Introduction
Abdominal aortic aneurysm (AAA) is defined as a
permanent and irreversible dilation of the abdominal aorta
(1). Age, male sex, personal history
of atherosclerotic cardiovascular disease, smoking and hypertension
are all associated with the occurrence of AAA. AAA accounts for
~15,000 deaths in the USA per year (2). In addition, 30–40% of patients with
ruptured AAA die without surgery (3–5). The
operative mortality rate of AAA is 40–50% (3–6),
however, the overall mortality of AAA rupture is 80–90%.
Since the first stent graft system designed for
endovascular aortic repair (EVAR) of AAA was approved by the US
Food and Drug Administration in 1999, EVAR has been a major
treatment for AAA (6). Compared to
traditional open surgery, EVAR has advantages, including lower
blood loss, less pain, shorter hospitalization, more rapid recovery
as well as lower morbidity and mortality (7). Its advantage is obvious, particularly
in patients with early-stage AAA. However, complications such as
rupture, endoleak, device migration and limb graft occlusion are
usually found after EVAR (2–4,7,8). In addition, patients receiving EVAR
have been reported to have high rates of re-intervention (8).
Limb graft occlusion is not rare and represents a
serious complication of EVAR. It is the third most common reason
for readmission after EVAR. The present study retrospectively
evaluated the incidence, causes, and methods of treatment and
prevention of limb graft occlusion following EVAR encountered at
our department.
Patients and methods
Patient data
A total of 66 patients who underwent EVAR at the
Department of Vascular and Endovascular Surgery (Henan Provincial
People's Hospital, Zhengzhou, China) from January 2005 to December
2013 were enrolled in the present study. Clinical data, including
computer tomographic angiography (CTA) data, digital subtraction
angiography (DSA) data as well as information on operative
management and outcome were collected and retrospectively analyzed.
Based on CTA, the standards of EVAR were as follows: i) An aneurysm
diameter of >50 mm; ii) an aneurysm diameter of <50 mm but
fulfillment of the following characteristics: a) An increase in the
aneurysm diameter of >5 mm over the last year; b) irregular
shape of the aneurysm associated with a high risk of rupture; c)
abdominal discomfort associated with the aneurysm; d) large amounts
of thrombosis in the aneurysm with a high risk of embolism. In
total, 61 bifurcated grafts and 5 aortauniilac (AUI) grafts (127
limbs in total) were assessed. Prior written and informed consent
was obtained from each patient and the study was approved by the
Ethics Review Board of Henan Provincial People's Hospital
(Zhengzhou, China).
Grafts
Talent, endurant and AUI grafts were purchased from
Medtronic (Medtronic Inc., Minneapolis, MN, USA). The Zenith graft
was from Cook Medical Inc. (Bloomington, IN, USA). The Wallstent
graft was from Boston Scientific Corp. (Boston, MA, USA).
EVAR treatment
After general anesthesia and bilateral inguinal
oblique incision, small and sub-centimeter incisions were made over
the femoral artery. Placement of a 6F femoral artery sheath was
performed. The 5F gold-labeled pigtail catheter was inserted into
the femoral artery, which was connected with a high-pressure
syringe and ventilator. Systemic heparinization was performed. A 4F
hunter catheter was then inserted and a Cook Lunderquist guide wire
(Cook Medical Inc.) was inserted into the femoral artery. A bracket
was guided by the wire and once the bracket was completely
released, the conveying system was carefully recycled into the
sheath and retreated to the bracket. A high-pressure syringe was
then connected and aortography was performed to compare the
conditions with those before stent implantation. The delivery
sheath was pulled out. Medtronic compliant balloon (AB46) expansion
of the stent ends and the interface part of the exit site was
performed. Angiography was performed once again when the bracket
was in a good position and state without obvious internal leakage.
After delivery, the guide wire and sheath were removed and the
wound was closed. Subsequent to surgery, the general condition of
the patients was monitored.
Post-operative treatment and
follow-up
Antiplatelet treatment was performed in all of the
66 patients during routine review after EVAR and heparin was used
in the case of thrombosis. The antiplatelet treatment was performed
for 12 months by oral administration of 75 mg PLAVIX (Sanofi
Winthrop S.A., Paris, France) daily for 6 months and 100 mg Aspirin
(Bayer, Leverkusen, Germany) daily for another 6 months. At 10
days, 3, 6 and 12 months and annually thereafter, duplex
ultrasonography or CTA was performed. Readmission was suggested if
the patient complained of any discomfort, particularly when
abdominal or limb symptoms appeared. Review examinations, including
physical examination, ankle-brachial index, CTA and if necessary
DSA were performed in patients with limb ischemia.
Statistical analysis
All statistical analyses were performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA) for Windows. Values are
expressed as the mean ± standard deviation. An independent-samples
t-test was performed for comparison between the two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Incidence of limb graft occlusion
following EVAR
To analyze the incidence of limb graft occlusion
following EVAR, the patients were followed up. As shown in Tables I and II, a total of 7 limbs in 7 patients were
occluded between 20 days and 12 months (average, 7.8±5.3 months)
after EVAR. The total incidence was 10.6% (7/66) in the patients
and 5.5% (7/127) in the limbs. Among the 7 patients, 6 bifurcated
grafts and 1 AUI graft were used and 5 right-limb grafts and 2
left-limb grafts were applied (Tables
I and II). For Patients with
limb graft occlusion, the follow-up time ranged from 1–38 months,
with an average follow-up time of 24.2±16.6 months. The time of the
appearance of limb occlusion showed a great variation, with the
shortest being 20 days in 1 case, 12 months in 4 cases and 3 months
in 2 cases. The clinical manifestation also varied within the
cohort; one case was asymptomatic, acute and severe ischemia was
found in 2 cases, claudication was found in 3 cases and asthenia
was found in 1 case. Taken together, these results showed that limb
graft occlusion following EVAR was not rare.
 | Table I.General information on the 7 cases of
limb graft occlusion following EVAR. |
Table I.
General information on the 7 cases of
limb graft occlusion following EVAR.
| Case no. | Sex | Age (years) | Aneurysm | Graft company | Graft type | Time of occlusion
after EVAR |
|---|
| 1 | Male | 64 | T | Medtronic | Bifurcated | 3 months |
| 2 | Male | 62 | T | Medtronic | Bifurcated | 12 months |
| 3 | Male | 77 | T | Medtronic | Bifurcated | 3 months |
| 4 | Male | 29 | F | Medtronic | Bifurcated | 12 months |
| 5 | Male | 76 | T | Medtronic | AUI | 20 days |
| 6 | Male | 43 | T | Cook | Bifurcated | 12 months |
| 7 | Female | 80 | T | Cook | Bifurcated | 12 months |
 | Table II.General information on the 7 cases
with limb graft occlusion following endovascular aortic repair. |
Table II.
General information on the 7 cases
with limb graft occlusion following endovascular aortic repair.
| Case no. | Symptom | Location of endograft
leg occlusion | Cause | Treatment | Outcome |
|---|
| 1 | Claudication | Right | D | Stenting and bypass;
routine antiplatelet therapy | Smooth blood
flow |
| 2 | Claudication | Left | A | Bypass; routine
antiplatelet therapy | Smooth blood
flow |
| 3 | Asymptomatic | Right | A | Conservative; routine
antiplatelet therapy | Non-aggravating |
| 4 | Asthenia | Right | D | Bypass; routine
antiplatelet therapy | Smooth blood
flow |
| 5 | Acute ischemia | Right | A, D | Thrombectomy; routine
antiplatelet therapy | Smooth blood
flow |
| 6 | Acute ischemia | Right | D | Thrombectomy;
angioplasty and stenting; routine antiplatelet therapy | Smooth blood
flow |
| 7 | Claudication | Left | A | Thrombectomy;
angioplasty and stenting; routine antiplatelet therapy | Smooth blood
flow |
Causes of limb graft occlusion
following EVAR
To investigate the causes of limb graft occlusion
following EVAR, clinical data of each patient were analyzed.
Anatomical factors were found in 3 patients. One case was a
77-year-old man with no symptoms. Regular examination was performed
at 3 months after EVAR and the pulse of the right femoral and
popliteal arteries was weak. CTA indicated that the right leg graft
was occluded. Another case was a 62-year-old man with left leg
graft occlusion occurring at 12 months after EVAR. The other case
was a 80-year-old female whose left leg graft was occluded because
of calcification and tortuousness of the iliac artery. This
occlusion was also found at 12 months after EVAR.
Device factors were found in 3 patients. One was a
29-year-old man who had a false aneurysm due to trauma. Due to the
small diameter of the abdominal aorta, a bifurcated stent graft was
unsuitable for him and ‘AUI and femoral-femoral bypass’ was
performed instead. At 6 months after EVAR, he complained of
asthenia in both legs; however, no obvious signs were detected by
ABI or CTA. At 12 months after EVAR, the symptoms had not
aggravated, but CTA examination indicated that AUI and artificial
graft were filled with thrombus. Another case was a 43-year-old man
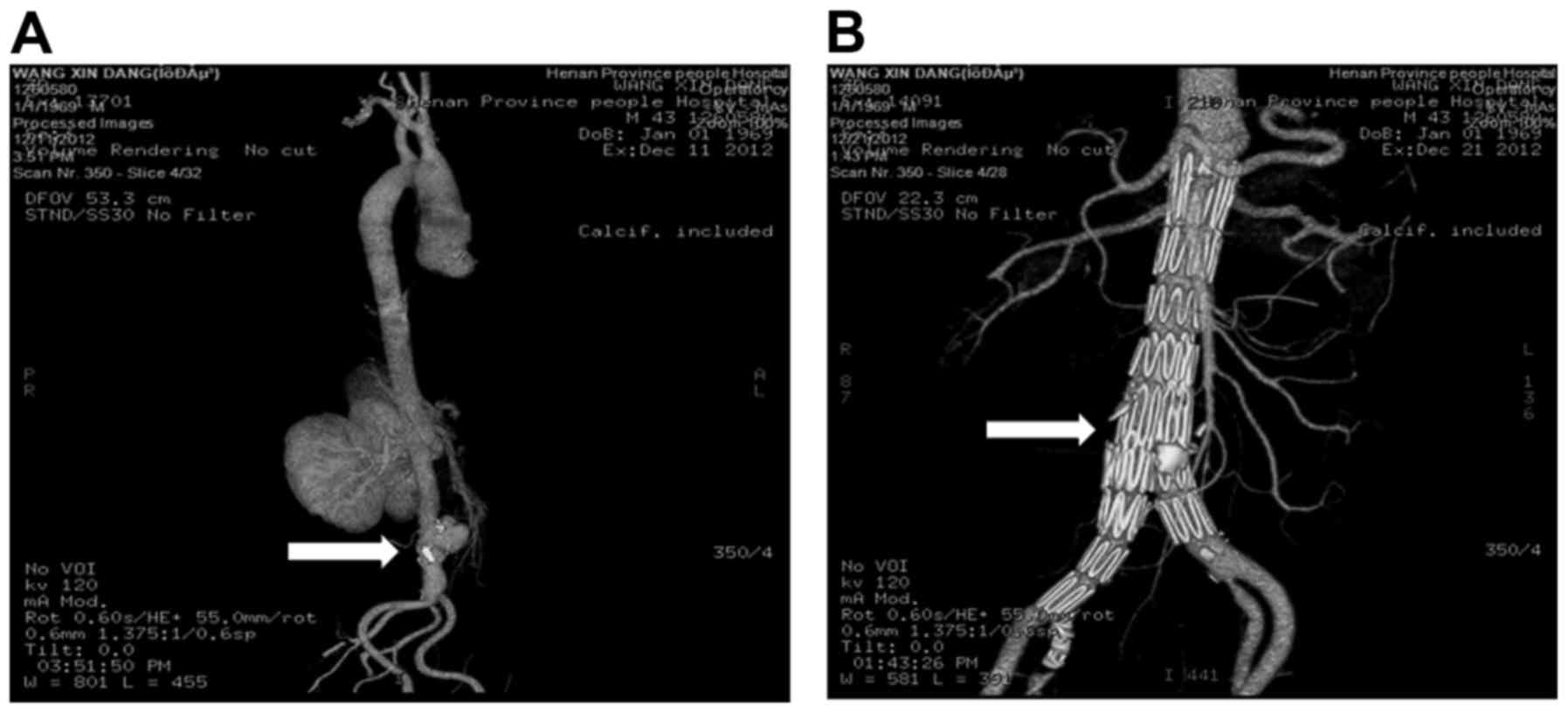
(Figs. 1–4). Aorta CTA confirms the formation of
abdominal aortic pseudoaneurysm (Fig.
1A). After detailed evaluation, EVAR surgery was performed. At
10 days after endovascular aortic repair, the pseudoaneurysm was
observed to be completed eradicated (Fig. 1B). After 12 months, the right lower
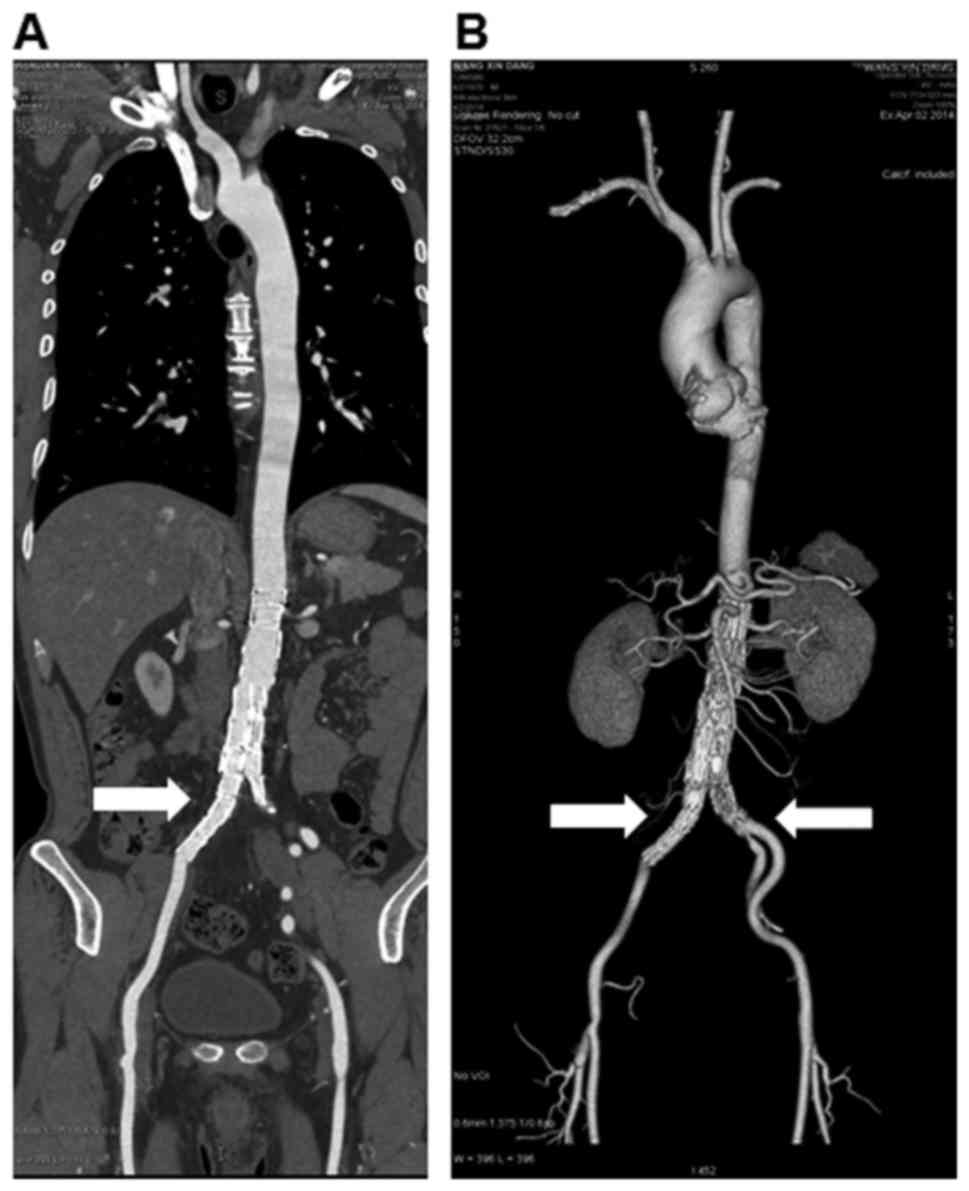
leg was cold with limited activity. The emergency CTA suggested
occlusion in the right iliac artery (Fig. 2A). The MIP indicated a right iliac
artery occlusion (Fig. 2B). Combined
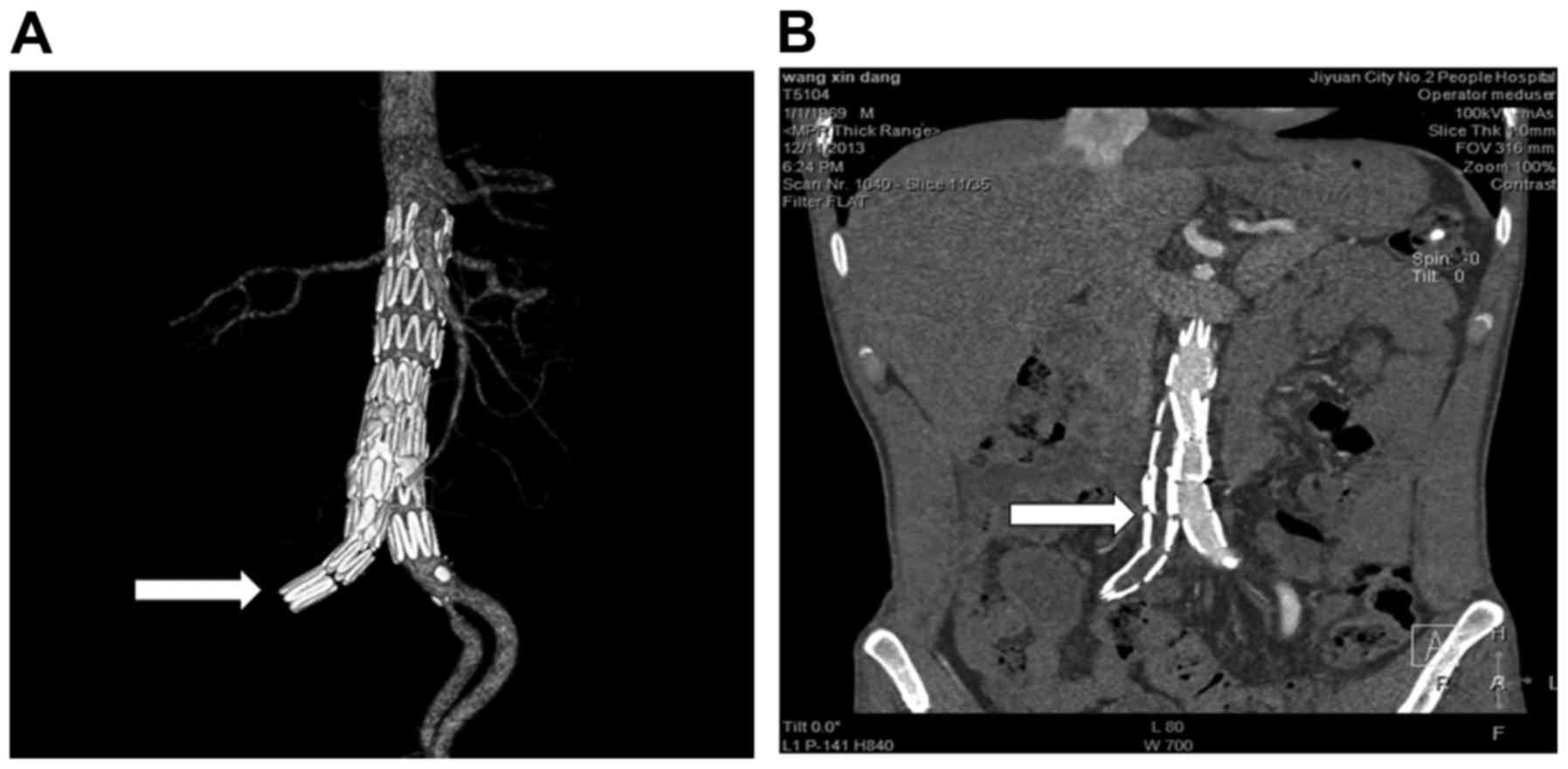
balloon dilatation and stent implantation was conducted. At 10 days
after reoperation, blood flow in the right iliac artery was
restored (Fig. 3A) and stent grafts
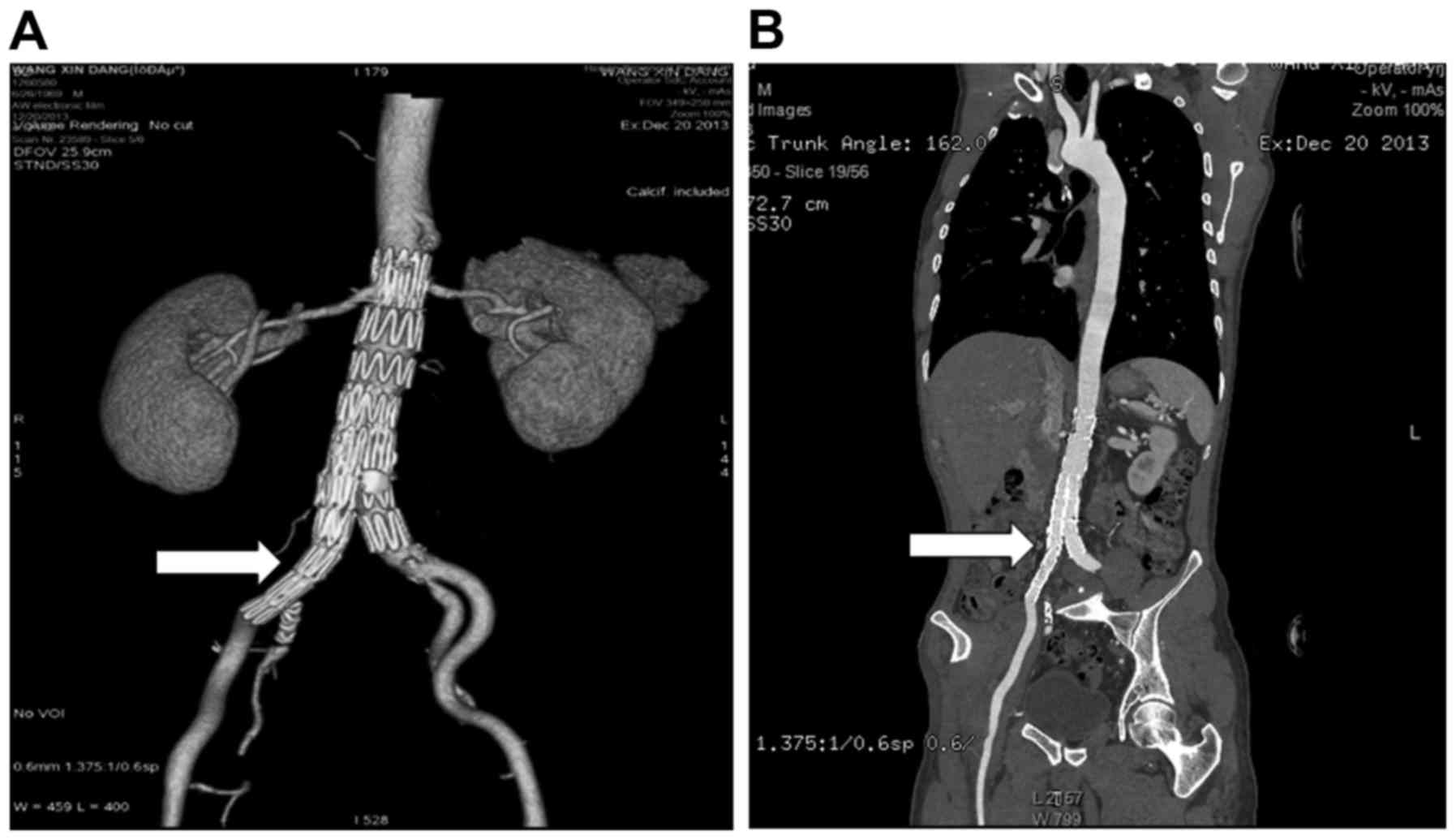
were well positioned and well shaped (Fig. 3B). The patient was examined by CTA 16
weeks after reoperation. The right iliac artery was restored
(Fig. 4A) and stent grafts were well
positioned and well shaped (Fig.
4B). The right leg was occluded at 12 months after EVAR. DSA
revealed a fibro cap at the exit of the stent graft. Meanwhile, the
right leg graft partly covered the left leg graft and affected the
blood flow of the left leg.
Occlusion due to anatomic as well as device factors
was found in a 76-year-old man. The right leg graft extended to the
external iliac artery and he suffered acute and severe ischemia in
the right leg at 20 days after EVAR. Symptoms of pallor, pain and
weak pulse were present in the right leg, whereas the left leg was
normal. An urgent angiography revealed that there was no blood flow
in the right leg graft. In summary, the causes of limb graft
occlusion following EVAR included anatomic factors, device factors
or their combination.
Treatment of limb graft occlusion
following EVAR
To identify the effects of various treatment methods
for limb graft occlusion following EVAR, the methods used and the
prognosis of the patients were analyzed. The thrombectomy,
aorto-right common iliac/left femoral artery bifurcated artificial
graft bypass, femoral-femoral artificial graft bypass,
femoral-femoral artery bypass and stent, and conservative treatment
were used in 1 case and thrombectomy together with a stent was used
in 2 cases. All patients except for the patient who received
conservative treatment had a good outcome. None of the patients
died and no amputation due to limb graft occlusion was required. In
conclusion, the results indicated that with appropriate treatment,
limb graft occlusion following EVAR has a favorable prognosis.
Discussion
Limb graft occlusion is not a rare but serious
complication following EVAR. Woody and Makaroun (9) reported that it is the third most common
reason for readmission after EVAR. Sivamurthy et al
(10) retrospectively reviewed 248
patients who underwent EVAR from 1999 to 2004, including 13
instances of limb thrombosis in 13 patients with a total incidence
of 5.2% in patients and 2.7% in limbs. Cochennec et al
(11) reviewed 460 AAA patients who
were selectively treated with a variety of commercially available
stent grafts from 1995 to 2005. They used 369 bifurcated and 91 AUI
grafts (829 limbs) in their study and found that 36 limbs in 33
patients (in 7.2% of patients and 4.3% of limbs) were occluded. The
total incidence in the present study was 10.6% in patients and 5.5%
in limbs, which was consistent with the results of previous
studies.
Limb graft occlusion following EVAR is a disease
caused by numerous factors, which may be divided into 3 major
categories. The first is anatomic factors, the second is device
(graft) factors and the final one contains the two aspects
combined. Anatomic factors includes small artery size (particularly
for female patients), aneurysm angulation of ≥60°, calcified and
narrowed aortic bifurcation, tortuous iliac artery and iliac artery
dissection. According to Woody and Makaroun (9), these anatomic characteristics led to
kinking of the iliac limb of the endograft. Extrinsic compression
of the endograft due to a significant thrombus load within the
aneurysm sac may lead to stenosis of the proximal portion of the
endograft limb. Likewise, a narrow aortic bifurcation may compress
the iliac graft limbs. In the present study 3 limbs in 3 patients
were occluded due to anatomic factors.
Carpenter et al (12) reported that each case of limb
thrombosis in their study was due to a mechanical cause. Device
factors included graft migration, insufficient graft size and low
radial force. Graft migration is a rare but serious complication,
and in the present study, a 64-year-old man who underwent EVAR 4
months previously was diagnosed with a ruptured AAA. During the
operation, it was found that the limb graft was incomplete and
another type of graft (Fluency; Bard Peripheral Vascular, Inc.,
Tempe, AZ, USA) was selected for him. One month after EVAR, CTA
showed that the graft was fluent; however, 3 month after EVAR,
claudication was found in his right leg. CTA indicated that the
right limb graft was occluded. During the operation, it was found
that the wire was not able to pass through the occluded graft and
that the limb graft had migrated from the main body. As a result, a
femoral-femoral artificial bypass was performed. Grafts lack
sufficient support with a weak radial force being a particularly
significant cause and kinking is common under these circumstances.
Previous studies have also described this problem (13). Excessive oversizing of the graft may
lead to infolding of the graft material within the graft lumen
(14,15).
Finally, twisting of the graft limbs may occur. This
is associated with anatomic factors as well as with characteristics
of the endograft delivery system. Twisting of grafted limbs narrows
the lumen and predisposes to thrombosis. It has been reported that
a graft extending into the external iliac artery is a high risk
factor for occlusion (16). When the
graft extends into the external iliac artery, the likelihood of
kinking is higher. Under these circumstances, the blood flow is
decreased.
Treatment can be divided into 4 aspects:
Endovascular, surgery, hybrid and conservative treatment. Certain
clinicians prefer endovascular treatment, insist that thrombosis
preserves the original shape of grafts and successfully implement
angiography or stenting (17).
However, artificial graft bypass is considered the first choice of
treatment for others. One important reason for the preference of
extra-anatomic bypass is that revascularization may prevent
embolization of the hypogastric artery secondary to thrombectomy or
thrombolysis. According to our experience, thrombolysis or
thrombectomy with adjunctive stenting is an effective treatment
method for limb graft occlusion, as thrombectomy combined with
stent implantation effectively addresses iliac artery stenosis
caused by stent displacement.
The best treatment method for limb graft occlusion
is prevention. It is better to select an individual therapeutic
schedule and an appropriate graft for individual patients at the
preoperative stage. According to previous clinical experiences,
antiplatelet therapy was observed to effectively reduce the
incidence of iliac occlusion after EVAR. Therefore, antiplatelet
therapy was performed in all patients of the current study for 12
months. For female patients or those with a small aortic size, open
surgery may be more suitable if no appropriate stent-graft is
available. The modalities of the operation are undoubtedly key to
preventing limb graft occlusion. During the final steps of the
operation, balloon dilation may be re-performed if angiography
reveals that the graft shows kinking or twisting. If the shape and
blood flow remain unsatisfactory, stenting is necessary. Sivamurthy
et al (10) used adjunctive
primary stenting during EVAR of lower limb graft occlusion, which
was efficient and safe. Kissing balloon during angiography is
another technique for preventing a previously normal contralateral
limb graft from being compromised. However, single-plane
angiography is inadequate for angiography. Multiplanar angiography
provides further details and may reveal limb problems.
Intra-vascular ultrasonography may provide valuable information, as
it may detect graft redundancy and the resultant infolding of graft
fabric into the lumen that is frequently undetected by angiography.
At present, EVAR is followed up at 1, 6 and 12 months, and yearly
thereafter. Sivamurthy et al (10) reported that most occlusions occur
within 6 months after endovascular AAA repair. Thus, the current
routine follow-up regimen appears to only have a small impact in
the prevention of limb thrombosis. In most cases, graft thrombosis
occurs within 6 months after stent-graft insertion and the patients
only undergo one routine imaging at 1 month. In the present study,
60% of occlusions occurred within 6 months and a different
follow-up schedule of 10 days, 3, 6 and 12 months and once annually
thereafter was employed. Furthermore, clinical experience of
authors of the present study suggests that routine antiplatelet
treatment after EVAR is a good preventive measure, particularly for
patients with peripheral artery disease.
References
|
1
|
Scali ST, Feezor RJ, Huber TS and Beck AW:
Acute bilateral renal artery chimney stent thrombosis after
endovascular repair of a juxtarenal abdominal aortic aneurysm. J
Vasc Surg. 61:1058–1061. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lim S, Halandras PM, Park T, Lee Y,
Crisostomo P, Hershberger R, Aulivola B and Cho JS: Outcomes of
endovascular abdominal aortic aneurysm repair in high-risk
patients. J Vasc Surg. 61:862–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Joannic D, Delassus P, Lalande A,
Juillion P and Fontaine JF: Comparison of the strain field of
abdominal aortic aneurysm measured by magnetic resonance imaging
and stereovision: A feasibility study for prediction of the risk of
rupture of aortic abdominal aneurysm. J Biomech. 48:1158–1164.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Törnqvist P, Dias N, Sonesson B,
Kristmundsson T and Resch T: Intra-operative cone beam computed
tomography can help avoid reinterventions and reduce CT follow up
after infrarenal EVAR. Eur J Vasc Endovasc Surg. 49:390–395. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Troisi N and Torsello G: Commentary:
New-generation devices and adjunctive procedures are the key
elements to expanding the indications for endovascular aneurysm
repair. J Endovasc Ther. 22:179–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
You JH, Park CB, Park HK, Jin ES and Kim
CJ: Endovascular repair of graft limb occlusion after endovascular
repair for abdominal aortic aneurysm using 0.014-inch guidewire and
coronary balloon. Int J Cardiol. 153:e37–e38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alder L, Al-Jarrah Q, Rahi MA, Wilde N and
Al-Khaffaf H: Percutaneous catheter-directed thrombolysis for
treatment of complete body and bilateral limb endovascular aortic
graft occlusion. EJVES Extra. 24:e37–e38. 2012. View Article : Google Scholar
|
|
8
|
Liaw JV, Clark M, Gibbs R, Jenkins M,
Cheshire N and Hamady M: Update: Complications and management of
infrarenal EVAR. Eur J Radiol. 71:541–551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Woody JD and Makaroun MS: Endovascular
graft limb occlusion. Semin Vasc Surg. 17:262–267. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sivamurthy N, Schneider DB, Reilly LM,
Rapp JH, Skovobogatyy H and Chuter TA: Adjunctive primary stenting
of Zenith endograft limbs during endovascular abdominal aortic
aneurysm repair: Implications for limb patency. J Vasc Surg.
43:662–670. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cochennec F, Becquemin JP, Desgranges P,
Allaire E, Kobeiter H and Roudot-Thoraval F: Limb graft occlusion
following EVAR: Clinical pattern, outcomes and predictive factors
of occurrence. Eur J Vasc Endovasc Surg. 34:59–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carpenter JP, Anderson WN, Brewster DC,
Kwolek C, Makaroun M, Martin J, McCann R, McKinsey J and Beebe HG:
Lifepath Investigators: Multicenter pivotal trial results of the
Lifepath System for endovascular aortic aneurysm repair. J Vasc
Surg. 39:34–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Erzurum VZ, Sampram ES, Sarac TP, Lyden
SP, Clair DG, Greenberg RK, Ohara PJ, Kashyap VS and Ouriel K:
Initial management and outcome of aortic endograft limb occlusion.
J Vasc Surg. 40:419–423. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Becquemin JP, Kelley L, Zubilewicz T,
Desgranges P, Lapeyre M and Kobeiter H: Outcomes of secondary
interventions after abdominal aortic aneurysm endovascular repair.
J Vasc Surg. 39:298–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wales L, Dunckley M, Bohm N, Kwok T,
Bratby M, Morgan R, Thompson M and Loftus I: Device-specific
outcomes following endovascular aortic aneurysm repair. Eur J Vasc
Endovasc Surg. 36:661–667. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maldonado TS, Rockman CB, Riles E, Douglas
D, Adelman MA, Jacobowitz GR, Gagne PJ, Nalbandian MN, Cayne NS,
Lamparello PJ, et al: Ischemic complications after endovascular
abdominal aortic aneurysm repair. J Vasc Surg. 40:703–710. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Becquemin JP, Allaire E, Desgranges P and
Kobeiter H: Delayed complications following EVAR. Tech Vasc Interv
Radiol. 8:30–40. 2005. View Article : Google Scholar : PubMed/NCBI
|