Introduction
Human T-lymphoma invasion and metastasis-inducing
protein 1 (TIAM1) is 1,591 amino acids long with a molecular weight
of 177 kD (1), and is detected at
the boundary between cells with actin-rich protrusions (2). TIAM1 has previously been demonstrated
to modulate the activity of Rho-like proteins and connect
extracellular signals to cytoskeletal activities through guanosine
diphosphate-guanosine triphosphate exchange activity (3). TIAM1 serves a critical role in the
regulation of cell adhesion, invasion and migration, which have
been directly implicated in the promotion of cancer progression and
metastasis (4). A previous clinical
investigation in patients with lung cancer has indicated that TIAM1
protein expression in lung tumor tissue is significantly higher
compared with that in normal lung tissue, and the expression of
TIAM1 is correlated with patient age, tumor size, tumor type and
tumor differentiation (5). In human
lung cancer cells, decreased expression of TIAM1 due to
ubiquitination-mediated degradation or short interfering RNA
(siRNA)-mediated knockdown has been reported to induce the
disassembly of cell junctions and cancer cell invasion (6). Furthermore, increased expression of
TIAM1 induces human lung cancer cell migration (4). In an orthotropic nude mouse model of
colon cancer, TIAM1 expression was reported to be upregulated in
colon carcinoma growths at metastatic sites, suggesting that
overexpression of TIAM1 contributes to the metastatic phenotype of
colon cancer cells (7). It has
previously been implicated that TIAM1 is a crucial component of the
Par complex in the regulation of neuronal and epithelial polarity
(8). In normal lung tissue,
mechanical stretch decreases the migration of alveolar epithelial
cells via the induction of TIAM1 translocation from the membrane to
the cytosol, suggesting that TIAM1 is involved in the repair
mechanisms of alveolar epithelial type 2 cells subjected to
mechanical strain (9). However, the
role of TIAM1 in lung fibroblasts during pulmonary fibrosis has not
been widely studied.
Idiopathic pulmonary fibrosis (IPF) affects more
than three million people worldwide (494.5 cases/100,000 people),
and half of these patients will succumb within 2–5 years of the
initial diagnosis due to limited effective treatments (10). Myofibroblasts are thought to be the
key effector cells in IPF and have several potential sources,
including epithelial to mesenchymal cell transition, resident
fibroblast differentiation and recruitment of circulating
fibrocytes (11). Lung fibroblast
proliferation, differentiation and accumulation in fibrotic foci
serve major roles in pulmonary fibrosis, stimulating the deposition
of extracellular matrix (ECM) proteins, including fibronectin (FN)
and collagen, in alveoli and resulting in lung dysfunction
(12). A previous study indicated
that various cytokines and lipid ligands regulate lung fibroblast
proliferation, differentiation and invasion (13). Transforming growth factor-β (TGF-β)
is one of the key factors that induce fibroblast differentiation
and lung fibrosis (14).
Adenovirus-induced TGF-β1 overexpression in a mouse model of lung
injury has been reported to induce pulmonary fibrosis (15). Typically, TGF-β1 signaling proceeds
via the activation of type I and type II serine/threonine kinase
receptors, which phosphorylates and translocates mothers against
decapentaplegic (Smad)2 and Smad3 to the nucleus to stimulate gene
expression in lung fibroblasts (16). TGF-β1 also activates nuclear factor
(NF)-κB-dependent pathways to induce gene expression in lung
fibroblasts during pulmonary fibrosis (17).
In the present study, a bleomycin (BLM)-induced
mouse model of pulmonary fibrosis was used to investigate whether
the expression of TIAM1 was increased in lung fibroblasts from lung
fibrotic foci. The results indicated that in vitro the
expression of TIAM1 in TGF-β1-challenged fibroblasts depends in
part on the NF-κB-mediated pathway, and overexpression of TIAM1
attenuates TGF-β1-induced lung fibroblast differentiation. In
summary, the results of the present study indicate that TIAM1 is
critical for fibroblast differentiation and pulmonary fibrosis.
Materials and methods
Antibodies and reagents
Mouse anti-α-smooth muscle actin (α-SMA; cat. no.
A5228), anti-β-actin antibodies (cat. no. A5441), Bay 11–7082
(NF-κB inhibitor; cat. no. B5556) and protease inhibitor cocktail
tablets (EDTA-free Complete) were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Recombinant human TGF-β1 was
obtained from PeproTech, Inc. (Rocky Hill, NJ, USA). Cell lysis
buffer and mouse anti-fibroblast specific protein 1 (FSP1; cat. no.
13018) antibody were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Rabbit anti-FN (cat. no. sc-69681), anti-TIAM1
(cat. no. sc-872), anti-GAPDH (cat. no. sc-47724), control mouse
IgG (cat. no. sc-2025) and control rabbit IgG (cat. no. sc-2051)
antibodies were all purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). The open reading frame clone of human TIAM1
(cat. no. 70631) and the control plasmid (pCDNA3.1; cat. no. 63560)
were purchased from Addgene, Inc. (Cambridge, MA, USA). Horseradish
peroxidase-conjugated anti-mouse IgG (cat. no. 170-6515) and
anti-rabbit IgG (cat. no. 170-6516) antibodies were obtained from
Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Mouse model of pulmonary fibrosis
A total of 20 wild-type (C57BL/6J) mice (male, 8–10
weeks, ~20 g) were purchased from Vital River Laboratory Animal
Technology (Beijing, China). Mice were provided with free access to
food and water, and housed under a 12 h light/dark cycle at 18–23°C
and 40–60% humidity. Mice were randomly divided into two groups;
BLM (n=15) and negative control (n=5). Mice were anesthetized (IP,
87.5 mg/kg of ketamine and 12.5 mg/kg of xylazine; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and then an intratracheal injection
of BLM (2 U/kg; Sigma-Aldrich; Merck KGaA) in saline (50 µl) or
saline alone was administered (18).
At 21 days following the BLM challenge, mice were sacrificed. Lungs
were harvested and the lobes were fixed at 25°C in 10% formalin for
24 h and 70% EtOH for 48 h, embedded in paraffin, cut into 5-µm
sections and subjected to hematoxylin and eosin staining (25°C 4
min hematoxylin and 2 min eosin) and trichrome staining (25°C,
trichrome, 10 min). All animal studies were approved by the Animal
Care Use Committee of Jilin Province Cancer Hospital (Changchun,
China).
Immunofluorescence microscopy
Immunofluorescence microscopy was used to assess the
expression of ECM-related protein and TIAM1 as previously described
(19). Briefly, paraffin-embedded
mouse lung tissue sections were dewaxed, rehydrated and subjected
to antigen retrieval. Antigen retrieval of tissue slides was
performed according to the EDTA buffer antigen retrieval protocol,
in which tissue slides were immersed in EDTA buffer containing 1 mM
EDTA and 0.05% Tween-20 (pH 8.0) at 95–100°C for 30 min. Sections
were subsequently blocked with TBST blocking buffer [2% bovine
serum albumin (BSA) and 1% fetal bovine serum (FBS); Santa Cruz
Biotechnology, Inc.] for 30 min at room temperature. Lung tissues
were then incubated with primary antibodies (1:200, rabbit
anti-TIAM1 (cat. no. sc-872; Santa Cruz Biotechnology, Inc.), mouse
anti-α-SMA (cat. no. A5228; Sigma-Aldrich; Merck KGaA), control IgG
from mouse (cat. no. sc-2025) and rabbit (cat. no. sc-2051) (both
from Santa Cruz Biotechnology, Inc.) for 1 h, followed by three
15-min washes with TBST. After this, tissues were stained with
Alexa Fluor secondary antibodies (1:200, cat. no. R37117 and
A-21202; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 25°C
for 1 h, followed by washing with TBST for 15 min. Slides were
incubated with mounting media (containing DAPI) at 25°C for 10 min,
and examined under a Nikon Eclipse TE2000-S fluorescence microscope
(Nikon, Tokyo, Japan). Images were captured using a digital camera
(Hamamatsu Photonics, Hamamatsu, Japan) with a 60X oil immersion or
4X objective lens. Fluorescence intensity was analyzed using the
ImageJ analysis system v1.45 (National Institutes of Health,
Bethesda, MD, USA). In brief, the expression of TIAM1 and α-SMA in
BLM-treated mice was normalized to the expression in control mice.
The number of FSP1(+) cells was quantified, and subsequently the
expression of TIAM1 in FSP1(+) cells was analyzed based on the
intensity of the merged color.
WI-38 lung fibroblasts were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA). Cells
were fixed with 3.7% formaldehyde for 10 min at 25°C, treated with
0.25% Triton X-100 for 10 min and subsequently blocked with 2.5%
BSA (Santa Cruz Biotechnology, Inc.) at 25°C for 1 h. Following
blocking, cells were incubated at 25°C with rabbit anti-TIAM1
(1:200) and mouse anti-α-SMA (1:400) antibodies for 1 h. Cells were
washed three times with PBS and subsequently incubated at 25°C with
Alexa Fluor-tagged secondary antibodies (1:200, cat. no. R37117 and
A-21202; Thermo Fisher Scientific, Inc.) at 25°C for 1 h. Finally,
the cells were mounted and examined using a Nikon Eclipse TE2000-S
fluorescence microscope using a 60X oil immersion lens.
Cell culture and TGF-β treatment
Primary mouse lung fibroblasts were isolated from
C57BL/6J mice with or without BLM treatment as previously described
(17). Isolated mouse lung
fibroblasts and human WI-38 fibroblasts were seeded and maintained
in 6-well plates with Dulbecco's modified Eagle's medium (DMEM)
containing 10% FBS (Sigma-Aldrich; Merck KGaA). Following serum
starvation for 24 h, lung fibroblast cells (~80% confluent) were
treated with TGF-β (5 ng/ml) or PBS for 0 to 48 h.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Following treatment with TGF-β (5 ng/ml, 12 h),
total RNA was extracted from WI-38 cells and purified using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
cDNA was synthesized (46°C, 20 min) by using a cDNA synthesis kit
(iScript cDNA synthesis kit, cat. no. 1708890) from Bio-Rad
Laboratories, Inc. A total of 1 µg RNA was converted to cDNA, and
this was used for qPCR. A SYBR Green qPCR kit and a CFX96 Touch
qPCR system (both from Bio-Rad Laboratories, Inc.) were used. For
real time qPCR, the amplification reactions were performed in
triplicate, and the thermal cycling conditions were as follows: 10
sec at 95°C followed by 40 cycles of 5 sec at 95°C and 30 sec at
60°C. GAPDH was used as the reference gene to normalize the
expression levels of TIAM1, FN and α-SMA. The primers used were as
follows: TIAM1 forward, 5′-GATCCACAGGAACTCCGAAGT-3′ and reverse,
5′-GCTCCCGAAGTCTTCTAGGGT-3′; FN forward,
5′-TCTGTGCCTCCTATCTATGTGC-3′ and reverse,
5′-GAGGGACCACGACAACTCTTC-3′; α-SMA forward,
5′-AAAAGACAGCTACGTGGGTGA-3′ and reverse,
5′-GCCATGTTCTATCGGGTACTTC-3′; GAPDH forward,
5′-TGTGGGCATCAATGGATTTGG-3′ and reverse,
5′-ACACCATGTATTCCGGGTCAAT-3′. Gene expression was analyzed using
CFX Manager software version 3.1 (Bio-Rad Laboratories, Inc.). The
2−∆∆Cq method was used for quantification (17).
NF-κB inhibitor treatment
Cells (human lung fibroblast, WI-38) were pretreated
with 10 µM Bay 11-7082 and vehicle solution (0.05%
dimethylsulfoxide), for 1 h. The cells were subsequently challenged
with TGF-β (5 ng/ml) for a subsequent 48 h prior to analysis using
western blotting.
Transfection of WI-38 cells with
siRNA
RNA smart pool targeting human TIAM1 (si-TIAM1) was
from GE Dharmacon, Inc. (Lafayette, CO, USA), and control scramble
(sc)-RNA was obtained from Santa Cruz Biotechnology, Inc.
Fibroblasts were seeded into 6-well plates until they reached a
confluence of 50–60%. They were then transfected with 200 nmol/l
si-TIAM1 or sc-RNA using siRNA transfection reagent (Huperfect
Transfection reagent, cat. no. 301704; Qiagen AB, Sollentuna,
Sweden). Briefly, the siRNAs were diluted in 900 µl basal DMEM and
incubated with the cells for at 37°C for 6 h. DMEM with 10% FBS was
refreshed every 24 h. At 48 h following transfection, the cells
were used for TGF-β treatment and analyzed via fluorescence
microscopy or western blot analysis.
Plasmid transfection of WI-38
cells
WI-38 cells grown to −70% confluence were
transiently transfected with 3 µg/ml of plasmids using plasmid
transfection reagent (Effectene Transfection reagent, cat. no.
301425; Qiagen AB) in serum-free DMEM medium at 25°C for 15 min,
and further incubated in DMEM medium with 10% FBS for 48 h. Cells
were subsequently used for TGF-β treatment and protein expression
analysis by western blot analysis.
Western blot analysis
Cells were disrupted by incubated with cell lysis
buffer (cat. no. 9806; Cell Signaling Technology, Inc.) for 10 min
on ice followed by centrifugation (5,000 × g, 15 min at 4°C).
Protein content in cell lysate was determined by using Micro BCA
Protein Assay kit (cat. no. 23235; Thermo Fisher Scientific, Inc.).
Briefly, 20–30 µg proteins in cell lysates were separated by
SDS-PAGE (10 or 4–20%). Proteins were transferred onto
nitrocellulose membranes (100 V, 1 h), blocked with blocking buffer
(TBS solution containing 0.1% Tween-20 and 5% BSA) at 25°C for 1 h
and then incubated with primary antibodies (anti-TIAM1, 1:1,000;
anti-FN, 1:2,000; anti-GAPDH, 1:2,000; anti-α-SMA, 1:5,000)
overnight at 4°C. The membranes were subsequently incubated with
secondary antibodies (1:2,000) at 25°C for 2 h. Secondary
antibodies used were horseradish peroxidase (HRP)-conjugated
anti-mouse IgG (cat. no. 170-6515) and HRP-conjugated anti-rabbit
IgG (cat. no. 170-6516) (both from Bio-Rad Laboratories, Inc.).
Finally, proteins were visualized using an enhanced
chemiluminescence kit (Bio-Rad Laboratories, Inc.) and analyzed
using ImageQuant 5.2 software (Molecular Devices, LLC, Sunnyvale,
CA, USA).
Statistical analysis
Data were analyzed using SPSS v16.0 statistical
software (SPSS Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard error of the mean from at least three independent
experiments. Data were analyzed using a two-tailed Student t-test
or two-way analysis of variance plus a multiple comparisons
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of TIAM1 in mouse lung
tissue
The expression of TIAM1 in lung tissues of mice with
or without BLM-challenge (2 U/kg) was assessed on day 21. BLM
treatment markedly induced lung injury and fibrosis in the
wild-type mice, as indicated by increased cell infiltration into
alveoli (Fig. 1A) and deposition of
collagen (Fig. 1B) compared with
control mice. Immunofluorescence (Fig.
1C and D) indicated that the expression of TIAM1 and α-SMA was
significantly increased in the lung fibrotic foci from
BLM-challenged mice compared with control mice (P<0.05).
 | Figure 1.Expression of TIAM1 in lung tissues
from control and BLM-treated mice. (A) H&E staining (scale bar,
500 µm), (B) trichrome staining (scale bar, 500 µm) and (C)
immunofluorescence staining of TIAM1, α-SMA and DAPI (scale bar,
200 µm) in lung tissue from BLM-treated and control mice. Red
arrows indicate lung injury, blue arrows indicate collagen
deposition and white arrows indicate colocalized TIAM1 and α-SMA
overexpression in fibrotic foci. (C) The upper panel shows the
representative immunofluorescence staining of negative control
using mouse and rabbit control IgG. (D) Quantification of
immunofluorescence staining. *P<0.05 as indicated. TIAM1, T-cell
lymphoma invasion and metastasis 1; BLM, bleomycin; H&E,
hematoxylin and eosin; α-SMA, α-smooth muscle actin; IgG,
immunoglobulin G. |
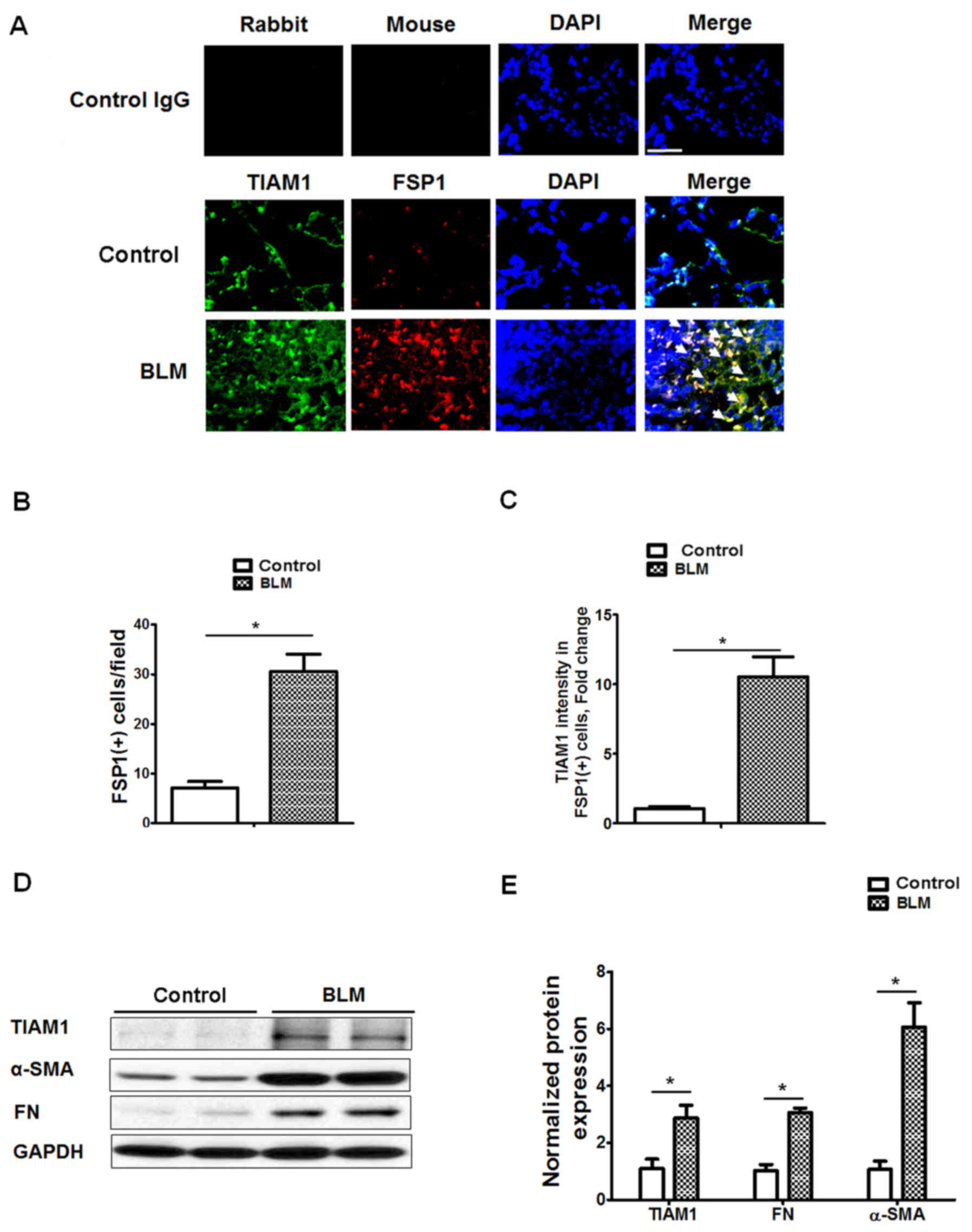
Expression of TIAM1 in mouse lung
fibroblasts
To investigate the expression of TIAM1 in mouse lung
fibroblasts, immunofluorescence staining for TIAM1 and FSP1 was
performed in mouse lung tissues from control and BLM-treated mice.
Staining indicated that the number FSP1(+) cells was significantly
increased in the fibrotic foci from BLM-treated mice compared with
control mice (P<0.05; Fig. 2A and
B), and the expression of TIAM1 was significantly increased in
the FSP1(+) cells (P<0.05; Fig. 2A
and C). Western blot results also indicated that the expression
levels of TIAM1, α-SMA and FN were significantly higher in lung
fibroblasts isolated from BLM-challenged mice compared with those
from control mice (P<0.05; Fig. 2D
and E). These data suggest that TIAM1 expression in lung
fibroblasts may be associated with fibroblast differentiation in
pulmonary fibrosis.
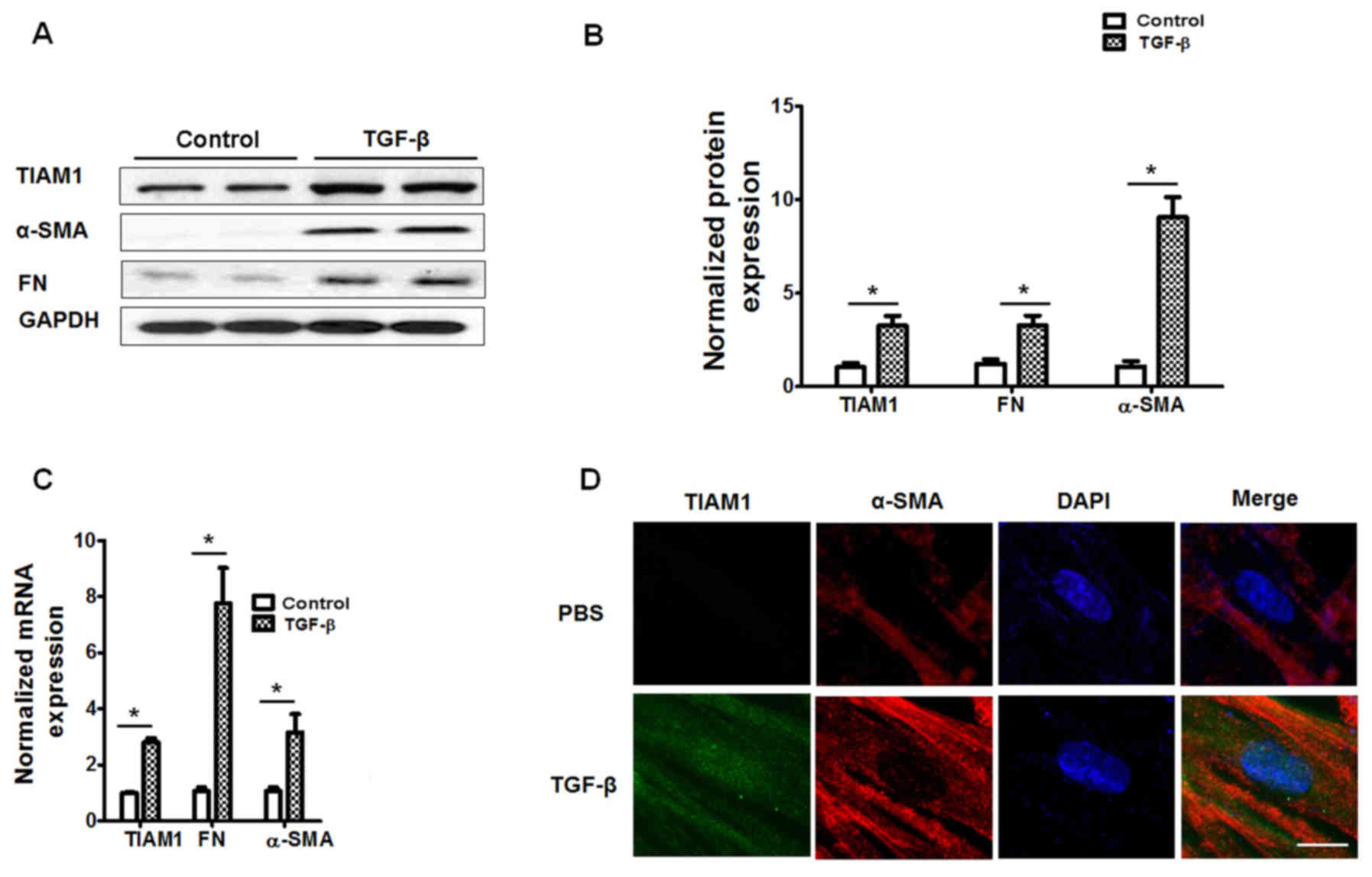
TIAM1 expression in TGF-β-treated
human lung fibroblasts
Human fibroblasts were challenged with TGF-β (5
ng/ml, 48 h) and the results demonstrated that TGF-β significantly
increased the mRNA and protein levels of FN and α-SMA (P<0.05;
Fig. 3A-C), indicating increased
fibroblast differentiation compared with control cells. Similarly,
cells challenged with TGF-β had significantly increased mRNA and
protein expression of TIAM1 compared with control cells (P<0.05;
Fig. 3A-C). Furthermore,
immunofluorescence staining indicated that the TGF-β challenge
increased the expression of TIAM1 in human lung fibroblasts
(Fig. 3D).
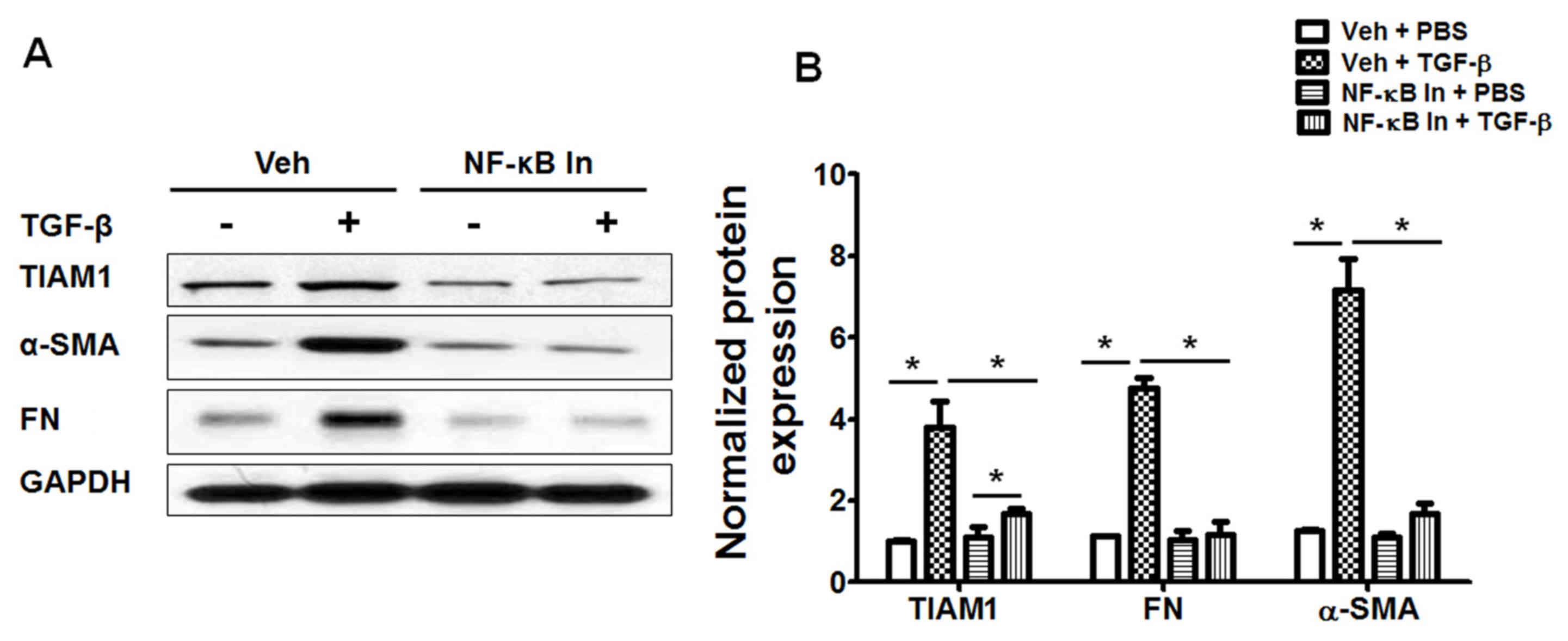
Inhibition of the NF-κB pathway
attenuates TGF-β-induced fibroblast differentiation and TIAM1
expression in human lung fibroblasts
It has previously been indicated that inhibiting the
NF-κB pathway via NF-κB inhibitor treatment or knockdown of the
NF-κB p65 subunit blocks TGF-β-induced differentiation in human
lung fibroblasts (17). In the
present study, fibroblasts were treated with Bay 11-7082, then
exposed to TGF-β treatment and assessed using western blotting. Bay
11-7082 treatment was demonstrated to significantly attenuate
TGF-β-induced fibroblast differentiation (P<0.05; Fig. 4) and markedly increase TIAM1
expression in human lung fibroblasts (Fig. 4A). These data suggest that TGF-β
induces TIAM1 expression in human lung fibroblasts via an
NF-κB-dependent pathway and may also be associated with fibroblast
differentiation.
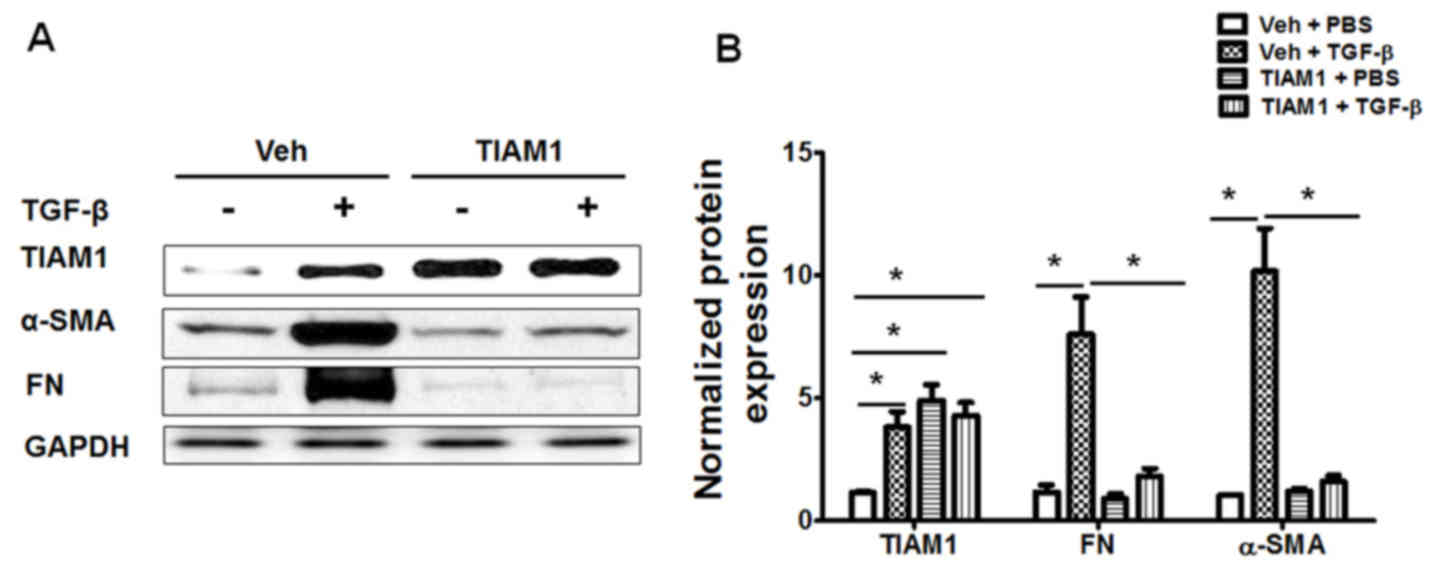
TIAM1 inhibits TGF-β-induced human
lung fibroblast differentiation
To assess the role of TIAM1 in TGF-β-induced
fibroblast differentiation, human lung fibroblasts were transfected
with plasmids coding human TIAM1 protein. Transfection with the
TIAM1 plasmid markedly increased the expression of TIAM1 protein in
human lung fibroblasts (Fig. 5A).
Furthermore, TIAM1 overexpression was observed to significantly
inhibit the TGF-β-induced overexpression of FN and α-SMA,
indicating that lung fibroblast differentiation was significantly
reduced compared with that of control cells (P<0.05; Fig. 5). The effect of TIAM1 knockdown was
also assessed via siRNA transfection. It was demonstrated that
TIAM1 knockdown significantly increased the TGF-β-induced
expression of FN and α-SMA in human lung fibroblasts (P<0.05;
Fig. 6). Together, these data
suggest that TIAM1 inhibits TGF-β-induced human lung fibroblast
differentiation.
Discussion
TIAM1 is a guanine nucleotide exchange factor, which
is crucially involved in tumor cell invasion and migration
(20,21). Clinical studies have suggested that
TIAM1 overexpression predicates poor overall survival in patients
with primary gallbladder carcinoma (22), prostate cancer (23) and hepatocellular carcinoma (24). In vitro studies in cancer cell
lines have indicated that the TIAM1 gene serves important roles in
the proliferation, invasion and metastasis of giant-cell lung
carcinoma (5), human breast cancer
(20), hepatocellular carcinoma
(25) and colorectal cancer
(26). However, there is limited
information available on the effects of TIAM1 on pulmonary
fibrosis. The results of the present study demonstrate that TIAM1
expression is associated with pulmonary fibrosis in a BLM-induced
mouse model of pulmonary fibrosis. Immunostaining and western
blotting data indicate that the expression of TIAM1 is
significantly higher in fibroblasts from fibrotic lung tissue
compared with those from controls. These data suggest that TIAM1
serves an important role in pulmonary fibrosis.
Fibroblasts are activated following tissue injury.
In physiological conditions, activated fibroblasts produce ECM
proteins followed by apoptosis; however, under pathological
conditions, including in IPF, the activated fibroblasts resist
apoptosis and exhibit high proliferation and differentiation
(13,27,28).
Activated fibroblasts induce the expression and secretion of TGF-β
and fibroblast growth factor (13,29,30),
which stimulate growth and proliferation in various cells,
including fibroblasts and breast cancer cells (31). In cancer tissues,
carcinoma-associated fibroblasts highly express α-SMA, fibroblast
activation protein and fibroblast surface protein, which are
protein markers of differentiated fibroblasts (32). Furthermore, activation of
carcinoma-associated fibroblasts has been demonstrated to inhibit
apoptosis in hepatocellular carcinoma cells (32). These observations suggest that
fibroblast differentiation and activation are critical to the
resistance of cancer cells to apoptosis.
Previous studies have indicated that the molecular
mechanisms of IPF may be associated with lung cancer (33,34).
Elderly male cigarette smokers with IPF were demonstrated to be at
a higher risk of developing lung cancer, and tumors were more
commonly observed in the lower lobes and peripheral regions of
lungs (35). The results of the
present study suggest that the oncogene TIAM1 also serves critical
roles in pulmonary fibrosis, specifically in lung fibroblasts. They
also indicate that the expression of TIAM1 is associated with
differentiation of lung fibroblasts, and that TGF-β, which is a key
factor of pulmonary fibrosis, directly stimulates fibroblast
differentiation and TIAM1 expression via an NF-κB-dependent
pathway. A previous study of lung fibroblasts indicated that
differentiation may be associated with cell invasion and lung
fibrogenesis (36), which suggests
that TIAM1 may be essential for fibrotic phenotype changes in
differentiated lung fibroblasts. TIAM1 knockdown in fibroblasts has
previously been reported to enhance the invasion of lung cancer
cells in vitro (5). The
results of the present study revealed that TIAM1 overexpression
blocks the TGF-β-induced differentiation of fibroblasts, which may
decrease fibrosis in vivo. Furthermore, knocking down the
expression of endogenous TIAM1 further augments fibroblast
differentiation. These data suggest that TIAM1 serves different
roles in fibroblast differentiation and cancer cell invasion.
However, further investigation is required to elucidate the role of
TIAM1 in fibroblast migration and invasion.
The present study indicates that the expression of
TIAM1 may be dependent on fibroblast differentiation.
Differentiated fibroblasts express higher level of ECM proteins,
including collagen and FN, compared with undifferentiated
fibroblasts (12,13). In pancreatic β-cells, ECM proteins
stimulate cell proliferation, and induce NF-κB nuclear
translocation and the expression of NF-κB inhibitor (37). Furthermore, blocking the NF-κB
pathway completely blocks ECM-induced cell proliferation (37) and the ECM-induced NF-κB pathway is
essential for maintaining glucose-induced expression and the
secretion of insulin in pancreatic β-cells (38). However, the effect of ECM in the
NF-κB pathway in fibroblasts remains to be elucidated. The results
of the present study demonstrate that inhibiting the NF-κB pathway
blocks the TGF-β-induced expression of FN and TIAM1, and indicated
that TIAM1 expression depends on NF-κB activation. During lung
fibrogenesis, differentiated fibroblasts display higher expression
level and nuclear translocation of the NF-κB p65 subunit (39), which suggests that differentiated
fibroblasts may induce activation of the NF-κB pathway. However,
the underlying molecular mechanisms require further study.
In conclusion, the results of the present study
suggest that TIAM1, an oncogene, is also associated with pulmonary
fibrosis. The expression of TIAM1 is upregulated in fibroblasts
during differentiation, whereas overexpression of TIAM1 attenuates
TGF-β-induced differentiation in human lung fibroblasts. The data
in the present study indicate that TIAM1 serves critical roles in
BLM-induced lung fibrosis in mice, and inhibits the differentiation
of pulmonary fibroblasts. These data suggest that TIAM1 is a
potential therapeutic target in pulmonary fibrosis.
Glossary
Abbreviations
Abbreviations:
|
TIAM1
|
T-cell lymphoma invasion and
metastasis 1
|
|
IPF
|
idiopathic pulmonary fibrosis
|
|
FN
|
fibronectin
|
|
ECM
|
extracellular matrix
|
|
α-SMA
|
α-smooth muscle actin
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
TGF-β
|
transforming growth factor-β
|
|
BLM
|
bleomycin
|
References
|
1
|
Bourguignon LY, Zhu H, Shao L and Chen YW:
Ankyrin-Tiam1 interaction promotes Rac1 signaling and metastatic
breast tumor cell invasion and migration. J Cell Biol. 150:177–191.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ceccarelli DF, Blasutig IM, Goudreault M,
Li Z, Ruston J, Pawson T and Sicheri F: Non-canonical interaction
of phosphoinositides with pleckstrin homology domains of Tiam1 and
ArhGAP9. J Biol Chem. 282:13864–13874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leeuwen FN, Kain HE, Kammen RA, Michiels
F, Kranenburg OW and Collard JG: The guanine nucleotide exchange
factor Tiam1 affects neuronal morphology; opposing roles for the
small GTPases Rac and Rho. J Cell Biol. 139:797–807. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paliwal S, Ho N, Parker D and Grossman SR:
CtBP2 promotes human cancer cell migration by transcriptional
activation of tiam1. Genes Cancer. 3:481–490. 2012.PubMed/NCBI
|
|
5
|
Wang HM and Wang J: Expression of Tiam1 in
lung cancer and its clinical significance. Asian Pac J Cancer Prev.
13:613–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaughan L, Tan CT, Chapman A, Nonaka D,
Mack NA, Smith D, Booton R, Hurlstone AF and Malliri A: HUWE1
ubiquitylates and degrades the RAC activator TIAM1 promoting
cell-cell adhesion disassembly, migration, and invasion. Cell Rep.
10:88–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minard ME, Herynk MH, Collard JG and
Gallick GE: The guanine nucleotide exchange factor Tiam1 increases
colon carcinoma growth at metastatic sites in an orthotopic nude
mouse model. Oncogene. 24:2568–2573. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mertens AE, Pegtel DM and Collard JG:
Tiam1 takes PARt in cell polarity. Trends Cell Biol. 16:308–316.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Desai LP, Chapman KE and Waters CM:
Mechanical stretch decreases migration of alveolar epithelial cells
through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell
Mol Physiol. 295:L958–L965. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
The Lancet Respiratory Medicine: The
changing landscape of idiopathic pulmonary fibrosis. Lancet Respir
Med. 2:5072014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang LS and Natarajan V: Sphingolipids in
pulmonary fibrosis. Adv Biol Regul. 57:55–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wynn TA: Integrating mechanisms of
pulmonary fibrosis. J Exp Med. 208:1339–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sime PJ, Xing Z, Graham FL, Csaky KG and
Gauldie J: Adenovector-mediated gene transfer of active
transforming growth factor-beta1 induces prolonged severe fibrosis
in rat lung. J Clin Invest. 100:768–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu P, Liu J and Derynck R:
Post-translational regulation of TGF-β receptor and Smad signaling.
FEBS Lett. 586:1871–1884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song N, Liu J, Shaheen S, Du L, Proctor M,
Roman J and Yu J: Vagotomy attenuates bleomycin-induced pulmonary
fibrosis in mice. Sci Rep. 5:134192015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi SH, Kim M, Lee HJ, Kim EH, Kim CH and
Lee YJ: Effects of NOX1 on fibroblastic changes of endothelial
cells in radiation-induced pulmonary fibrosis. Mol Med Rep.
13:4135–4142. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minard ME, Kim LS, Price JE and Gallick
GE: The role of the guanine nucleotide exchange factor Tiam1 in
cellular migration, invasion, adhesion and tumor progression.
Breast Cancer Res Treat. 84:21–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu S, Li Y, Qi W, Zhao Y, Huang A, Sheng
W, Lei B, Lin P, Zhu H, Li W and Shen H: Expression of Tiam1
predicts lymph node metastasis and poor survival of lung
adenocarcinoma patients. Diagn Pathol. 9:692014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Du X, Wang S, Lu J, Wang Q, Song N, Yang
T, Dong R, Zang L, Yang Y, Wu T and Wang C: Clinical value of
Tiam1-Rac1 signaling in primary gallbladder carcinoma. Med Oncol.
29:1873–1878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Engers R, Mueller M, Walter A, Collard JG,
Willers R and Gabbert HE: Prognostic relevance of Tiam1 protein
expression in prostate carcinomas. Br J Cancer. 95:1081–1086. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Ye X, Guan J, Chen B, Li Q, Zheng
X, Liu L, Wang S, Ding Y, Ding Y and Chen L: Tiam1 is associated
with hepatocellular carcinoma metastasis. Int J Cancer. 132:90–100.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ding Y, Chen B, Wang S, Zhao L, Chen J,
Ding Y, Chen L and Luo R: Overexpression of Tiam1 in hepatocellular
carcinomas predicts poor prognosis of HCC patients. Int J Cancer.
124:653–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Wu DH and Ding YQ: Tiam1 gene
expression and its significance in colorectal carcinoma. World J
Gastroenterol. 11:705–707. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nho RS and Polunovsky V: Translational
control of the fibroblast-extracellular matrix association: An
application to pulmonary fibrosis. Translation (Austin).
1:e239342013.PubMed/NCBI
|
|
28
|
Darby IA, Laverdet B, Bonté F and
Desmoulieré A: Fibroblasts and myofibroblasts in wound healing.
Clin Cosmet Investig Dermatol. 7:301–311. 2014.PubMed/NCBI
|
|
29
|
Gohda E, Matsunaga T, Kataoka H, Takebe T
and Yamamoto I: Induction of hepatocyte growth factor in human skin
fibroblasts by epidermal growth factor, platelet-derived growth
factor and fibroblast growth factor. Cytokine. 6:633–640. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Strutz F, Zeisberg M, Renziehausen A,
Raschke B, Becker V, van Kooten C and Müller G: TGF-beta 1 induces
proliferation in human renal fibroblasts via induction of basic
fibroblast growth factor (FGF-2). Kidney Int. 59:579–592. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andre F and Cortés J: Rationale for
targeting fibroblast growth factor receptor signaling in breast
cancer. Breast Cancer Res Treat. 150:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song T, Dou C, Jia Y, Tu K and Zheng X:
TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor
apoptosis by activating SDF1/CXCR4 signaling in hepatocellular
carcinoma. Oncotarget. 6:12061–12079. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mizuno K, Mataki H, Seki N, Kumamoto T,
Kamikawaji K and Inoue H: MicroRNAs in non-small cell lung cancer
and idiopathic pulmonary fibrosis. J Hum Genet. 62:57–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stella GM, Inghilleri S, Pignochino Y,
Zorzetto M, Oggionni T, Morbini P and Luisetti M: Activation of
oncogenic pathways in idiopathic pulmonary fibrosis. Transl Oncol.
7:650–655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Archontogeorgis K, Steiropoulos P,
Tzouvelekis A, Nena E and Bouros D: Lung cancer and interstitial
lung diseases: A systematic review. Pulm Med. 2012:3159182012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Y, Jiang D, Liang J, Meltzer EB, Gray
A, Miura R, Wogensen L, Yamaguchi Y and Noble PW: Severe lung
fibrosis requires an invasive fibroblast phenotype regulated by
hyaluronan and CD44. J Exp Med. 208:1459–1471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Parnaud G, Hammar E, Ribaux P, Donath MY,
Berney T and Halban PA: Signaling pathways implicated in the
stimulation of beta-cell proliferation by extracellular matrix. Mol
Endocrinol. 23:1264–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hammar EB, Irminger JC, Rickenbach K,
Parnaud G, Ribaux P, Bosco D, Rouiller DG and Halban PA: Activation
of NF-kappaB by extracellular matrix is involved in spreading and
glucose-stimulated insulin secretion of pancreatic beta cells. J
Biol Chem. 280:30630–30637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun X, Chen E, Dong R, Chen W and Hu Y:
Nuclear factor (NF)-κB p65 regulates differentiation of human and
mouse lung fibroblasts mediated by TGF-β. Life Sci. 122:8–14. 2015.
View Article : Google Scholar : PubMed/NCBI
|