Introduction
Hepatocellular carcinoma (HCC), which is one of the
most common and fatal tumor types in the world, has a poor
prognosis and is responsible for numerous cases of
cancer-associated mortality (1).
Hepatitis B virus (HBV) and HCV are generally considered to be risk
factors for HCC and jointly account for its most common etiology
(2). Furthermore, it has been
suggested that HCC arising from HBV or HCV infection has an
increased mortality rate, although the exact underlying mechanisms
of virus-mediated hepatocarcinogenesis have remained elusive
(3,4).
Polypyrimidine tract-binding protein-associated
splicing factor (PSF) is an abundant ubiquitous nuclear protein
that serves critical regulatory roles in vertebrates (5,6). One of
its primary roles is as a component of spliceosomes and it is
mediated by its RNA-binding domains (RBD) (7). In addition, PSF has been identified as
a transcriptional regulator of the gene encoding for P450-linked
side chain cleaving enzyme, which initiates the steroidogenic
pathway, and binds to its regulatory region through its DNA-binding
domain (DBD) (8,9). The RBD and DBD function independently
and their novel mechanisms for reversible gene transcription
mediated by PSF protein and noncoding RNA have been investigated.
It has been demonstrated that their transcription is activated by
the release of PSF protein from the repressed proto-oncogene G
antigen 6 (GAGE6) by five PSF-binding noncoding RNA fragments
(10). This indicates that the
regulatory activity of noncoding RNA in tumorigenesis occurs
through its binding to the RBD of PSF and the consequent release of
the transcriptionally repressed proto-oncogene from its DBD.
Long interspersed nuclear element 1 (LINE-1; L1) is
a repetitive element, which constitutes 17–25% of the human genome,
and comprises a 5′-untranslated region (UTR), two open-reading
frames and a 3′-UTR (11). The
genome contains numerous copies of L1s, which have often been
referred to as junk DNA sequences, as their function was unknown.
However, it has been revealed that L1s serve important
transcriptional regulatory functions, which is tuned by changes in
their methylation status (12).
Hypomethylation in the promoter region of L1 leads to
transcriptional activation of the L1 element, causing transposition
of the retro-element and chromosomal alteration (13). This indicates that physiological
processes are regulated by the amount of L1 RNA expression;
however, the exact mechanism has remained elusive.
It has been demonstrated that hypomethylation of L1
is associated with HBV infection, a larger tumor size and a more
advanced disease stage (14).
Pyrosequencing analysis of the methylation level of L1 revealed
that increased hypomethylation of L1 is associated with an
increased risk of developing HCC (15). Therefore, the present study aimed to
assess the interaction between L1 RNA and PSF protein. Lau et
al (16) have previously
indicated that transcripts of the genome integration sites of
viral-human fusion genes which function as hybrid RNAs with
tumor-promoting properties. However, it has remained elusive
whether L1 RNA affects the processes of tumorigenesis in HCC
without HBV infection or of carcinogenesis in general, including
lung carcinogenesis.
The aim of the present study was to investigate
whether, apart from the chimeric HBx-LINE-1 transcript,
interactions between L1 RNA and PSF protein and the changes
occurring in the regulatory activities of PSF have a role in lung
carcinogenesis. The binding of L1 RNA to the RBD of PSF, the
release of the DNA promoter region of GAGE6, and the effects on the
proliferation of the A549 lung cancer and the 16HBE normal
bronchial cell lines were assessed in order to identify the
potential role of L1 RNA in these processes.
Materials and methods
Cell lines
The A549 human non-small cell lung carcinoma cell
line, the 16HBE human bronchial epithelial cell line and the 293
human embryonic kidney (HEK293) cell line were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and all cells were obtained
from Dr Xu Song (Department of Center for Functional Genomics and
Bioinformatics, College of Life Science, Sichuan University,
Chengdu, China). All cells were supplemented with 10% (v/v) fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin in an atmosphere containing 5%
CO2 at 37°C.
Construction of plasmid encoding L1
RNA
The L1 complementary (c) DNA fragment was amplified
by polymerase chain reaction (PCR) using the genome of A549 cells
as a template and primers were designed to generate HindIII
and XbaI restriction sites at the 5′ and 3′ ends of the
amplified fragments, respectively. The primer sequences used were
as follows: L1 forward, 5′-CCCAAGCTTGTGTTGTTGAGGATGTGAAG-3′ and
reverse, 5′-TGCTCTAGATCCTGTGATTGATTTATTTTACTTA-3′ [Beijing Genomics
Institute (BGI), Beijing, China]. The PCR mixture contained forward
and reverse primers (0.2 µM), DNA template (1 ng/µl), dNTP, Taq
polymerase and PCR buffer (primeSTAR; Takara Bio., Inc., Otsu,
Japan). The PCR procedure consisted of 34 cycles of denaturation at
95°C for 10 sec, annealing at 55°C for 10 sec, and extension at
72°C for 30 sec, with initial denaturation of template DNA at 95°C
for 5 min. The amplified L1 cDNA was subsequently digested with
HindIII and XbaI (Takara Bio, Inc.) and cloned into
multiple cloning sites of pcDNA3.1 (+) (Thermo Fisher Scientific,
Inc.).
Stable transfection of cell lines
The A549-L1 and 16HBE-L1 cell lines were constructed
by stably transfecting plasmid pcDNA3.1-L1 encoding the 380-nt L1
RNA fragment into A549 or 16HBE cells. The A549-vector or
16HBE-vector cell lines were constructed by transfecting the empty
vector, pcDNA3.1, into A549 or 16HBE cells. All transfections were
performed using the transfection reagent Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's instructions. After 2 days of incubation, stably
transfected A549 or 16HBE cells were selected with 450 or 900 g/ml
geneticin (G418; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany), respectively. Following selection, cells were maintained
with half-doses of G418. The expression levels of target genes were
analyzed using reverse transcription-quantitative PCR
(RT-qPCR).
RNA extraction and RT-qPCR
TRIzol reagent (Thermo Fisher Scientific, Inc.) was
used to isolate total RNA from cells according to the
manufacturer's instructions. A sample of 500 ng RNA was reverse
transcribed into cDNA in a 10-µl reaction volume using M-MLV
reverse transcriptase reagent (Vazyme Biotech Co., Ltd., Nanjing,
China) and qPCR was performed using 0.25 µl cDNA and SsoFast
EvaGreen™ Supermix (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
on a Bio-Rad CFX96 Real-Time PCR detection system (Bio-Rad
Laboratories, Inc.). Data were analyzed using Bio-Rad CFX 2.1
Manager software (Bio-Rad Laboratories, Inc.) and GAPDH gene
expression was used as an internal reference. Data was normalized
using the 2−ΔΔCq method (17). The forward and reverse primers used
for PCR were as follows: GAGE6 forward,
5′-GCCTCCTGAAGTGATTGGGCCTA-3′ and reverse,
5′-CAGGCGTTTTCACCTCCTCTGGA-3′; PSF forward,
5′-ATGTCTCGGGATCGGTTCCGGA-3′ and reverse,
5′-CCAACAAACAACCGACATCGCTG-3′; L1 forward,
5′-TGAAGTAAAGAAAACCCTTGCCT-3′ and reverse,
5′-TCTTGGTCATTGTGAATAGTGCT-3′; and GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′.
All primers were supplied by BGI. The thermocycling conditions were
1 min at 95°C, followed by 40 cycles of 95°C for 10 sec, 60°C for
30 sec and 72°C for 20 sec.
Purification of His-tagged
proteins
Construction of a prokaryotic expression vector
carrying the intact structural gene of human PSF protein was
amplified by PCR, inserted into the plasmid pET-28a and expressed
in Escherichia coli (E. coli) BL21 cells (Vazyme
Biotech Co., Ltd.). The DBD, RBD-1 and RBD-2 of the PSF protein
gene were inserted into the plasmid pET-42a and expressed in E.
coli BL21 cells (Vazyme Biotech Co., Ltd.). Cells were cultured
in LB medium at 37°C with shaking at 200 rpm until the inoculum
optical density at 600 nm reached 0.6, and then shaken overnight at
25°C and 100 rpm in the presence of 0.1 mM isopropyl
β-D-1-thiogalactopyranoside. Proteins were purified using
Ni-nitrilotriacetic acid agarose (Qiagen, Inc., Valencia, CA,
USA).
Electrophoretic mobility shift assays
(EMSA)
The RNA probe was transcribed in vitro using
T7 RNA polymerase (Promega Corp., Madison, WI, USA) from the PCR
product containing the T7 RNA polymerase promoter. The 61-bp DNA
probe was the PSF binding motif in the GAGE6 promoter (18) and was synthesized with the following
sequence:
5′-GCCTTCTGCAAAGAAGTCTTGCGCATCTTTTGTGAAGTTTATTTCTAGCTTTTTGATGCTG-3′,
and was synthesized by BGI. The RNA or DNA probe was biotin-labeled
using an RNA 3′ End Biotinylation kit (Pierce; Thermo Fisher
Scientific, Inc.) or a Biotin 3′ End DNA labeling kit (Pierce;
Thermo Fisher Scientific, Inc.), respectively. A total of 5 ng
biotin-labeled RNA or DNA fragments were mixed with 100–500 ng
recombinant PSF protein, DBD of PSF, RBD-1 of PSF, RBD-2 of PSF or
the total protein of E. coli lysates. EMSA was performed
using a LightShift Chemiluminescent EMSA kit (Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions.
Cell proliferation assay
A total of 5×103 cells per well were
cultured in a 12-well plate at 37°C as attached monolayers in DMEM
with 10% FBS. Each day, the cell numbers in three wells were
counted with a hemocytometer following detachment with trypsin.
Each test had two replicates (n=3).
For the 5-ethynyl-2′-deoxyuridine (EdU) assay,
~4×103 cells per well were grown in a 96-well dish and
processed with the EdU labeling kit from RiboBio Co., Ltd.
(Guangzhou, China).
Soft-agar colony assay
A total of 1×103 cells were suspended in
1 ml 0.3% BD Difco™ Agar (BD Biosciences, Franklin Lakes, NJ, USA)
in DMEM with 10% FBS and seeded in each well of 6-well plates
containing 1 ml of a solidified layer of 0.6% agar in the same
medium. Each test had two replicates (n=3). Following 21 days of
incubation, 100 µl 1.5 mM nitro blue tetrazolium in
phosphate-buffered saline (PBS) was added to the dishes and after 4
h, the colonies were counted and images were captured with an Epson
4990 scanner.
Chromatin immunoprecipitation (ChIP)
and RNA immunoprecipitation (RIP) assays
ChIP assays were performed using a ChIP assay kit
(Upstate Biotechnology, Inc., Lake Placid, NY, USA) according to
the manufacturer's protocol. Cells were cultured in 100-mm plates
to 70–80% confluence, reversibly cross-linked with formaldehyde and
immunoprecipitated with an anti-PSF antibody (P2860; Sigma-Aldrich;
Merck Millipore). The GAGE6 promoter DNA fragments in PSF-DNA
complexes were analyzed by qPCR using the following primers: GAGE6
forward, 5′-GCCTTCTGCAAAGAAGTCTTGCGC-3′ and reverse,
5′-ATGCGAATTCGAGGCTGAGGCAGACAAT-3′. Dihydrofolate reductase (DHFR)
5′ UTR DNA was used as a negative control and the primers used were
as follows: Forward, 5′-CTGATGTCCAGGAGGAGAAAGG-3′ and reverse,
5′-AGCCCGACAATGTCAAGGACTG-3′. All primers were obtained from BGI.
RIP assays were performed using an RIP-Assay kit (MBL International
Co., Woburn, MA, USA) following the manufacturer's instructions.
The pcDNA3.1-PSF (encoding human PSF protein) and pcDNA3.1-L1
plasmids mixed with Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.) were transiently transformed into HEK293 cells. After 4 h of
transfection, the cells were washed once with PBS and fresh medium
was added. The cells were cultured for 48 h prior to testing. L1
RNA in PSF-RNA complexes was analyzed by extracting the RNA with
TRIzol reagent and assaying by RT-qPCR (as described above). Rabbit
IgG (provided in the kit) was compared with anti-PSF antibody to
determine the fold enrichment of the target gene. DHFR 5′ UTR DNA
was compared with GAGE6 DNA, and GAPDH mRNA with L1 RNA to assess
the specific binding of PSF with GAGE6 DNA or L1 RNA.
Statistical analysis
All results are presented as the mean ± standard
error of the mean. Student's t-test was used for comparisons
between two groups. P<0.05 was considered to indicate a
statistically significant difference. Data were analyzed using
GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA,
USA).
Results
Binding ability of L1 RNA and PSF
protein in vitro
It has been suggested that HBV may cause HCC by
integrating into L1 retrotransposons and lead to L1 transcription
(16). Therefore, it was
hypothesized that transcripts of retrotransposons L1 may also
influence lung carcinogenesis. The present study focused on the
role L1 RNA may serve in lung carcinogenesis without HBV infection,
which is different from HBx-LINE-1 causing hepatocarcinogenesis
(16). It has previously been
demonstrated that L1PA16 RNA binds to PSF protein, which is part of
the LINE retrotransposons family (10). Therefore, L1 RNA, although it shares
no sequence similarity with L1PA16 RNA, may bind to PSF. The
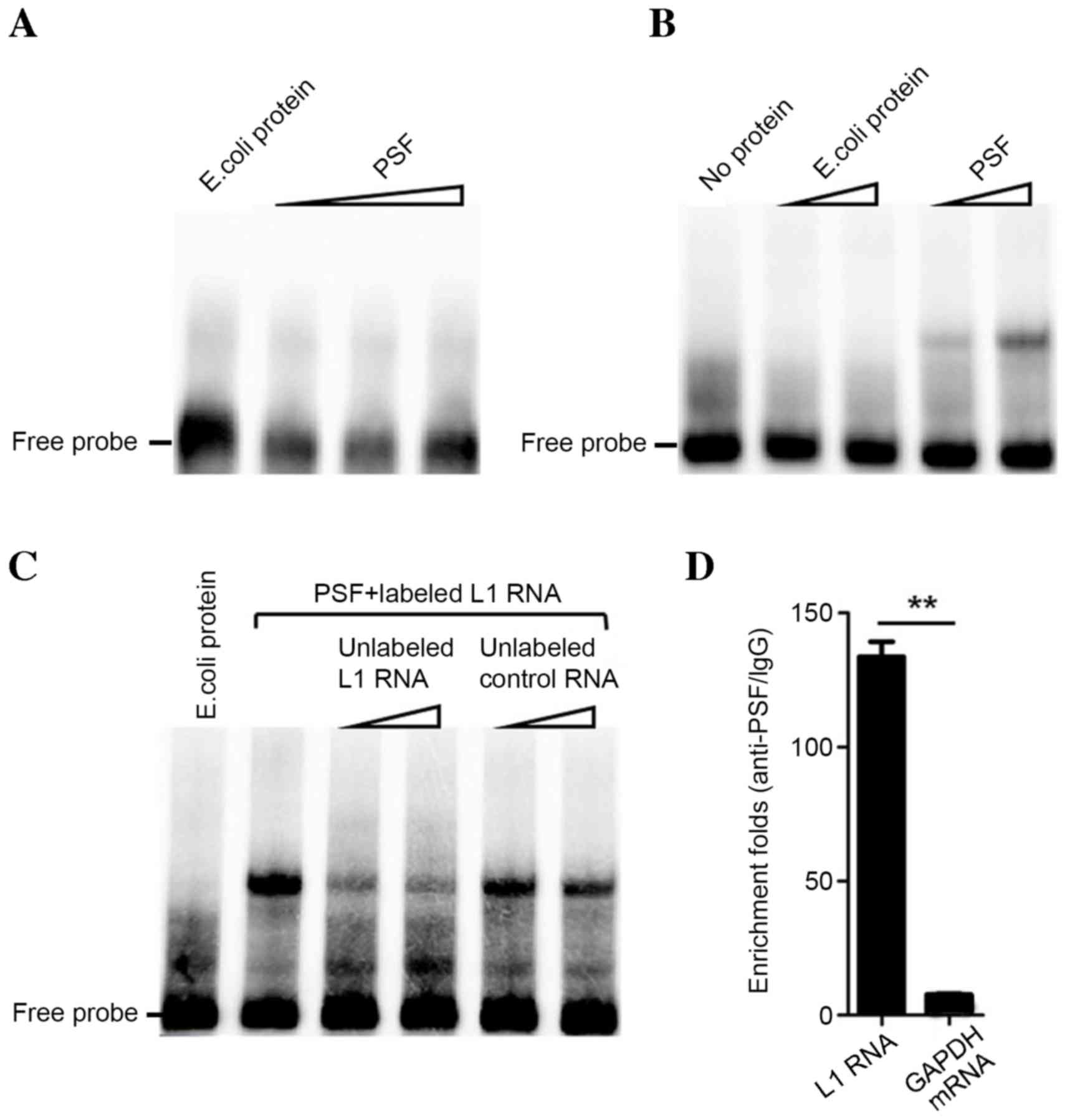
results of in vitro testing by EMSA indicated that L1 RNA
binds to PSF protein, while RNA control pcDNA3.1 RNA exhibits no
detectable binding (Fig. 1A and B).
Addition of unlabeled L1 RNA probe reduced the binding band of
L1/PSF, indicating its binding capacity and specificity to PSF
(Fig. 1C). An in vitro RIP
assay revealed that L1 RNA and PSF have a strong binding
interaction. Compared with the enrichment fold of GAPDH mRNA
(negative control), L1 RNA was significantly enriched by
immunoprecipitation with anti-PSF antibody (P<0.01; Fig. 1D). Therefore, the results indicated
that L1 RNA strongly and specifically binds to PSF in
vitro.
Identification of the interacting
domain of PSF protein and L1 RNA
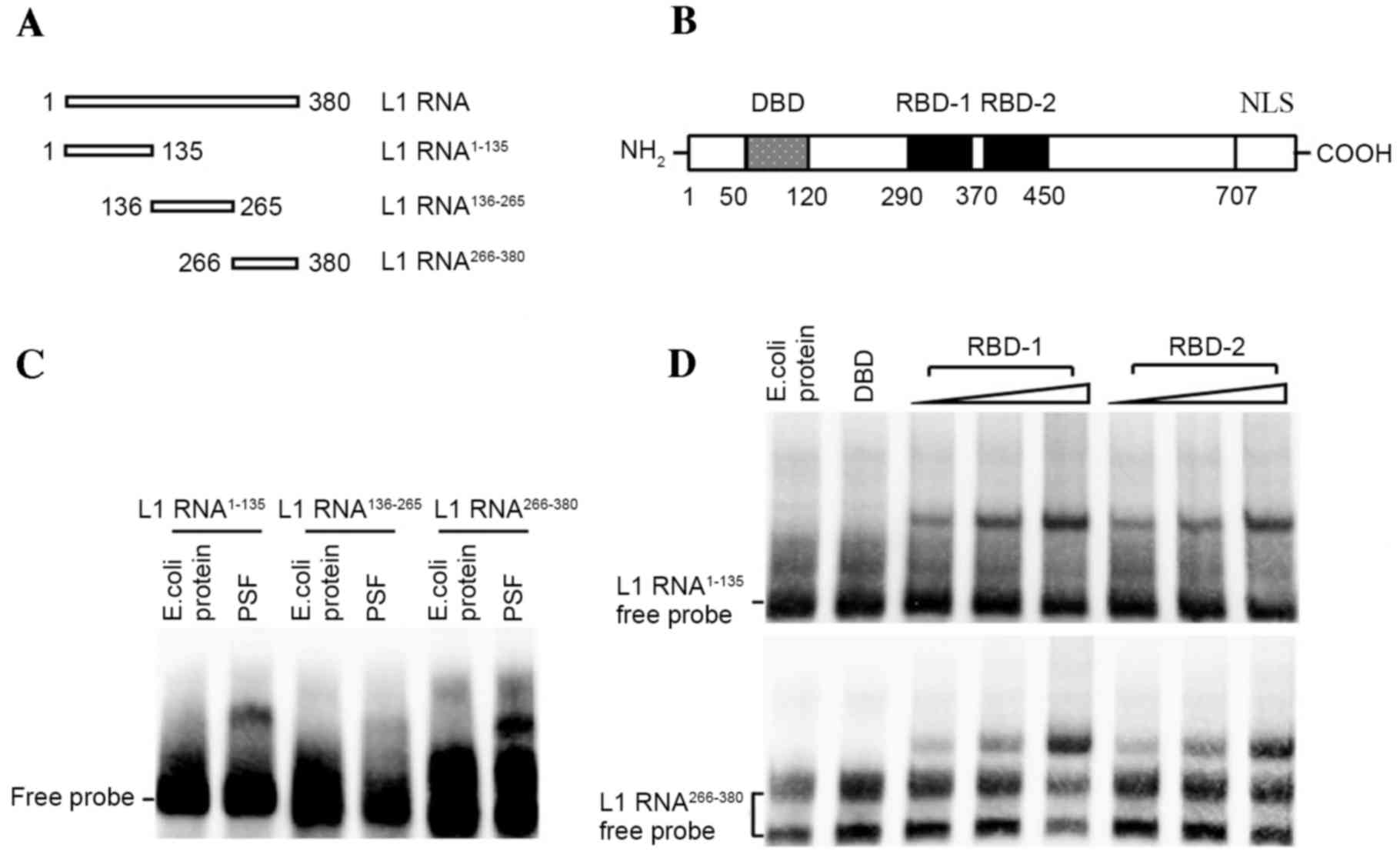
To biochemically analyze the interaction between PSF
and L1 RNA, several independent RNA-protein interaction assays were
performed. First, to define a region within L1 RNA sufficient for
PSF binding, biotin-labeled RNAs corresponding to different regions
of the L1 RNA were incubated with purified PSF protein and the
reactions were analyzed by EMSA (Fig. 2A
and B). Fragments 1 (L1 RNA1–135) and 3 (L1
RNA266–380) efficiently bound the intact PSF protein
(Fig. 2C). By contrast, no
interacting bands between fragment 2 (L1 RNA136–265) and
PSF were observed. Two RBDs, referred to as RBD-1 and RBD-2, have
been detected in PSF, which is responsible for binding non-coding
RNA (18). The release of the target
DNA fragment from the DBD of PSF by RNA binding is controlled by
RBD-1 but not RBD-2. This means that it is critical to identify
which RBD is bound by L1 RNA. For this, biotin-labeled L1 RNA
fragments were incubated with increasing amounts of the respective
RBDs (amino acids 290–370 of RBD-1 and amino acids 370–455 of
RBD-2) of PSF for subsequent EMSAs. The results demonstrated that,
with increasing amounts of RBD-1 or RBD-2 truncate, stronger bands
of protein-RNA complex were observed, indicating the effective
interactions (Fig. 2D).
Binding of L1 RNA to PSF competitively
inhibits PSF from binding to the downstream target gene GAGE6
It has been suggested that PSF regulates mouse Rab23
expression by binding to the Rab23 promoter and repressing its
transcriptional activity (19).
However, certain types of non-coding RNA, including VL30-1, may
bind to the PSF protein and suppress its ability to bind to the
Rab23 response element (19). The
effect of L1 RNA on the expression of the Rab23 human homolog GAGE6
was assessed by competitive EMSA. The unlabeled L1 RNA probe
reduced the binding band of biotin-DNA/PSF, while it remained
unaffected by the plasmid encoding control RNA (Fig. 3A). This result indicated that L1 RNA
inhibits the binding of biotin-DNA with PSF protein in
vitro. Subsequently, A549 cells were transfected with
L1-encoding plasmid or with the empty pcDNA3.1 plasmid as a control
and subjected to a CHIP assay. qPCR demonstrated that compared with
the control, overexpression of L1 RNA reduced the binding of the
PSF/GAGE6 promoter (P<0.01; Fig.
3B). These results demonstrated that L1 RNA exerts its
function, at least in part, by binding to PSF and reversing
PSF-mediated gene repression (Fig.
3C). Therefore, overexpression of L1 RNA leads to a release of
PSF from the promoter region of GAGE6 and causes an increase in the
expression of GAGE6 mRNA (P<0.05; Fig. 3C).
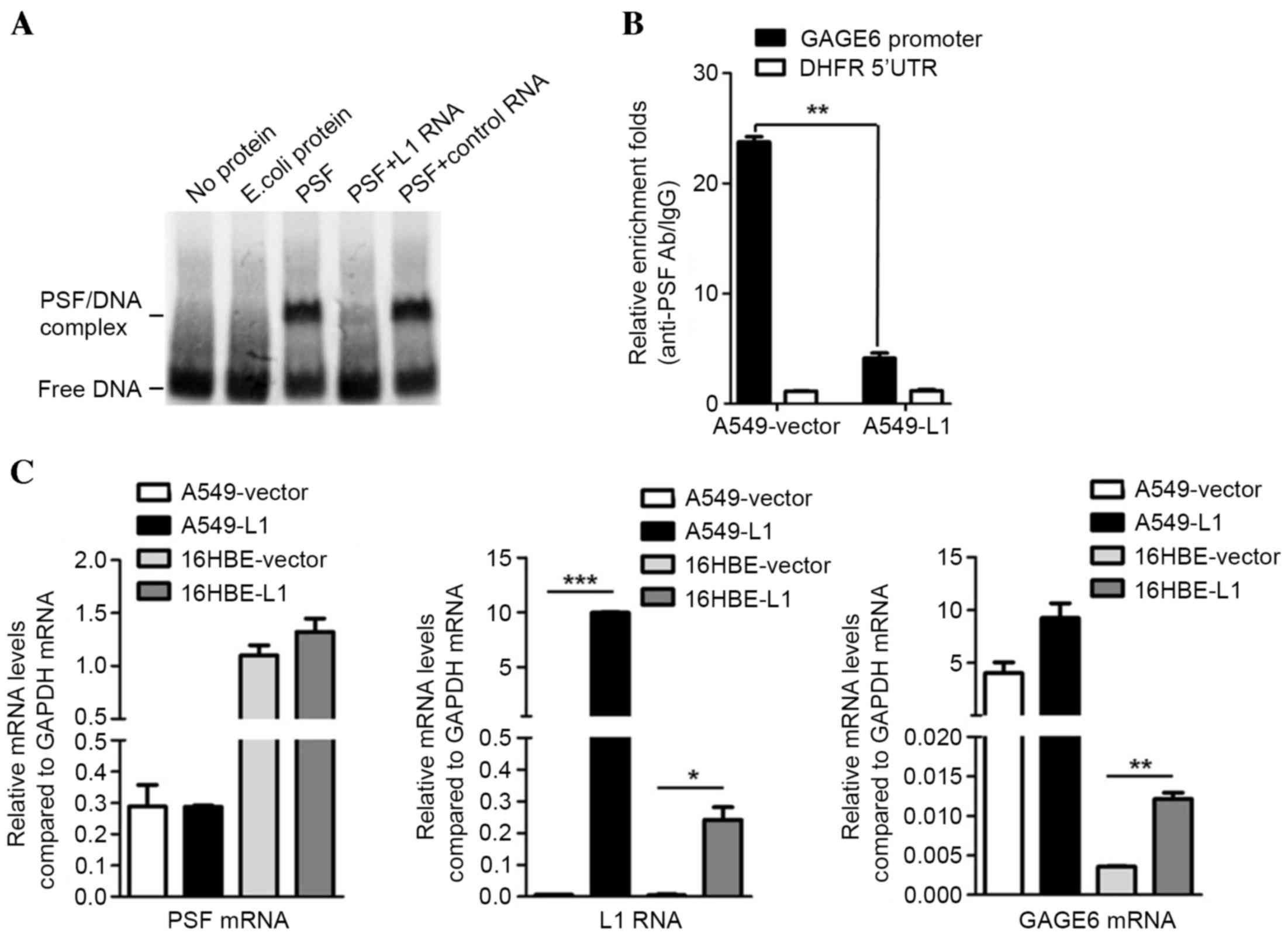 | Figure 3.Effects of L1 RNA binding to PSF on
GAGE6. (A) Binding of L1 RNA releases GAGE6 promoter DNA from PSF.
(B) Release of GAGE6 promoter region from PSF by overexpression of
L1 RNA demonstrated by chromatin immunoprecipitation. (C)
Expression levels of PSF mRNA, L1 RNA and GAGE6 mRNA in A549 and
16HBE cells transfected with L1 RNA overexpression or empty vector
measured using reverse-transcription quantitative polymerase chain
reaction. *P<0.05, **P<0.01, ***P<0.001. GAGE6, G antigen
6; 5′UTR, 5′ untranslated region; Ab, antibody; IgG, immunoglobulin
G; E. coli, Escherichia coli; L1, long interspersed nuclear element
1; PSF, polypyrimidine tract-binding protein-associated splicing
factor; DHFR, dihydrofolate reductase. |
Effects of L1 RNA overexpression on
the proliferation and colony formation of A549 and 16HBE cells
As a previous study has indicated the regulatory
activities of GAGE6 in cell proliferation and tumor formation
(18), the effects of L1 RNA on cell
proliferation and colony formation were investigated in the present
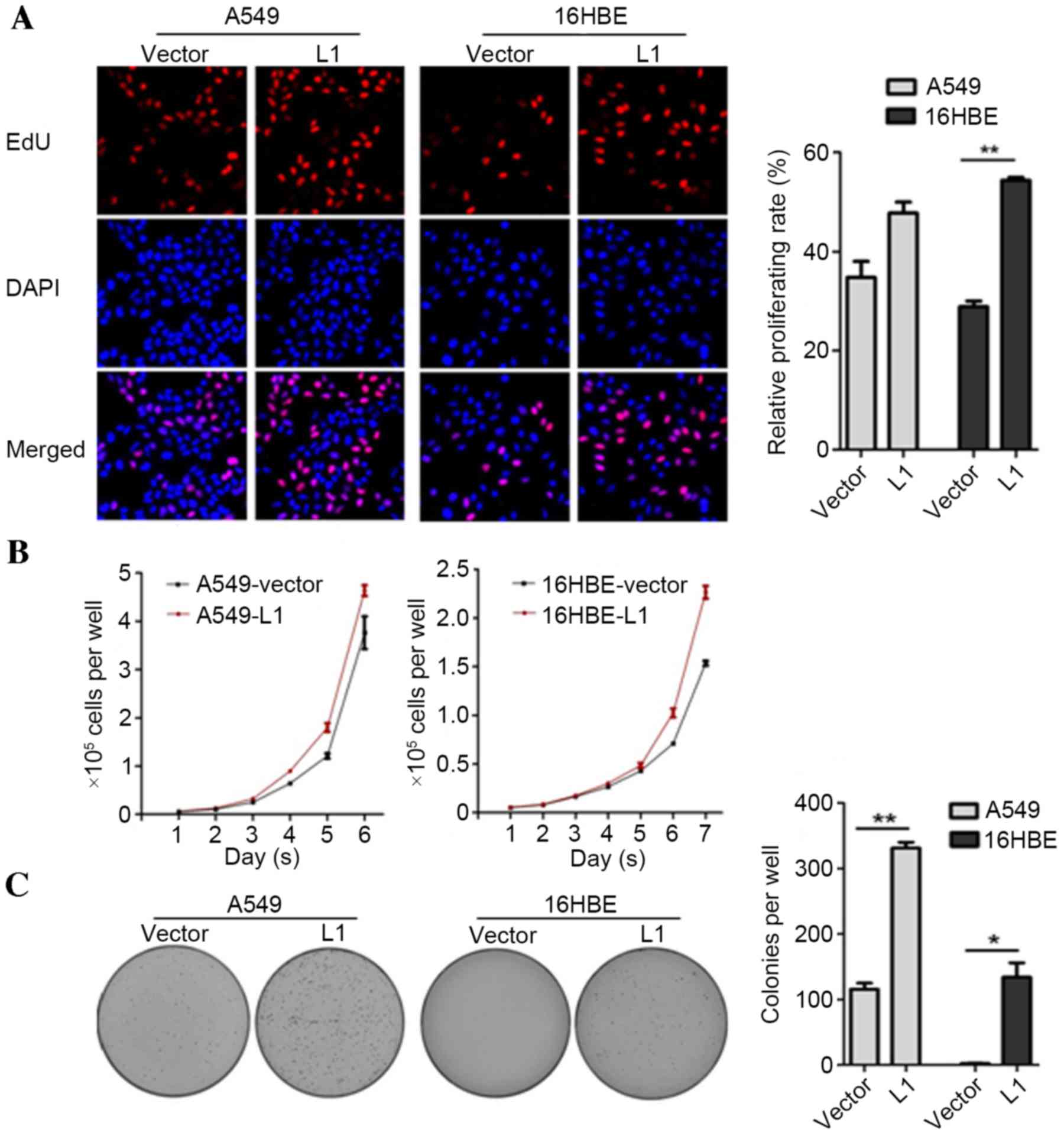
study. An EdU incorporation assay was employed to detect whether
overexpression of L1 RNA may affect the number of proliferating
cells. The results demonstrated that the number of EdU-positive
cells in the A549-L1 RNA and 16HBE-L1 RNA groups were increased
compared with the A549-vector and 16HBE-vector groups, respectively
(P<0.01; Fig. 4A). Consistent
with the EdU staining results, cell proliferation and colony
formation rates were significantly higher in A549-L1 RNA and
16HBE-L1 RNA cells compared with the control groups (P<0.05;
Fig. 4B and C). Taken together, the
results demonstrated that overexpression of L1 RNA promoted cell
proliferation and colony formation.
Discussion
The results of the present study expand on a
previous study demonstrating that the chimeric HBx-LINE-1 functions
as a hybrid RNA and promotes tumorigenesis in HCC (16). A lower hypomethylation level of L1
and higher transcriptional level of L1 RNA has been detected in
HBV-transfected tumor samples compared with adjacent tissues
(20). The present study described a
mechanism of gene regulation involving formation of a complex
between L1 RNA and the regulatory protein PSF, accounting for the
effect of L1 RNA on cell proliferation.
L1 is biologically significant not only as a
retrotransposon that can function as a genetic modifier, but also
as a transcript which is associated with tumorigenesis (13). Furthermore, the transcription of L1
is epigenetically controlled by hypomethylation. L1 elements
contain a 5′-C-phosphate-G-3′ island in their 5′-UTR, which is
usually heavily methylated in normal somatic cells (21). The importance of hypomethylation in
this region has been highlighted in various types of human cancer,
including HCC, chronic myeloid leukemia, bladder cancer,
gastrointestinal stromal tumors, gastric cancer and ovarian cancer
(12). Transcription of L1 induced
by hypomethylation significantly correlates with the degree of
malignancy, particularly in colorectal cancer (21–23).
This suggests that L1 hypomethylation may serve a more important
role in human cancer than the hypomethylation-induced inhibition of
transcription of specific tumor suppressor genes.
PSF is a multifunctional protein, containing two RBD
and one DBD. Originally, PSF was identified as a component of
spliceosomes and observed to serve a transcriptional regulatory
role on GAGE6, which functions as an oncogene (7,24).
Repression of GAGE6 expression involves PSF binding to its
regulatory region. The complex formed with L1 RNA inhibits and
dissociates the binding of PSF to the GAGE6 regulatory region,
allowing transcription to proceed (24). Complex formation with the RBDs on PSF
and the resulting regulation of gene transcription was mapped to
two fragments in L1 RNA. Binding of these two fragments to the RBDs
of PSF may cause allosteric modification, resulting in weaker
affinity of the DBD for the GAGE6 DNA motif. It was observed that
increasing the concentration of L1 RNA increased its inhibitory
effect on PSF/DNA binding affinity. L1 RNA is transcribed from
endogenous genomic retrotransposons and the level varies depending
on the cell type and the level of global hypomethylation (25). Thus, the key parameters that
determine whether GAGE6 expression is repressed by PSF or induced
by L1 RNA are the type of cell and the global hypomethylation
status. It has been determined that human L1 retrotransposons are a
major source of endogenous mutagens (26). The present study demonstrated the
individual oncogenic activity of L1 RNA, which potentially
specifies a functionally distinct class of long non-coding RNA-like
transcripts.
In conclusion, the present study described a
mechanism controlling cell proliferation and tumorigenesis in human
cells, which involves the activation of oncogene GAGE6
transcription by L1 RNA through binding to PSF protein and its
simultaneous release from the GAGE6 promoter region. In addition,
overexpression of L1 exerted strong regulatory effects on the
proliferation and colony formation of lung cancer and normal lung
cells without HBV infection. Therefore, endogenous L1 RNA may
potentially serve an important regulatory role in
tumorigenesis.
Acknowledgements
The authors would like to thank Mr. Huimin Shi
(Molecular Biology Laboratory, The Food and Drug Inspection
Institute, Chengdu, China) for editing the draft manuscript. The
current study was supported by the Sichuan Provincial Science and
Technology Supporting Program (grant no. 2016FZ0093).
References
|
1
|
Guo XL, Li D, Hu F, Song JR, Zhang SS,
Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, et al: Targeting
autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Lett.
320:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ng J and Wu J: Hepatitis B- and hepatitis
C-related hepatocellular carcinomas in the United States:
similarities and differences. Hepat Mon. 12:e76352012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McGivern DR and Lemon SM: Virus-specific
mechanisms of carcinogenesis in hepatitis C virus associated liver
cancer. Oncogene. 30:1969–1983. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai WL and Chung RT: Viral
hepatocarcinogenesis. Oncogene. 29:2309–2324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kowalska E, Ripperger JA, Muheim C, Maier
B, Kurihara Y, Fox AH, Kramer A and Brown SA: Distinct roles of
DBHS family members in the circadian transcriptional feedback loop.
Mol Cell Biol. 32:4585–4594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lowery LA, Rubin J and Sive H:
Whitesnake/sfpq is required for cell survival and neuronal
development in the zebrafish. Dev Dyn. 236:1347–1357. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patton JG, Porro EB, Galceran J, Tempst P
and Nadal-Ginard B: Cloning and characterization of PSF, a novel
pre-mRNA splicing factor. Genes Dev. 7:393–406. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Urban RJ, Bodenburg Y, Kurosky A, Wood TG
and Gasic S: Polypyrimidine tract-binding protein-associated
splicing factor is a negative regulator of transcriptional activity
of the porcine P450scc insulin-like growth factor response element.
Mol Endocrinol. 14:774–782. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Urban RJ, Bodenburg YH and Wood TG: NH2
terminus of PTB-associated splicing factor binds to the porcine
P450scc IGF-I response element. Am J Physiol Endocrinol Metab.
283:E423–E427. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Feng TT, Lian Y, Zhang G, Garen A
and Song X: Role of human noncoding RNAs in the control of
tumorigenesis. Proc Natl Acad Sci USA. 106:pp. 12956–12961. 2009;
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kazazian HH Jr: Mobile elements: Drivers
of genome evolution. Science. 303:1626–1632. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aporntewan C, Phokaew C, Piriyapongsa J,
Ngamphiw C, Ittiwut C, Tongsima S and Mutirangura A:
Hypomethylation of intragenic LINE-1 represses transcription in
cancer cells through AGO2. PLoS One. 6:e179342011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chowdhury S, Cleves MA, MacLeod SL, James
SJ, Zhao W and Hobbs CA: Maternal DNA hypomethylation and
congenital heart defects. Birth Defects Res A Clin Mol Teratol.
91:69–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tangkijvanich P, Hourpai N, Rattanatanyong
P, Wisedopas N, Mahachai V and Mutirangura A: Serum LINE-1
hypomethylation as a potential prognostic marker for hepatocellular
carcinoma. Clin Chim Acta. 379:127–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu HC, Wang Q, Yang HI, Tsai WY, Chen CJ
and Santella RM: Global DNA methylation levels in white blood cells
as a biomarker for hepatocellular carcinoma risk: A nested
case-control study. Carcinogenesis. 33:1340–1345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lau CC, Sun T, Ching AK, He M, Li JW, Wong
AM, Co NN, Chan AW, Li PS, Lung RW, et al: Viral-human chimeric
transcript predisposes risk to liver cancer development and
progression. Cancer Cell. 25:335–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song X, Sun Y and Garen A: Roles of PSF
protein and VL30 RNA in reversible gene regulation. Proc Natl Acad
Sci USA. 102:pp. 12189–12193. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G, Cui Y, Zhang GF, Garen A and Song
X: Regulation of proto-oncogene transcription, cell proliferation,
and tumorigenesis in mice by PSF protein and a VL30 noncoding RNA.
Proc Natl Acad Sci USA. 106:pp. 16794–16798. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YJ, Wu HC, Yazici H, Yu MW, Lee PH
and Santella RM: Global hypomethylation in hepatocellular carcinoma
and its relationship to aflatoxin B(1) exposure. World J Hepatol.
4:169–175. 2012. View Article : Google Scholar PubMed/NCBI
|
|
21
|
Ogino S, Kawasaki T, Nosho K, Ohnishi M,
Suemoto Y, Kirkner GJ and Fuchs CS: LINE-1 hypomethylation is
inversely associated with microsatellite instability and CpG island
methylator phenotype in colorectal cancer. Int J Cancer.
122:2767–2773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goel A, Xicola RM, Nguyen TP, Doyle BJ,
Sohn VR, Bandipalliam P, Reyes J, Cordero C, Balaguer F, Castells
A, et al: Aberrant DNA methylation in hereditary nonpolyposis
colorectal cancer without mismatch repair deficiency.
Gastroenterology. 138:1854–1862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sunami E, De Maat M, Vu A, Turner RR and
Hoon DS: LINE-1 hypomethylation during primary colon cancer
progression. PLoS One. 6:e188842011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eynde B, Peeters O, De Backer O, Gaugler
B, Lucas S and Boon T: A new family of genes coding for an antigen
recognized by autologous cytolytic T lymphocytes on a human
melanoma. J Exp Med. 182:689–698. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trivedi M, Shah J, Hodgson N, Byun HM and
Deth R: Morphine induces redox-based changes in global DNA
methylation and retrotransposon transcription by inhibition of
excitatory amino acid transporter type 3-mediated cysteine uptake.
Mol Pharmacol. 85:747–757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burns KH and Boeke JD: Human transposon
tectonics. Cell. 149:740–752. 2012. View Article : Google Scholar : PubMed/NCBI
|