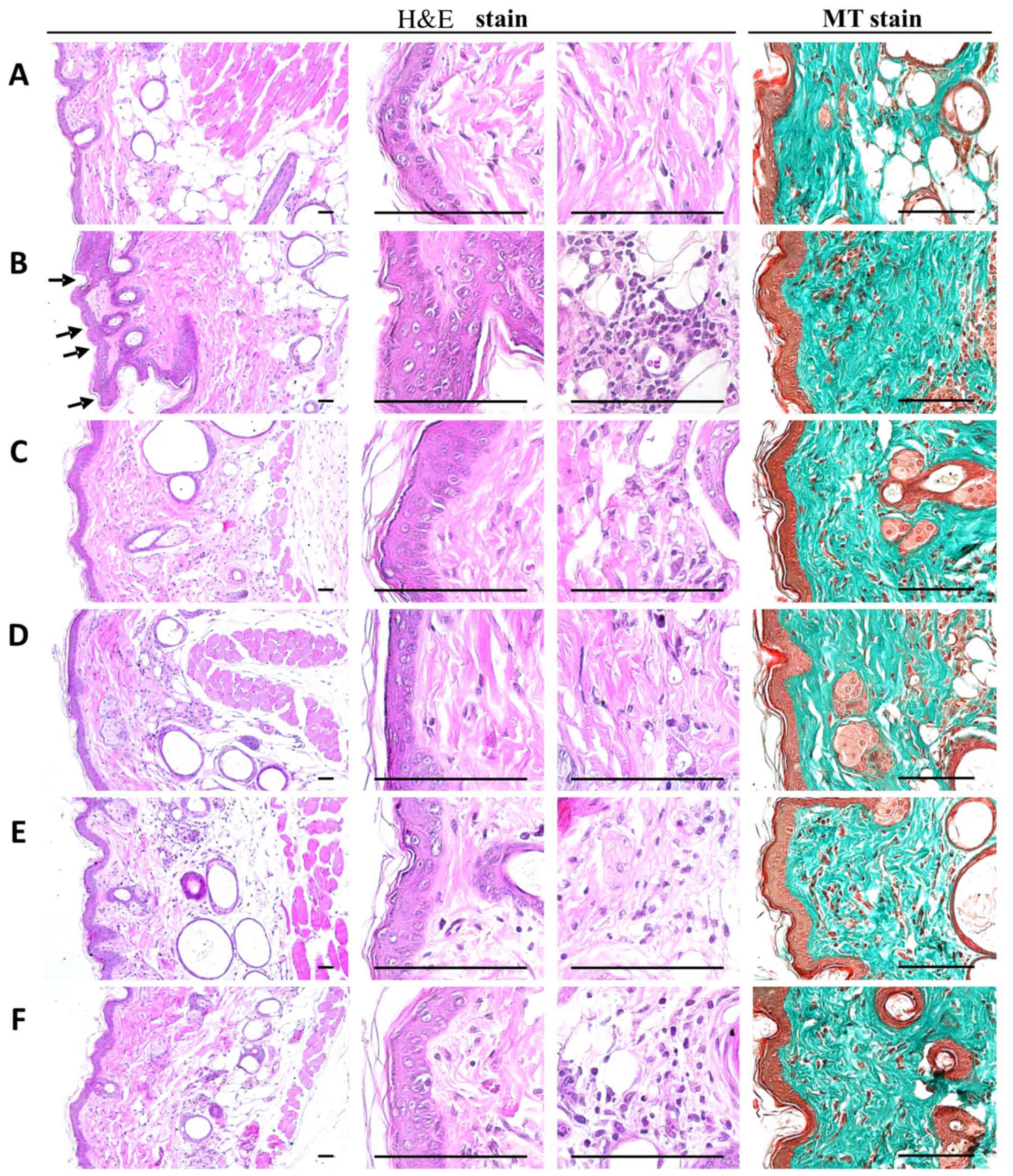
PCP decreases histopathological dermis
sclerosis and inflammatory signs in UVB-exposed mice
When the mean epithelial thicknesses of UVB-exposed
mice and intact control mice were compared, it was determined that
UVB-exposed control mice exhibited higher mean epithelial
thicknesses of dorsal back skin tissues compared with intact
control mice. In addition, there was a cellular infiltration of
inflammatory cells into the epidermis, an abnormal accumulation of
collagen in the dermis and the formation of microfolds on the
surface of the epithelial lining was observed (Fig. 4).
These findings were confirmed by histomorphometric
analysis; significant increases in the number of epithelial surface
microfolds, mean epithelial thickness, numbers of dermal
infiltrating inflammatory cells and the percentages of collagen
fiber-occupied dermal regions were detected in UVB-exposed mice
compared with unexposed intact vehicle controls (P<0.01).
However, significant dose-dependent decreases in UVB-induced
histopathological dermis sclerosis and inflammatory signs were
observed in mice orally administered 100, 200 and 400 mg/kg PCP
compared with UVB control mice (P<0.01). At an oral dose of 2
ml/kg, PCS was also associated with significant decreases in
epithelial surface microfolds, mean epithelial thickness, numbers
of dermal infiltrating inflammatory cells and percentages of
collagen fiber-occupied dermal regions compared with the UVB
control (P<0.05; Table
VII).
 | Table VII.General histomorphometrical analysis
of skin following 15 weeks continuous oral administration of PCP or
PCS in UVB-exposed mice. |
Table VII.
General histomorphometrical analysis
of skin following 15 weeks continuous oral administration of PCP or
PCS in UVB-exposed mice.
| Groups | No. of microfolds
(folds/mm of epidermis) | Mean epithelial
thickness (µm/epidermis) | Mean inflammatory
cells (cells/mm2 of dermis) | Collagen fiber
occupied regions (%/mm2 of dermis) |
|---|
| Controls |
|
|
|
|
|
Intact | 8.75±4.40 | 19.11±2.12 | 10.25±4.20 | 42.52±4.59 |
|
UVB |
70.88±14.38a |
47.39±8.29a |
241.25±39.15a |
76.51±7.06a |
| Reference |
|
|
|
|
| PCS 2
ml/kg |
46.00±11.93a,b |
30.60±5.51a,b |
186.93±24.73a,c |
64.92±5.26a,b |
| PCP |
|
|
|
|
| 100
mg/kg |
40.75±10.05a,b |
29.00±4.44a,b |
171.50±28.35a,b |
60.98±10.95a,b |
| 200
mg/kg |
33.25±10.07a,b |
25.72±5.61b,d |
149.38±32.95a,b |
58.64±4.25a,b |
| 400
mg/kg |
23.88±11.24a,b |
23.86±4.54b |
113.88±26.82a,b |
54.71±4.28a,b |
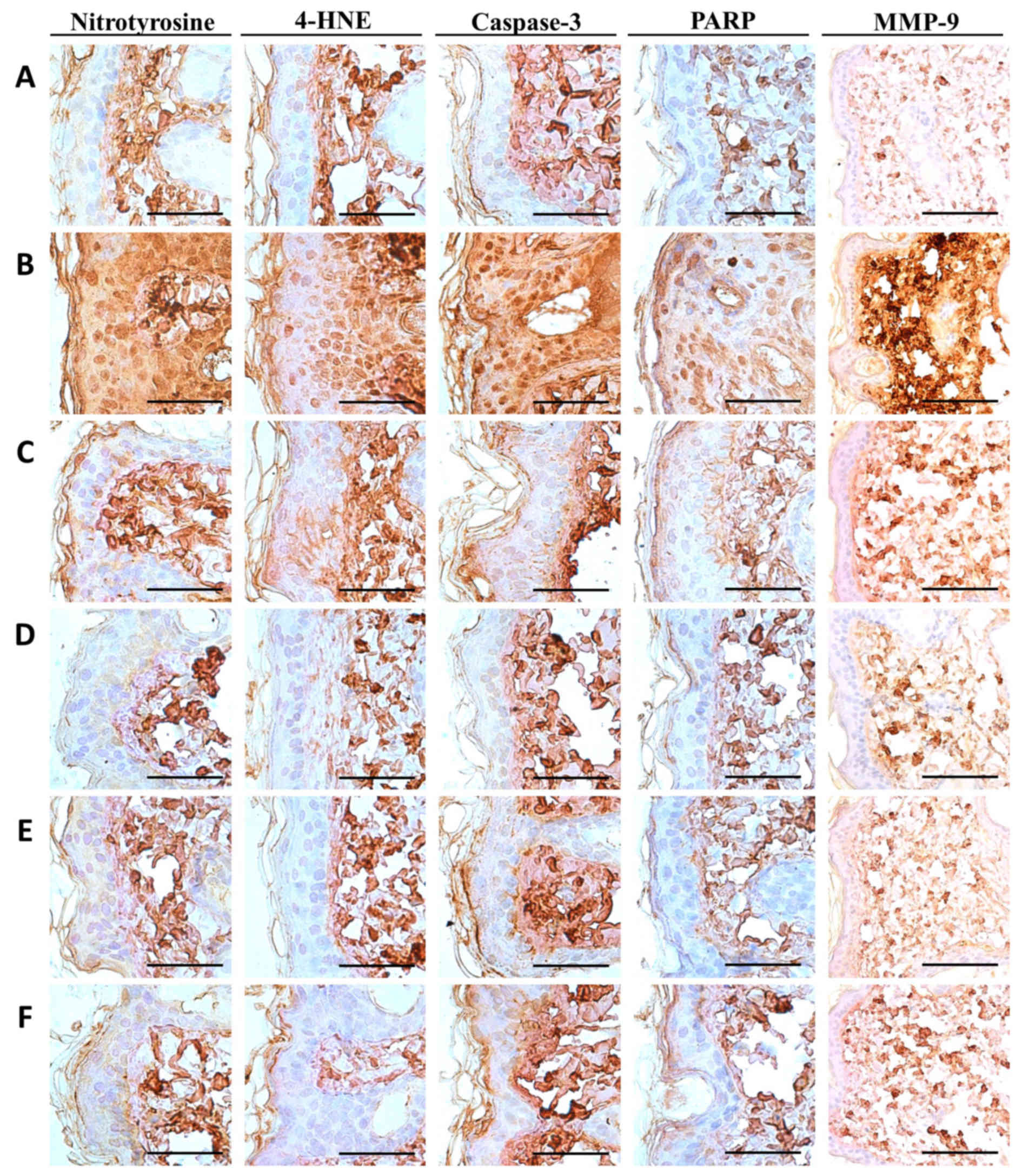
PCP decreases NT-4-HNE-, caspase-3-
and PARP-positive cells and decreases dermal MMP-9-immunoreactivity
cells in UVB-exposed mice
Notable increases in the number of cells
immunolabeled for oxidative stress (NT and 4-HNE) and apoptosis
(caspase-3 and PARP) markers were detected among epidermal
keratinocytes on the dorsal back skin tissues in UVB-exposed
control mice, with marked increases in dermal MMP-9
immunoreactivity (Fig. 5). These
changes were confirmed by histomorphometric analyses; significant
increases in epithelial NT-, 4-HNE-, caspase-3- and
PARP-immunoreactive cells as well as dermal MMP-9 immunoreactivity,
were observed in UVB-exposed control mice compared with unexposed
control mice (P<0.01; Table
VIII). However, significant decreases in oxidative stress and
apoptosis markers and dermal MMP-9 immunoreactivity were observed
in mice treated with 2 ml/kg PCS and 100, 200 and 400 mg/kg PCP
compared with UVB-exposed mice (P<0.01; Table VII). In particular, PCP induced
clear dose-dependent decreases in epithelial NT-, 4-HNE-,
caspase-3- and PARP-positive cells, as well as dermal
MMP-9-immunoreactive cells (Table
VIII and Fig. 5).
 | Table VIII.Immuno-histomorphometrical analysis
of skin following 15 weeks continuous oral administration of PCP or
PCS in UVB-exposed mice. |
Table VIII.
Immuno-histomorphometrical analysis
of skin following 15 weeks continuous oral administration of PCP or
PCS in UVB-exposed mice.
|
| Epidermal
immunoreactive cells (cells/100 epithelial cells) |
|
|---|
|
|
|
|
|---|
| Groups | NT | 4-HNE | Caspase-3 | PARP | Dermis MMP-9
immunoreactivity (%) |
|---|
| Controls |
|
|
|
|
|
|
Intact | 11.38±5.83 | 12.50±4.87 | 16.13±6.64 | 15.38±7.33 | 28.88±10.82 |
|
UVB |
82.50±11.17a |
71.75±10.43a |
83.00±10.20a |
80.75±10.98a |
75.88±13.27a |
| Reference |
|
|
|
|
|
| PCS 2
ml/kg |
52.00±11.12a,b |
53.38±11.86a,b |
58.25±10.95a,b |
52.63±11.29a,b |
56.63±11.48a,b |
| PCP |
|
|
|
|
|
| 100
mg/kg |
47.50±8.99a,b |
42.13±10.36a,b |
41.25±10.96a,b |
43.88±12.32a,b |
47.38±13.14a,b |
| 200
mg/kg |
35.00±6.19a,b |
38.00±11.16a,b |
34.50±10.38a,b |
36.38±10.25a,b |
42.38±11.67b,c |
| 400
mg/kg |
19.88±3.23b,c |
28.13±12.56a,b |
24.75±7.36b |
27.88±10.37b,c |
32.75±12.83b |
Discussion
Prolonged human exposure to sunlight results in
unwanted and deleterious effects, including the development of
cancer, wrinkles, scales, dryness and mottled pigment abnormalities
(hyper- or hypopigmentation) (4–6).
Furthermore, acute exposure to UV radiation may stimulate the
migration of inflammatory cells, such as neutrophils (38). Inflammation may cause skin damage,
premature skin aging and skin cancer (39). Additionally, chronic photoaging
triggers marked hyperplasia of epidermal cells with hyperkeratosis,
leading to wrinkle formation (40).
The results of the current study demonstrated that PCP (100, 200
and 400 mg/kg) significantly inhibits UVB-induced wrinkle formation
and hyperplasia/hypertrophy of the epithelial keratocytes.
Pomegranate extracts contain anthocyanins, ellagitannins and
hydrolyzable tannins, and oral administration of these extracts
reduces UVB-induced carcinogenesis in mice (41,42).
Ellagic acids contained in pomegranates exhibit antimutant,
antiviral, antioxidant and skin-whitening activity and are already
used in Japan as a food additive (43). It has also been suggested that punic
acid in pomegranate seed oil may prevent
7,12-dimethylbenz(a)anthracene (DMBA)- and
12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin
cancer (44,45).
Increased vascular permeability and blood flow are
induced by inflammatory chemical mediators, including the
cyclooxygenase-derived metabolites of arachidonic acid (46). UVB light-induced edema in hairless
mice and erythema in humans can be explained by the same mechanism
(4,6). In the current study, the administration
of PCP was able to inhibit skin edema in animals exposed to UVB
irradiation.
Generally, UVB irradiation stimulates the production
of ROS, such as superoxide anions, are important factors (46) in the process of neutrophil
recruitment to inflammatory foci. Neutrophils are the first cells
to be recruited from the peripheral blood to inflammatory sites
(4,47). Inflammatory cells serve an important
role in eliminating the causes of inflammation (48), whereas activated polymorphonuclear
leukocytes (PMNs) are a potential source of oxygen metabolites and
may exacerbate inflammation (49).
UVB irradiation increases the activity of MPO, an activating
cytotoxic enzyme released from PMNs (4,14,15,47).
Thus, reduced neutrophil influx into the skin tissue may be
confirmed by a reduction in MPO activity (32). Nox enzymes are involved in the
generation of endogenous ROS in response to inflammatory mediators,
including cytokines, growth factors and hypoxic conditions
(50,51). Nox2 is predominantly expressed in
myeloid white blood cells (52). The
results of the current study indicated that PCP decreased
UVB-induced MPO activity and dermis inflammatory cell infiltration.
Additionally, all three doses of PCP and PCS inhibited the
expression of Nox2 mRNA and the production of superoxide anions
(46,53). These results support the hypothesis
that the antioxidant effect of PCP inhibits the inflammatory
processes induced by UVB irradiation.
Following exposure to UVB, human keratinocytes
exhibit increased expression of cytokines, including TNF-α, IL-1α,
IL-1β and IL-6 (4,6,17).
IL-1β-stimulated neutrophils and other cells upregulate the
expression of intercellular adhesion molecules (54). UVB-induced inflammasomes serve an
important in regulating the activation of IL-1β. Activation of
nucleotide-binding oligomerization domain-containing protein (NOD)
1 and NOD2 by UVB leads to the recruitment of the adapter
apoptosis-associated speck-like protein, resulting in the
activation of pro-caspase-1 into its cleaved form (55). Consequently, caspase-1-depedent
cleavage stimulates the activation of IL-1β by pro-IL-1β (56). The results of the current study
indicated that IL-1β levels in the PCP- and PCS-treated groups were
lower than those in the UVB-exposed group. The inhibition of IL-1β
production by PCP is consistent with the inhibition of MPO
activity, as IL-1β is chemotactic for neutrophils (57). Inhibition of IL-1β production by PCP
may lead to a reduction in neutrophil recruitment and consequently,
to a reduction in MPO activity. Furthermore, PCP inhibited
gp91phox mRNA expression and the production of superoxide
anions; these findings suggest that preventing ROS-mediated IL-1β
production may be a mechanism of action of PCP against the effects
of UVB irradiation.
IL-10 is a potent anti-inflammatory cytokine that
blocks NF-κB activity. It regulates inflammatory signaling by
reducing levels of UVB radiation-induced pro-inflammatory cytokines
(54). Decreases in the levels of
UVB-induced IL-10 in the present study were consistent with the
results reported by Campanini et al (4). These decreases were reversed following
treatment with PCP; thus the protective mechanism of PCP against
UVB may be associated with an increase in IL-10 production.
GSH is a sensitive marker of oxidative stress caused
by UVB irradiation (58). 4-HNE is a
tissue lipid peroxidation marker and is regarded as a potential
causal agent in many diseases including chronic inflammation,
neurodegenerative disease, adult respiratory distress syndrome,
atherogenesis, diabetes and certain types of cancer (59). NT has been identified in the process
of tyrosine nitration that is mediated by reactive nitrogen
species, including the peroxynitrite anion and nitrogen dioxide,
and can be generated by the myeloperoxidase system (60). NT is considered to be an
iNOS-dependent marker (61). The
results of the current study indicated that PCP inhibits the
depletion of endogenous antioxidants. These results suggest that
PCP exerts antioxidant activity by maintaining the GSH system and
defending against the oxidative stress associated with exposure to
UVB.
Previous studies have demonstrated that, in hairless
mice, prolonged exposure to UVB causes significant increases in the
levels of MMP-1, −9, and −13 (62,63).
MMPs are directly responsible for the skin photoaging process;
thus, the direct inhibition of MMP activity with a specific
inhibitor or the indirect inhibition of MMP by reduction of its
expression may be an effective therapeutic method of counteracting
photoaging (9,37). In the present study, UVB treatment
increased MMP activity and associated abnormal dermal collagen
deposition was significantly inhibited following treatment with PCP
and PCS. These results indicate that PCP may be effective at
preventing photoaging by reducing the expression of MMPs.
UV-mediated mass apoptosis may damage the normal
barrier function of the skin and exacerbate skin photoaging
(64). It has been demonstrated that
UVB-induced apoptosis enhances the expression of caspase-3 and
PARP-immunoreactive keratinocytes in the skin epithelium (7,64). The
results of the present study determined that increases in epidermal
apoptotic markers were dose-dependently inhibited by oral
administration of the three doses of PCP and PCS. These
observations suggest that PCP may be effective against photoaging
by inhibiting epidermal keratinocyte apoptosis (7,64).
Normal human keratin layers contain 10–20% water,
however loss of water content increases wrinkle formation and
itching (50). The COL1A1 and COL1A2
genes encode the component COL1, part of the major structural
protein type I procollagen (65).
Collagen destruction is thought to be responsible for the
appearance of aged skin and changes resulting from chronic sun
exposure (66). It has been
suggested that skin wrinkles and ECM degradation are associated
with an increase in the activities of dermal enzymes, including
hyaluronidase, collagenase, elastase and MMP-1 (67). Hyaluronan, a major ECM component,
serves an important role in maintaining water homeostasis in the
skin (23,30) and the synthesis of Has 1, 2, and 3
(30). According to the results of
the current study, the groups receiving PCP exhibited increased
skin water, COL1 and hyaluronan content. Furthermore, the groups
exhibited upregulation of COL1A1 and 2, and of Has 1, 2, and 3 mRNA
expression. In particular, levels of COL1A1 and 2, and Has 1, 2,
and 3 in mice receiving 100 mg/kg PCP increased more than those in
mice receiving 2 ml/kg PCS.
There were a few limitations of the present study.
SKH-1 hairless mice are wildly used to examine the mechanism and
protection of age-related skin changes (40) however, UV-developed wrinkles differ
from those in humans as they arise following chronic ultraviolet
radiation (UVR) exposure and are associated with epidermal rather
than dermal changes (68,69). The UVR-exposed dermis in SKH1 mice
exhibits elastic fiber hyperplasia, collagen degradation and
elevated glycosaminoglycans, which are associated with alterations
in the activity of MMPs. UVR-developed wrinkles in SKH1 mice occur
as prominent horizontal creases on the dorsum. In addition,
experimental results from the mice model cannot directly be applied
to humans due to differences between evaluations of animal target
studies and clinically usable evaluations.
Taken together, the results of the current study
indicate that protection against UVB-induced photoaging was
achieved by treatment with different doses of PCP (100, 200 and 400
mg/kg) and 2 ml/kg PCS. This was due to the active cytoprotective
and anti-apoptotic effects, MMP activity inhibition and ECM (COL1
and hyaluronan) synthesis-related moisturizing, anti-inflammatory
and anti-oxidative effects of PCP and PCS. PCP may therefore be
developed as a functional protective agent against UVB-induced skin
photoaging. According to the results of the current study, skin
aging induced by UVB is most effectively suppressed by treatment
with 200 mg/kg PCP. In humans, the equivalent dose is 1 g/person
for humans. Therefore, the clinical efficacy of 1 g/person PCP
against skin photoaging in humans should be investigated
further.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean government
(grant no. 2012R1A5A2A42671316). The authors Mr Beom-Rak Choi, Mrs
Seung-Hee Kim, Mrs Hae-Yeon Yi and Mrs Hye-Rim Park are employees
at Health-Love Co., Ltd.
Glossary
Abbreviations
Abbreviations:
|
4-HNE
|
4-hydroxynonenal
|
|
ABC
|
avidin-biotin complex
|
|
COL1
|
collagen, type I
|
|
COL1A1
|
COL1 α 1
|
|
COL1A2
|
COL1 α 2
|
|
ECM
|
extracellular matrix
|
|
ELISA
|
enzyme linked immunosorbent assay
|
|
GSH
|
glutathione
|
|
Has
|
hyaluronic acid synthase
|
|
IL
|
interleukin
|
|
MDA
|
malondialdehyde
|
|
MMP
|
matrix metalloproteinase
|
|
MPO
|
myeloperoxidase
|
|
MT
|
Masson's trichrome
|
|
NBT
|
nitroblue tetrazolium
|
|
Nox2
|
Gp91phox subunit of the
phagocyte NADPH oxidase
|
|
NT
|
nitrotyrosine
|
|
PARP
|
cleaved poly(ADP-ribose)
polymerase
|
|
PCP
|
dried pomegranate juice concentrated
powder
|
|
PCS
|
pomegranate juice concentrated
solution
|
|
ROS
|
reactive oxygen species
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
UV
|
ultraviolet
|
References
|
1
|
Chaquour B, Seité S, Coutant K, Fourtanier
A, Borel JP and Bellon G: Chronic UVB- and all-trans
retinoic-acid-induced qualitative and quantitative changes in
hairless mouse skin. J Photochem Photobiol B. 28:125–135. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher GJ, Kang S, Varani J, Bata-Csorgo
Z, Wan Y, Datta S and Voorhees JJ: Mechanisms of photoaging and
chronological skin aging. Arch Dermatol. 138:1462–1470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilchrest BA: A review of skin ageing and
its medical therapy. Br J Dermatol. 135:867–875. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campanini MZ, Pinho-Ribeiro FA, Ivan AL,
Ferreira VS, Vilela FM, Vicentini FT, Martinez RM, Zarpelon AC,
Fonseca MJ, Faria TJ, et al: Efficacy of topical formulations
containing Pimenta pseudocaryophyllus extract against UVB-induced
oxidative stress and inflammation in hairless mice. J Photochem
Photobiol B. 127:153–160. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ono R, Fukunaga A, Masaki T, Yu X, Yodoi J
and Nishigori C: Suppressive effect of administration of
recombinant human thioredoxin on cutaneous inflammation caused by
UV. Bioengineered. 4:254–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duncan FJ, Martin JR, Wulff BC, Stoner GD,
Tober KL, Oberyszyn TM, Kusewitt DF and Van Buskirk AM: Topical
treatment with black raspberry extract reduces cutaneous
UVB-induced carcinogenesis and inflammation. Cancer Prev Res
(Phila). 2:665–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hasegawa T, Shimada S, Ishida H and
Nakashima M: Chafuroside B, an Oolong tea polyphenol, ameliorates
UVB-induced DNA damage and generation of photo-immunosuppression
related mediators in human keratinocytes. PLoS One. 8:e773082013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lei X, Liu B, Han W, Ming M and He YY:
UVB-Induced p21 degradation promotes apoptosis of human
keratinocytes. Photochem Photobiol Sci. 9:1640–1648. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin XJ, Kim EJ, Oh IK, Kim YK, Park CH and
Chung JH: Prevention of UV-induced skin damages by 11, 14,
17-eicosatrienoic acid in hairless mice in vivo. J Korean Med Sci.
25:930–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang TH, Park HM, Kim YB, Kim H, Kim N, Do
JH, Kang C, Cho Y and Kim SY: Effects of red ginseng extract on UVB
irradiation-induced skin aging in hairless mice. J Ethnopharmacol.
123:446–451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kawada C, Kimura M, Masuda Y and Nomura Y:
Oral administration of hyaluronan prevents skin dryness and
epidermal thickening in ultraviolet irradiated hairless mice. J
Photochem Photobiol B. 153:215–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bae JY, Choi JS, Choi YJ, Shin SY, Kang
SW, Han SJ and Kang YH: (−)Epigallocatechin gallate hampers
collagen destruction and collagenase activation in
ultraviolet-B-irradiated human dermal fibroblasts: Involvement of
mitogen-activated protein kinase. Food Chem Toxicol. 46:1298–1307.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casagrande R, Georgetti SR, Verri WA Jr,
Dorta DJ, dos Santos AC and Fonseca MJ: Protective effect of
topical formulations containing quercetin against UVB-induced
oxidative stress in hairless mice. J Photochem Photobiol B.
84:21–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hanada K, Sawamura D, Tamai K, Hashimoto I
and Kobayashi S: Photoprotective effect of esterified glutathione
against ultraviolet B-induced sunburn cell formation in the
hairless mice. J Invest Dermatol. 108:727–730. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Obermüller-Jevic UC, Schlegel B, Flaccus A
and Biesalski HK: The effect of beta-carotene on the expression of
interleukin-6 and heme oxygenase-1 in UV-irradiated human skin
fibroblasts in vitro. FEBS Lett. 509:186–190. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Abaseri TB, Hammiller B, Repertinger SK
and Hansen LA: The epidermal growth factor receptor increases
cytokine production and cutaneous inflammation in response to
ultraviolet irradiation. ISRN Dermatol. 2013:8487052013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kvietys PR and Granger DN: Role of
reactive oxygen and nitrogen species in the vascular responses to
inflammation. Free Radic Biol Med. 52:556–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Verri WA Jr, Vicentini FTMC, Baracat MM,
Georgetti SR, Cardoso RDR, Cunha TM, Ferreira SH, Cunha FQ, Fonesca
MJV and Casagrande R: Flavonoids as anti-inflammatory and analgesic
drugs: Mechanisms of action and perspectives in the development of
pharmaceutical formsStudies in Natural Products Chemistry:
Bioactive Natural Products. (Part P). Atta-Ur Rahman: Elsevier;
Amsterdam: pp. 297–322. 2012, View Article : Google Scholar
|
|
20
|
Gil MI, Tomás-Barberán FA, Hess-Pierce B,
Holcroft DM and Kader AA: Antioxidant activity of pomegranate juice
and its relationship with phenolic composition and processing. J
Agric Food Chem. 48:4581–4589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noda Y, Kaneyuki T, Mori A and Packer L:
Antioxidant activities of pomegranate fruit extract and its
anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J Agric
Food Chem. 50:166–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim ND, Mehta R, Yu W, Neeman I, Livney T,
Amichay A, Poirier D, Nicholls P, Kirby A, Jiang W, et al:
Chemopreventive and adjuvant therapeutic potential of pomegranate
(Punica granatum) for human breast cancer. Breast Cancer Res Treat.
71:203–217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang SJ, Choi BR, Kim SH, Yi HY, Park HR,
Park SJ, Song CH, Park JH, Lee YJ and Kwang S: Inhibitory effects
of pomegranate concentrated solution on the activities of
hyaluronidase, tyrosinase, and metalloproteinase. J Cosmet Sci.
66:145–159. 2015.PubMed/NCBI
|
|
24
|
Kang SJ, Choi BR, Kim SH, Yi HY, Park HR,
Song CY, Park SJ, Ku SK and Lee YJ: Effect of pomegranate
concentration solution on photoaging. J Soc Preventive Korean Med.
19:109–116. 2015.
|
|
25
|
Kang SJ, Choi BR, Lee EK, Kim SH, Yi HY,
Park HR, Song CH, Lee YJ and Ku SK: Inhibitory effect of dried
pomegranate concentration powder on melanogenesis in B16F10
melanoma cells; involvement of p38 and PKA signaling pathways. Int
J Mol Sci. 16:24219–24242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Committee for the Update of the Guide for
the Care and Use of Laboratory Animals, Institute for Laboratory
Animal Resources, Division on Earth and Life Studies and National
Research Council of the National Academies: Guide for the Care and
Use of Laboratory Animals. The National Academies Press;
Washington, DC: 2011
|
|
27
|
Jung SK, Lee KW, Kim HY, Oh MH, Byun S,
Lim SH, Heo YS, Kang NJ, Bode AM, Dong Z and Lee HJ: Myricetin
suppresses UVB-induced wrinkle formation and MMP-9 expression by
inhibiting Raf. Biochem Pharmacol. 79:1455–1461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim KH, Park SJ, Lee JE, Lee YJ, Song CH,
Choi SH, Ku SK and Kang SJ: Anti-skin-aging benefits of exopolymers
from Aureobasidium pullulans SM2001. J Cosmet Sci. 65:285–298.
2014.PubMed/NCBI
|
|
29
|
Kim YH, Chung CB, Kim JG, Ko KI, Park SH,
Kim JH, Eom SY, Kim YS, Hwang YI and Kim KH: Anti-wrinkle activity
of ziyuglycoside I isolated from a Sanguisorba officinalis root
extract and its application as a cosmeceutical ingredient. Biosci
Biotechnol Biochem. 72:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamane T, Nakagami G, Yoshino S, Muramatsu
A, Matsui S, Oishi Y, Kanazawa T, Minematsu T and Sanada H:
Hydrocellular foam dressing promotes wound healing along with
increases in hyaluronan synthase 3 and PPARα gene expression in
epidermis. PLoS One. 8:e739882013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
32
|
Botelho MA, Rao VS, Carvalho CB,
Bezerra-Filho JG, Fonseca SG, Vale ML, Montenegro D, Cunha F,
Ribeiro RA and Brito GA: Lippia sidoides and Myracrodruon urundeuva
gel prevents alveolar bone resorption in experimental periodontitis
in rats. J Ethnopharmacol. 113:471–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jamall IS and Smith JC: Effects of cadmium
on glutathione peroxidase, superoxide dismutase, and lipid
peroxidation in the rat heart: A possible mechanism of cadmium
cardiotoxicity. Toxicol Appl Pharmacol. 80:33–42. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barbosa DS, Cecchini R, El Kadri MZ,
Rodríguez MA, Burini RC and Dichi I: Decreased oxidative stress in
patients with ulcerative colitis supplemented with fish oil omega-3
fatty acids. Nutrition. 19:837–842. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Harrigan TJ, Abdullaev IF, Jourd'heuil D
and Mongin AA: Activation of microglia with zymosan promotes
excitatory amino acid release via volume-regulated anion channels:
The role of NADPH oxidases. J Neurochem. 106:2449–2462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KH, Park SJ, Lee YJ, Lee JE, Song CH,
Choi SH, Ku SK and Kang SJ: Inhibition of UVB-induced skin damage
by exopolymers from Aureobasidium pullulans SM-2001 in hairless
mice. Basic Clin Pharmacol Toxicol. 116:73–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Burns T, Breathnach S, Cox N and Griffiths
C: Rook's Textbook of Dermatology. Blackwell Science;
Massachusetts, MA: 2004, View Article : Google Scholar
|
|
39
|
Xu Y, Shao Y, Voorhees JJ and Fisher GJ:
Oxidative inhibition of receptor-type protein-tyrosine phosphatase
kappa by ultraviolet irradiation activates epidermal growth factor
receptor in human keratinocytes. J Biol Chem. 281:27389–27397.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kligman LH: The hairless mouse model for
photoaging. Clin Dermatol. 14:183–195. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Afaq F, Khan N, Syed DN and Mukhtar H:
Oral feeding of pomegranate fruit extract inhibits early biomarkers
of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse
epidermis. Photochem Photobiol. 86:1318–1326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bosch R, Philips N, Suárez-Pérez JA,
Juarranz A, Devmurari A, Chalensouk-Khaosaat J and González S:
Mechanisms of photoaging and cutaneous photocarcinogenesis, and
photoprotective strategies with phytochemicals. Antioxidants
(Basel). 4:248–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shaygannia E, Bahmani M, Zamanzad B and
Rafieian-Kopaei M: A review study on Punica granatum L. J Evid
Based Complementary Altern Med. 21:221–227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schubert SY, Lansky EP and Neeman I:
Antioxidant and eicosanoid enzyme inhibition properties of
pomegranate seed oil and fermented juice flavonoids. J
Ethnopharmacol. 66:11–17. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aviram M, Dornfeld L, Rosenblat M, Volkova
N, Kaplan M, Coleman R, Hayek T, Presser D and Fuhrman B:
Pomegranate juice consumption reduces oxidative stress, atherogenic
modifications to LDL, and platelet aggregation: Studies in humans
and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin
Nutr. 71:1062–1076. 2000.PubMed/NCBI
|
|
46
|
Witko-Sarsat V, Rieu P, Descamps-Latscha
B, Lesavre P and Halbwachs-Mecarelli L: Neutrophils: Molecules,
functions and pathophysiological aspects. Lab Invest. 80:617–653.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zimmerman BJ, Grisham MB and Granger DN:
Role of oxidants in ischemia/reperfusion-induced granulocyte
infiltration. Am J Physiol. 258:G185–G190. 1990.PubMed/NCBI
|
|
49
|
Sullivan GW, Sarembock IJ and Linden J:
The role of inflammation in vascular diseases. J Leukoc Biol.
67:591–602. 2000.PubMed/NCBI
|
|
50
|
Kim HJ, Kim CH, Ryu JH, Joo JH, Lee SN,
Kim MJ, Lee JG, Bae YS and Yoon JH: Crosstalk between
platelet-derived growth factor-induced Nox4 activation and MUC8
gene overexpression in human airway epithelial cells. Free Radic
Biol Med. 50:1039–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
O'Leary DP, Bhatt L, Woolley JF, Gough DR,
Wang JH, Cotter TG and Redmond HP: TLR-4 signalling accelerates
colon cancer cell adhesion via NF-κB mediated transcriptional
up-regulation of Nox-1. PLoS One. 7:e441762012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Krause KH: Tissue distribution and
putative physiological function of NOX family NADPH oxidases. Jpn J
Infect Dis. 57:S28–S29. 2004.PubMed/NCBI
|
|
53
|
Hattori H, Subramanian KK, Sakai J and Luo
HR: Reactive oxygen species as signaling molecules in neutrophil
chemotaxis. Commun Integr Biol. 3:278–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Weiss E, Mamelak AJ, La Morgia S, Wang B,
Feliciani C, Tulli A and Sauder DN: The role of interleukin 10 in
the pathogenesis and potential treatment of skin diseases. J Am
Acad Dermatol. 50:657–675; quiz 676–658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Benko S, Tozser J, Miklossy G, Varga A,
Kadas J, Csutak A, Berta A and Rajnavolgyi E: Constitutive and UV-B
modulated transcription of Nod-like receptors and their functional
partners in human corneal epithelial cells. Mol Vis. 14:1575–1583.
2008.PubMed/NCBI
|
|
56
|
Takeishi A, Kuranaga E and Miura M:
Sensing and reacting to dangers by caspases: Caspase activation via
inflammasomes. Drug Discov Ther. 2:14–23. 2008.PubMed/NCBI
|
|
57
|
Bellosta S, Dell'Agli M, Canavesi M, Mitro
N, Monetti M, Crestani M, Verotta L, Fuzzati N, Bernini F and
Bosisio E: Inhibition of metalloproteinase-9 activity and gene
expression by polyphenolic compounds isolated from the bark of
Tristaniopsis calobuxus (Myrtaceae). Cell Mol Life Sci.
60:1440–1448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
de Gruijl FR: Skin cancer and solar UV
radiation. Eur J Cancer. 35:2003–2009. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Smathers RL, Galligan JJ, Stewart BJ and
Petersen DR: Overview of lipid peroxidation products and hepatic
protein modification in alcoholic liver disease. Chem Biol
Interact. 192:107–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kettle AJ, van Dalen CJ and Winterbourn
CC: Peroxynitrite and myeloperoxidase leave the same footprint in
protein nitration. Redox Rep. 3:257–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pacher P, Beckman JS and Liaudet L: Nitric
oxide and peroxynitrite in health and disease. Physiol Rev.
87:315–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Inomata S, Matsunaga Y, Amano S, Takada K,
Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y and Fukuda M:
Possible involvement of gelatinases in basement membrane damage and
wrinkle formation in chronically ultraviolet B-exposed hairless
mouse. J Invest Dermatol. 120:128–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Kim HH, Lee MJ, Lee SR, Kim KH, Cho KH,
Eun HC and Chung JH: Augmentation of UV-induced skin wrinkling by
infrared irradiation in hairless mice. Mech Ageing Dev.
126:1170–1177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
El-Mahdy MA, Zhu Q, Wang QE, Wani G,
Patnaik S, Zhao Q, Arafa el-S, Barakat B, Mir SN and Wani AA:
Naringenin protects HaCaT human keratinocytes against UVB-induced
apoptosis and enhances the removal of cyclobutane pyrimidine dimers
from the genome. Photochem Photobiol. 84:307–316. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wallis GA, Sykes B, Byers PH, Mathew CG,
Viljoen D and Beighton P: Osteogenesis imperfecta type III:
Mutations in the type I collagen structural genes, COL1A1 and
COL1A2, are not necessarily responsible. J Med Genet. 30:492–496.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Liao PL, Li CH, Chang CY, Lu SR, Lin CH,
Tse LS and Cheng YW: Anti-ageing effects of alpha-naphthoflavone on
normal and UVB-irradiated human skin fibroblasts. Exp Dermatol.
21:546–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Maity N, Nema NK, Abedy MK, Sarkar BK and
Mukherjee PK: Exploring Tagetes erecta Linn flower for the
elastase, hyaluronidase and MMP-1 inhibitory activity. J
Ethnopharmacol. 137:1300–1305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kambayashi H, Yamashita M, Odake Y, Takada
K, Funasaka Y and Ichihashi M: Epidermal changes caused by chronic
low-dose UV irradiation induce wrinkle formation in hairless mouse.
J Dermatol Sci. 27 Suppl 1:S19–S25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Moloney SJ, Edmonds SH, Giddens LD and
Learn DB: The hairless mouse model of photoaging: Evaluation of the
relationship between dermal elastin, collagen, skin thickness and
wrinkles. Photochem Photobiol. 56:505–511. 1992. View Article : Google Scholar : PubMed/NCBI
|