Introduction
Dendritic cells (DCs) are the most potent
antigen-presenting cells and they have a critical role in innate
and adaptive immunity (1,2). Notably, they have a unique ability to
initiate naive T cells (3). Immature
DCs (imDC), which are located in peripheral tissues (such as the
skin, capture and process antigens), migrate to the draining
lymphoid organs where they are able to prime cluster of
differentiation (CD)4+ and CD8+ T cells (4). However, whether they induce T
cell-mediated immune response or tolerance is determined by the
status of DCs (5).
Over the past few years, great interest has been
focused on the development of DC-based immunotherapy due to the
unique capacity of DCs to initiate naive T cells. It is now
straight-forward to generate monocyte-derived DCs (moDCs) in
vitro using granulocyte-macrophage colony-stimulating factor
(GM-CSF) and IL-4 (6,7). A number of protocols have been tested
for their capacity to induce DC maturation (8–11) due to
the fact that fully mature DCs are more powerful than imDC at
inducing immune responses (5). Among
these protocols, IL-1β, IL-6, TNF-α and prostaglandins E2 (PGE2),
which was developed by Jonuleit et al (8), has become the gold standard cocktail
for DC maturation. To date, DCs matured with this standard cocktail
have been applied in the treatment of patients with different
malignant tumors and promising results have been demonstrated in
several clinical studies (12–16).
Although this standard cocktail has been widely
used, the appropriate time span for DC maturation has not been
determined. It has been reported that DCs gradually lose their
function over a few days after maturation (17). Therefore, shortening the time to
mature DCs in vitro may be beneficial for the effectiveness
of DC-based therapeutic vaccine in vivo. Therefore, the
present study comprehensively compared DCs matured for 24 and 48 h
using the standard cocktail to determine the appropriate time span
for DC maturation.
Materials and methods
Isolation of PBMCs and positive
selection of CD14+ monocytes
Peripheral blood mononuclear cells (PBMCs) were
isolated by Ficoll-Paque Plus (GE Healthcare, Chicago, IL, USA)
density gradient centrifugation from healthy human heparinized
blood (Beijing 307 Hospital of Chinese People's Liberation Army,
Beijing, China). All subjects were recruited in August 2015 (n=3;
male: Female, 1:2; age 27.33±1.53 years). Inclusion criteria for
subjects in this study were i) no fever; ii) normal renal and
hepatic function; iii) no drug use within one month. Exclusion
criteria were i) infection with hepatitis B virus, hepatitis C
virus, hepatitis D virus or human immunodeficiency virus; ii) liver
cirrhosis or hepatocellular carcinoma, fatty liver or alcoholic
hepatitis. PBMCs were washed by PBS twice before CD14+ monocytes
isolation using human CD14+ microbeads (Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany) according to the manufacturer's
instructions. The purity of the isolated CD14+ monocytes was
>90%. The Ethics Committee at the Second Affiliated Hospital,
Zhejiang University School of Medicine (Hangzhou, China) approved
this study. Informed consent was obtained from all
participants.
DCs generation
Monocyte-derived DCs were generated as previously
described with minor modifications (9). CD14+ monocytes were re-suspended in
serum-free AIM-V medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 100 U/ml penicillin and 100
µg/ml streptomycin and placed in 24-well plates (Corning, Inc.,
Corning, NY, USA) for incubation at 37°C in a humidified atmosphere
containing 5% CO2 for 2 h. Following complete aspiration
of the supernatant, fresh AIM-V medium supplemented with GM-CSF
(1,000 IU/ml) and IL-4 (500 IU/ml; both PeproTech, Inc., Rocky
Hill, NJ, USA) was added to the cells. The cells were supplied
every 2 days with fresh medium. On day 5, imDC were harvested and
cultured in the presence of IL-1β, IL-6, TNF-α (1,000 IU/ml;
PeproTech, Inc.) and PGE2 (1 µg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) for a further 24 or 48 h, respectively, to
obtain mature DCs (mDC). Supernatants were collected and retained
for cytokine analysis.
Flow cytometric analysis
Flow cytometry was performed using FACS Calibur (BD
Biosciences). Cells were stained with the following monoclonal
antibodies: Fluorescein isothiocyanate (FITC)-labeled antibodies
against CD40 and CD86, phycoerythrin (PE)-labeled antibodies
against CD80, CD83 and programmed death-ligand 1 (PD-L1), peridinin
chlorophyll protein (PerCP)-labeled antibodies against human
leukocyte antigen-D related (HLA-DR), allophycocyanin-labeled
antibodies against CD14, CD11c, and isotype matched control
antibodies (BD Biosciences). FACS data were analyzed using FlowJo
software (version 5.7.2; Tree Star, Inc.).
Apoptosis assay
Freshly harvested mDC (1×105) were washed
twice with cold PBS and incubated with Annexin-V-PE and
7-amino-actinomycin D (7-AAD) for 15 min before
fluorescence-activated cell sorting (FACS) analysis. FACS data were
analyzed using FlowJo software (version 5.7.2; Tree Star, Inc.,
Ashland, OR, USA).
Endocytic ability during the
maturation of DCs
ImDC and mDC (1×105) cells were suspended
in 100 µl of AIM-V and incubated with FITC-dextran (1 mg/ml) for 60
min either at 37°C or 4°C (negative control). Afterwards, cells
were washed three times in cold PBS prior to FACS analysis. FACS
data were analyzed using FlowJo software (version 5.7.2).
Allogeneic mixed lymphocyte reaction
(MLR)
mDC matured for 24 or 48 h were treated with 50
µg/ml mitomycin-C at 37°C in a humidified 5% CO2
atmosphere for 45 min. Afterwards, DCs were washed three times and
added to allogeneic CD14+ monocytes depleted PBMCs (105
cells) at a ratio 1:10 (DCs:PBMCs) in 96-well plates (Corning,
Inc.) for 4 days, then 20 µl CellTiter 96 Aqueous non-radioactive
reagent (Promega Corp., Madison, WI, USA) was added to each well
and cultures were continued for another 4 h. Following this,
absorbance at 490 nm was recorded using an ELISA plate reader.
Cytokines secretion analysis
Production of IL-12p40, IL-12p70 and IL-10 was
assayed by ELISA kit (BioLegend, Inc., San Diego, CA, USA)
according to the manufacturer's instructions.
Statistical analysis
Comparisons between groups of quantitative variables
were performed using the Mann-Whitney U test. The test was
two-sided and differences were considered significant if P<0.05.
Data handling and analysis were performed with SPSS software for
Windows, version 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Purity of CD14+ monocytes and mature
DCs
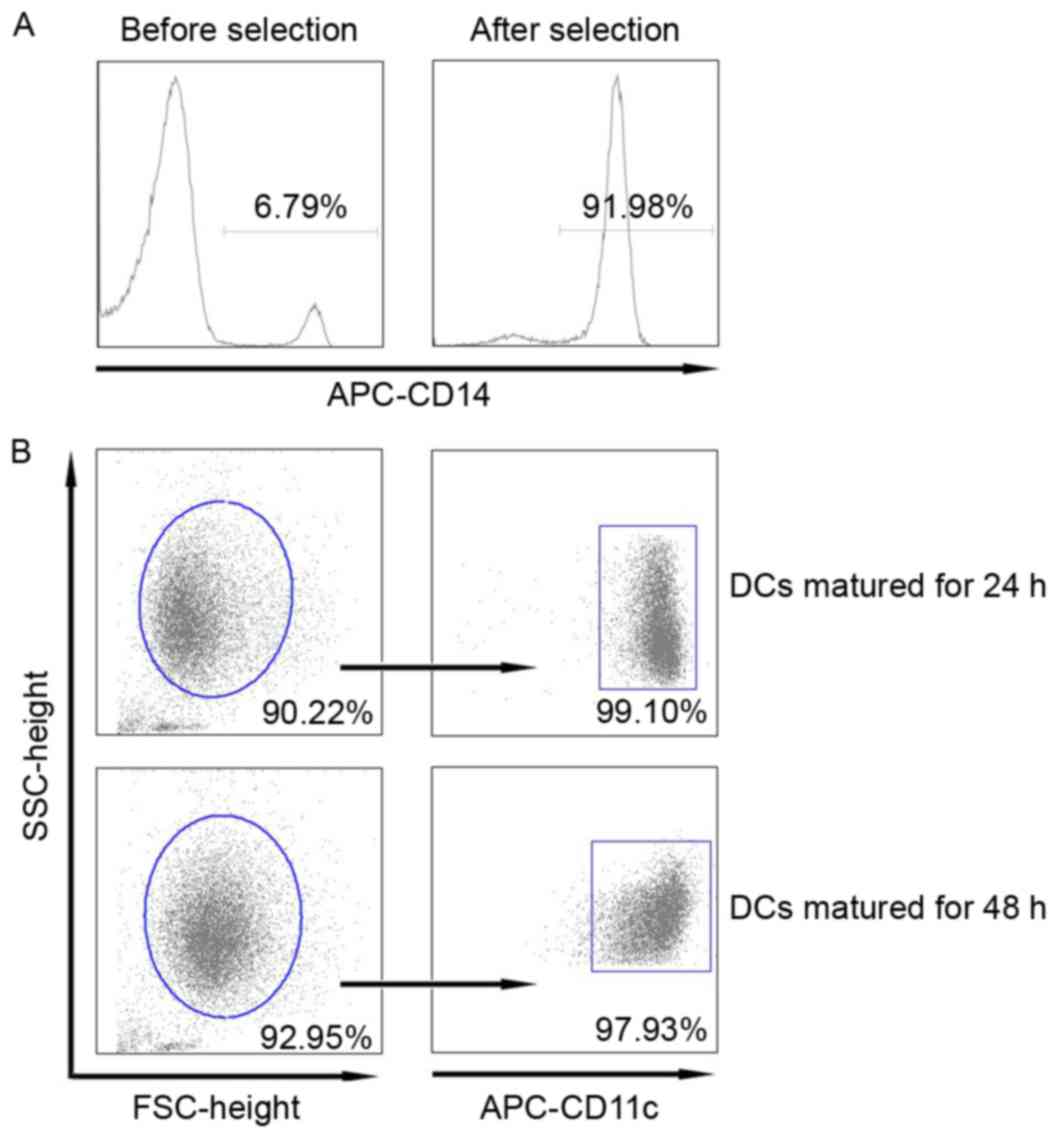
In order to improve the purity of monocyte-derived
DCs, we first selected the CD14+ monocytes from PBMCs by using
human CD14+ microbeads instead of the conventional cell adherent
technique. Our data showed that the proportion of CD14+ monocytes
in PBMCs was approximately 6.79% prior to selection. However, this
number increased to 91.98% following selection (Fig. 1A). The purity of DCs matured for 24
and 48 h was 90.22 and 92.95%, respectively, of which the majority
of mature DCs were CD11c+ DCs (Fig.
1B).
Phenotypic characteristics of mature
DCs
In the present study, co-stimulatory and
co-inhibitory surface markers, including CD40, CD80, CD83, CD86,
HLA-DR and PD-L1, were compared between DCs matured via a standard
cocktail for 24 and 48 h by flow cytometry. Compared to DCs matured
for 24 h, DCs matured for 48 h expressed higher levels of CD80,
both in frequency and mean fluorescence intensity (MFI). For CD83
and CD86 exhibited higher levels of frequency when matured for 48 h
instead of 24 h, however no differences were found in MFI. Notably,
similar expression levels of CD40 were found in frequency after 48
h, whereas MFI was higher in DCs matured for 24 h. No differences
of HLA-DR were found in terms of frequency and MFI. However, for
the inhibitory molecule, PD-L1, a higher frequency was also
observed in DCs matured for 48 h. The frequencies of co-stimulatory
molecules for both DCs all exceeded 90%, with the exception of CD83
(Table I).
 | Table I.Phenotypic characteristics of mature
DCs. |
Table I.
Phenotypic characteristics of mature
DCs.
|
|
| Mature DCs |
|---|
|
|
|
|
|---|
| Markers | Value | 24 h | 48 h |
|---|
| CD40 | % |
92.24 |
96.57 |
|
| MFI |
67.21 |
59.22a |
| CD80 | % |
93.94 |
98.82a |
|
| MFI | 149.24 | 247.51a |
| CD83 | % |
83.55 |
94.98a |
|
| MFI |
81.90 |
89.17 |
| CD86 | % |
94.47 |
99.52a |
|
| MFI | 198.56 | 216.83 |
| PD-L1 | % |
95.03 |
98.01a |
|
| MFI | 105.18 | 135.52 |
| HLA-DR | % |
97.22 |
99.57 |
|
| MFI | 629.59 | 675.37 |
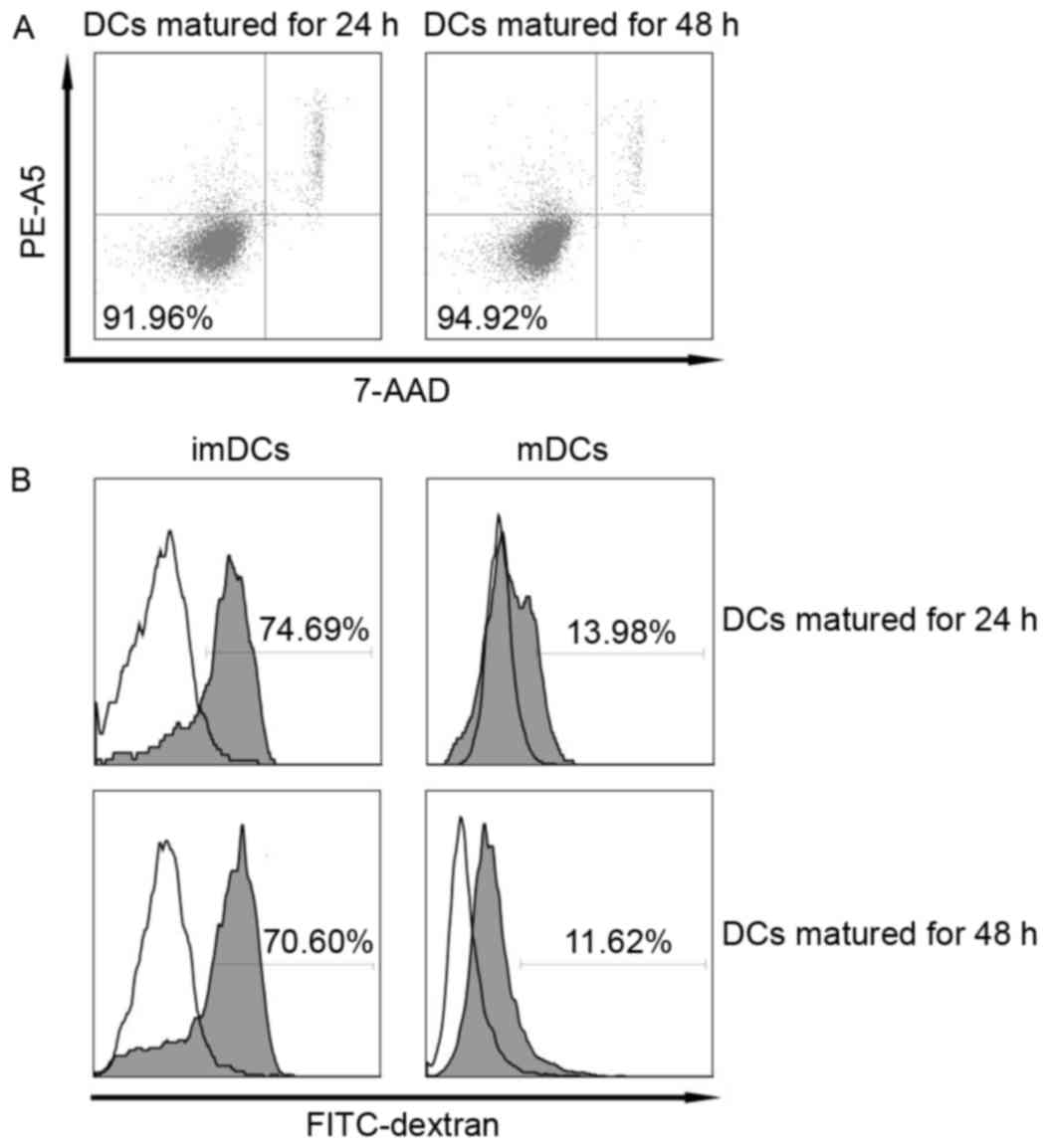
Viability and endocytosis of mature
DCs
High viability is important for the preparation of
effective DC-based therapeutic vaccines. Therefore, we determined
and compared the viability of DCs matured via the standard cocktail
for different time spans. Our data showed that the viability of DCs
matured for 24 and 48 h, respectively, were similarly high and
exceeded 90% (Fig. 2A). It is known
that the ability to take up antigens is one of the most important
functions of immature DCs and this capacity decreases quickly upon
maturation (18). Consistent with
this, we found that imDCs showed high endocytosis while the
endocytic capacity of DCs matured for 24 and 48 h both decreased
rapidly to a similar extent (Fig.
2B).
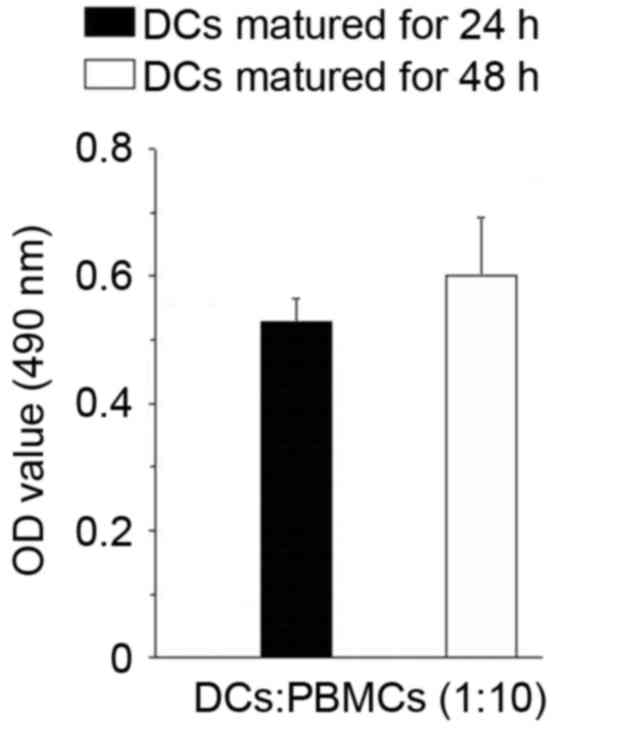
T cell stimulatory capacity and
cytokine productions of mature DCs
The T cell stimulatory capacity of DCs matured for
different time spans was assessed via an allogeneic mixed
lymphocyte reaction. Our data showed that the T cell stimulatory
capacity of DCs matured for 24 and 48 h, respectively, was
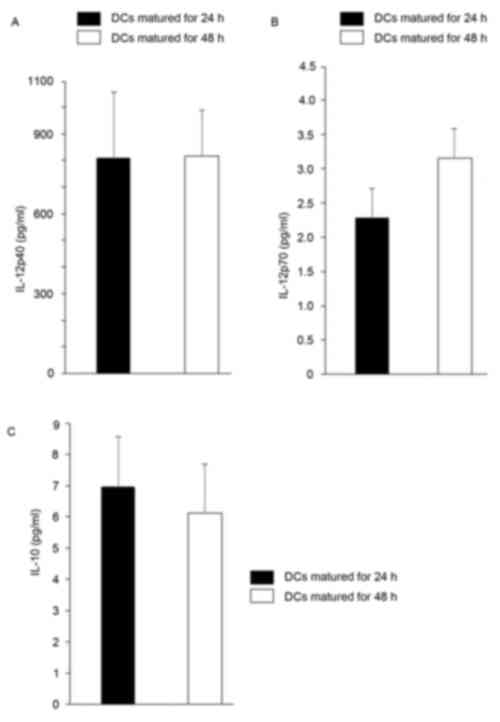
comparable (Fig. 3). We subsequently
detected the cytokine production of mDCs from both time points and
found that mDCs matured for 24 and 48 h, respectively, secreted
comparable high levels of IL-12p40, which is a subunit of IL-12p70.
Both groups of cells secreted comparable low levels of IL-10;
however, mDCs matured for 24 h secreted relatively lower levels of
bioactive IL-12p70 (Fig. 4).
Discussion
IL-1β, IL-6, TNF-α and PGE2 has been widely used as
a standard cocktail for in vitro generation of mature DCs
(8,12–16,19,20).
However, the optimal time for DC maturation using this standard
cocktail has not been established. In the present study, we found
that DCs matured for 24 h were, phenotypically speaking, also fully
mature compared to DCs matured for 48 h. DCs matured for 24 h
expressed even higher levels of the CD40 co-stimulatory molecule in
terms of MFI, whereas lower levels of the co-inhibitory molecule,
PD-L1, were detected in terms of frequency. Notably, the viability,
endocytosis, T-cell stimulatory capacity and cytokine production
were all comparable between DCs matured for 24 and 48 h,
respectively.
Pioneering studies indicating the possibility of
culturing murine DCs ex vivo from bone marrow precursors
initiated DC vaccine development in the 1990s (21). Human applications followed soon
thereafter and it was demonstrated that peripheral blood-derived
monocytes and CD34+ hematopoietic progenitors are suitable for
generating human DCs (22). In
previous studies, DCs have been induced from adherent monocytes by
washing out non-adherent cells, such as T and B cells. However, the
purity of DCs obtained by this method is ~60%. In the present
study, we induced DCs from CD14+ monocytes selected by using human
CD14+ microbeads. The purity of DCs obtained from this method
exceeds 90%, which is crucial for the improved effectiveness of
DC-based immunotherapy (23).
The maturation state of DCs has been considered as a
decisive factor in immune responses. Previous clinical studies have
demonstrated that improved clinical outcomes were more frequently
observed in trials using mature DCs in the therapeutic vaccination
of patients with cancer, including prostate cancer, melanoma and
glioblastoma (24–26), although moderate clinical benefit was
also reported in trials using IL-4 immature DCs (27). Due to their low co-stimulatory and
MHC class I and II molecule expression, immature and semi-mature
DCs are prone to inducing suboptimal T-cell priming and causing
T-cell tolerance. Fully mature DCs (for example, matured with
proinflammatory cytokines or TLR agonists) are able to prime CD4+ T
and CD8+ T cells (5,28). It is well-known that co-stimulatory
molecules, such as CD80 and CD86, have a key role in the induction
of effective T cell responses (29).
Our data showed that DCs matured for 24 and 48 h, respectively,
expressed high levels of CD80 and CD86, which are important for the
initiation of a robust immune response. Furthermore, CD40, which
also has a critical role in T cell activation (30,31)
expressed even higher levels in DCs matured for 24 h. Notably,
PD-L1, which is well-known for its inhibitory role in T cell
activation (32–34), expressed relatively lower levels on
DCs matured for 24 h and this may be beneficial for T cell
priming.
It is increasingly recognized that abundant
production of IL-12, particularly IL-12p70 during DC maturation has
a crucial role in the differentiation and expansion of Th1 cell and
Th1-polarized immunity (13,35,36). In
clinical trials of melanoma (37)
and glioblastoma (38), favorable
outcomes were observed to be related to DC1-derived IL-12p70
production and Th1-polarized immunity. Similar to previous studies
(39,40), the present study found that although
DCs matured with a different time span secreted higher levels of
IL-12p40, and secreted little bioactive IL-12p70. Therefore,
studies to further improve the capacity of DCs to produce bioactive
IL-12p70 are necessary. IL-10, known as an anti-inflammatory and
immunosuppressive cytokine, was first described as a product of Th2
cells that inhibited cytokine synthesis in Th1 cells (41). It is now known that multiple immune
cells, including macrophages, dendritic cells (DC), B cells, and
various subsets of CD4+ and CD8+ T cells, are able to produce IL-10
(42). IL-10 inhibits the capacity
of antigen-presenting cells, including DCs and macrophages, to
present antigens to T cells in various ways to modulate immune
responses (43). Recently, tumor
cell-secreted IL-10 has been demonstrated to counteract the
immunity of modified DCs in an established tumor model, which
indicated that the high level of IL-10 within tumor
microenvironment may impair DC vaccine functions (44). In the present study, DCs matured with
the standard cocktail for different time spans (24 and 48 h)
secreted minimal IL-10, which is a positive factor for DCs exerting
immune responses.
High viability is another important factor in
DC-based immunotherapy. In fact, DCs will gradually lose their
function in a few days after maturation owing to apoptosis
(17). In our present study, DCs
matured for 24 and 48 h, respectively, exhibited high viability.
The high viability may be due to the addition of PGE2 to the
cocktail as previous studies have shown that PGE2 promotes
apoptotic resistance and survival of DCs (45,46).
In conclusion, our preliminary results indicated
that 24-h stimulation is sufficient for DC maturation when using
IL-1β, IL-6, TNF-α and PGE2. Reducing the time to mature DCs in
vitro from 48 to 28 h may be beneficial for the optimal
preparation of tumor-pulsed DC therapeutic vaccine and improve its
in vivo effectiveness.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81400621)
and the Mega-projects of Science Research (grant no.
008ZX10002-008).
Glossary
Abbreviations
Abbreviations:
|
DCs
|
dendritic cells
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
GM-CSF
|
granulocyte-macrophage
colony-stimulating factor
|
|
PGE2
|
prostaglandin E2
|
|
PD-L1
|
programmed cell death ligand 1
|
References
|
1
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kushwah R and Hu J: Complexity of
dendritic cell subsets and their function in the host immune
system. Immunology. 133:409–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schraml BU and e Sousa C Reis: Defining
dendritic cells. Curr Opin Immunol. 32:13–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guermonprez P, Valladeau J, Zitvogel L,
Théry C and Amigorena S: Antigen presentation and T cell
stimulation by dendritic cells. Annu Rev Immunol. 20:621–667. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lutz MB and Schuler G: Immature,
semi-mature and fully mature dendritic cells: Which signals induce
tolerance or immunity? Trends Immunol. 23:445–449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Romani N, Gruner S, Brang D, Kampgen E,
Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM and
Schuler G: Proliferating dendritic cell progenitors in human blood.
J Exp Med. 180:83–93. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sallusto F and Lanzavecchia A: Efficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony-stimulating factor
plus interleukin 4 and downregulated by tumor necrosis factor
alpha. J Exp Med. 179:1109–1118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jonuleit H, Kühn U, Müller G, Steinbrink
K, Paragnik L, Schmitt E, Knop J and Enk AH: Pro-inflammatory
cytokines and prostaglandins induce maturation of potent
immunostimulatory dendritic cells under fetal calf serum-free
conditions. Eur J Immunol. 27:3135–3142. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng Z, Takahashi M, Narita M, Toba K,
Liu A, Furukawa T, Koike T and Aizawa Y: Generation of dendritic
cells from adherent cells of cord blood by culture with
granulocyte-macrophage colony-stimulating factor, interleukin-4,
and tumor necrosis factor-alpha. J Hematother Stem Cell Res.
9:453–464. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boullart AC, Aarntzen EH, Verdijk P,
Jacobs JF, Schuurhuis DH, Benitez-Ribas D, Schreibelt G, van de
Rakt MW, Scharenborg NM, de Boer A, et al: Maturation of
monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8
ligands combined with prostaglandin E2 results in high
interleukin-12 production and cell migration. Cancer Immunol
Immunother. 57:1589–1597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anguille S, Smits EL, Cools N, Goossens H,
Berneman ZN and Van Tendeloo VF: Short-term cultured,
interleukin-15 differentiated dendritic cells have potent
immunostimulatory properties. J Transl Med. 7:1092009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schuler-Thurner B, Schultz ES, Berger TG,
Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO,
Romani N and Schuler G: Rapid induction of tumor-specific type 1 T
helper cells in metastatic melanoma patients by vaccination with
mature, cryopreserved, peptide-loaded monocyte-derived dendritic
cells. J Exp Med. 195:1279–1288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu S, Koski GK, Faries M, Bedrosian I,
Mick R, Maeurer M, Cheever MA, Cohen PA and Czerniecki BJ: Rapid
high efficiency sensitization of CD8+ T cells to tumor antigens by
dendritic cells leads to enhanced functional avidity and direct
tumor recognition through an IL-12-dependent mechanism. J Immunol.
171:2251–2261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ellebaek E, Engell-Noerregaard L, Iversen
TZ, Froesig TM, Munir S, Hadrup SR, Andersen MH and Svane IM:
Metastatic melanoma patients treated with dendritic cell
vaccination, Interleukin-2 and metronomic cyclophosphamide: Results
from a phase II trial. Cancer Immunol Immunother. 61:1791–1804.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC,
Due-Tønnesen P, Suso EM, Sæbøe-Larssen S, Sandberg C, Brinchmann
JE, Helseth E, et al: Therapeutic vaccination against autologous
cancer stem cells with mRNA-transfected dendritic cells in patients
with glioblastoma. Cancer Immunol Immunother. 62:1499–1509. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berntsen A, Trepiakas R, Wenandy L,
Geertsen PF, Straten P thor, Andersen MH, Pedersen AE, Claesson MH,
Lorentzen T, Johansen JS and Svane IM: Therapeutic dendritic cell
vaccination of patients with metastatic renal cell carcinoma: A
clinical phase 1/2 trial. J Immunother. 31:771–780. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lanzavecchia A and Sallusto F: Regulation
of T cell immunity by dendritic cells. Cell. 106:263–266. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mellman I: Dendritic cells: Master
regulators of the immune response. Cancer Immunol Res. 1:145–149.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leverkus M, Walczak H, McLellan A, Fries
HW, Terbeck G, Bröcker EB and Kämpgen E: Maturation of dendritic
cells leads to up-regulation of cellular FLICE-inhibitory protein
and concomitant down-regulation of death ligand-mediated apoptosis.
Blood. 96:2628–2631. 2000.PubMed/NCBI
|
|
20
|
Krause P, Singer E, Darley PI,
Klebensberger J, Groettrup M and Legler DF: Prostaglandin E2 is a
key factor for monocyte-derived dendritic cell maturation: Enhanced
T cell stimulatory capacity despite IDO. J Leukoc Biol.
82:1106–1114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Young JW and Steinman RM: Accessory cell
requirements for the mixed-leukocyte reaction and polyclonal
mitogens, as studied with a new technique for enriching blood
dendritic cells. Cell Immunol. 111:167–182. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Markowicz S and Engleman EG:
Granulocyte-macrophage colony-stimulating factor promotes
differentiation and survival of human peripheral blood dendritic
cells in vitro. J Clin Invest. 85:955–961. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Li J, Wu K, Azhati B and Rexiati
M: Culture and identification of mouse bone marrow-derived
dendritic cells and their capability to induce T lymphocyte
proliferation. Med Sci Monit. 22:244–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Draube A, Klein-González N, Mattheus S,
Brillant C, Hellmich M, Engert A and von Bergwelt-Baildon M:
Dendritic cell based tumor vaccination in prostate and renal cell
cancer: A systematic review and meta-analysis. PLoS One.
6:e188012011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Vries IJ, Lesterhuis WJ, Scharenborg
NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM,
Torensma R, Adema GJ, et al: Maturation of dendritic cells is a
prerequisite for inducing immune responses in advanced melanoma
patients. Clin Cancer Res. 9:5091–5100. 2003.PubMed/NCBI
|
|
26
|
Yamanaka R, Homma J, Yajima N, Tsuchiya N,
Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M and
Tanaka R: Clinical evaluation of dendritic cell vaccination for
patients with recurrent glioma: Results of a clinical phase I/II
trial. Clin Cancer Res. 11:4160–4167. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anguille S, Smits EL, Lion E, van Tendeloo
VF and Berneman ZN: Clinical use of dendritic cells for cancer
therapy. Lancet Oncol. 15:e257–e267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cintolo JA, Datta J, Mathew SJ and
Czerniecki BJ: Dendritic cell-based vaccines: Barriers and
opportunities. Future Oncol. 8:1273–1299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Greenwald RJ, Freeman GJ and Sharpe AH:
The B7 family revisited. Annu Rev Immunol. 23:515–548. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taraban VY, Rowley TF and Al-Shamkhani A:
Cutting edge: A critical role for CD70 in CD8 T cell priming by
CD40-licensed APCs. J Immunol. 173:6542–6546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma DY and Clark EA: The role of CD40 and
CD154/CD40L in dendritic cells. Semin Immunol. 21:265–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carter L, Fouser LA, Jussif J, Fitz L,
Deng B, Wood CR, Collins M, Honjo T, Freeman GJ and Carreno BM:
PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells
and is overcome by IL-2. Eur J Immunol. 32:634–643. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selenko-Gebauer N, Majdic O, Szekeres A,
Höfler G, Guthann E, Korthäuer U, Zlabinger G, Steinberger P, Pickl
WF, Stockinger H, et al: B7-H1 (programmed death-1 ligand) on
dendritic cells is involved in the induction and maintenance of T
cell anergy. J Immunol. 170:3637–3644. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trinchieri G: Interleukin-12 and the
regulation of innate resistance and adaptive immunity. Nat Rev
Immunol. 3:133–146. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Strioga MM, Felzmann T, Powell DJ Jr,
Ostapenko V, Dobrovolskiene NT, Matuskova M, Michalek J and Schijns
VE: Therapeutic dendritic cell-based cancer vaccines: The state of
the art. Crit Rev Immunol. 33:489–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carreno BM, Becker-Hapak M, Huang A, Chan
M, Alyasiry A, Lie WR, Aft RL, Cornelius LA, Trinkaus KM and
Linette GP: IL-12p70-producing patient DC vaccine elicits
Tc1-polarized immunity. J Clin Invest. 123:3383–3394. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okada H, Kalinski P, Ueda R, Hoji A,
Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK,
et al: Induction of CD8+ T-cell responses against novel
glioma-associated antigen peptides and clinical activity by
vaccinations with {alpha}-type 1 polarized dendritic cells and
polyinosinic-polycytidylic acid stabilized by lysine and
carboxymethylcellulose in patients with recurrent malignant glioma.
J Clin Oncol. 29:330–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lee AW, Truong T, Bickham K, Fonteneau JF,
Larsson M, Da Silva I, Somersan S, Thomas EK and Bhardwaj N: A
clinical grade cocktail of cytokines and PGE2 results in uniform
maturation of human monocyte-derived dendritic cells: Implications
for immunotherapy. Vaccine. 20 Suppl 4:A8–A22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Trepiakas R, Pedersen AE, Met O, Hansen
MH, Berntsen A and Svane IM: Comparison of alpha-Type-1 polarizing
and standard dendritic cell cytokine cocktail for maturation of
therapeutic monocyte-derived dendritic cell preparations from
cancer patients. Vaccine. 26:2824–2832. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fiorentino DF, Bond MW and Mosmann TR: Two
types of mouse T helper cell. IV. Th2 clones secrete a factor that
inhibits cytokine production by Th1 clones. J Exp Med.
170:2081–2095. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Couper KN, Blount DG and Riley EM: IL-10:
The master regulator of immunity to infection. J Immunol.
180:5771–5777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mittal SK and Roche PA: Suppression of
antigen presentation by IL-10. Curr Opin Immunol. 34:22–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song S, Wang Y, Wang J, Lian W, Liu S,
Zhang Z, Liu F and Wei L: Tumour-derived IL-10 within tumour
microenvironment represses the antitumour immunity of
Socs1-silenced and sustained antigen expressing DCs. Eur J Cancer.
48:2252–2259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vassiliou E, Sharma V, Jing H, Sheibanie F
and Ganea D: Prostaglandin E2 promotes the survival of bone
marrow-derived dendritic cells. J Immunol. 173:6955–6964. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baratelli F, Krysan K, Heuzé-Vourc'h N,
Zhu L, Escuadro B, Sharma S, Reckamp K, Dohadwala M and Dubinett
SM: PGE2 confers survivin-dependent apoptosis resistance in human
monocyte-derived dendritic cells. J Leukoc Biol. 78:555–564. 2005.
View Article : Google Scholar : PubMed/NCBI
|