Introduction
Myocardial infarction (MI), commonly referred to as
a heart attack, occurs when blood flow stops to part of the heart
causing damage to the heart muscle (1). With increasing morbidity and death
rates, it has become a great threat to human health. The leading
cause of MI at the acute phase is acute heart failure (2). With the development of medical
techniques, thrombolysis, interventional stent and bypass surgery
have greatly decreased mortality rates of the conversion from acute
MI to acute heart failure (3).
However, heart failure can be become chronic. Priority is given to
remodeling of cardiac fibrosis for chronic MI. Excessive cardiac
fibrosis remodeling causes heart failure.
As an inhalation anesthetic, isoflurane is does not
irritate the respiratory system (4).
When used as an anesthesia for teenagers, adults and the elderly,
it is stable in induction with high recovery quality (5). N2O is not toxic, has strong
analgesic effects and patients are quickly awoken (5). Consequently, it is widely employed in
clinics.
With high lipid solubility and low water solubility,
blood concentrations of N2O peak after intravenous
injection of 2.5 mg/kg of propofol after 2 min (6). Redistribution quickly occurs, resulting
in a quick decrease in blood concentration (5). Characterized by quickly taking effect
without drug accumulation and patients being easily awoken,
propofol metabolizes in the liver and its elimination half life is
30–60 min (7). Fentanyl is a newly
discovered narcotic opiate analgesic, which has similar
pharmacological functions to other opiates, such as relieving pain
and calming (8). The efficacy of
fentanyl peaks 3–5 min after intravenous injection and metabolizes
through the liver. Its terminal half time is 2 to 4 h (9). With specific pharmacokinetic features,
it takes effect fast and peaks after 1.6 min of intravenous
injection. It can be degraded via non-specific esterase in red
blood cells and tissues (10). With
a terminal half life of 0.1 to 0.6 h, its clearance rates are not
influenced by hepatic and renal functions, which is a key advantage
in addition to its high safety index, short waking time, reduced
respiratory depression and stable hemodynamics (11).
In the present study, the effect of isoflurane +
N2O inhalation and propofol + fentanyl anesthesia on
myocardial function was investigated, as assessed by cardiac
troponin in patients or the established rat model.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee
(grant no. 20081009) of Zhongshan Hospital affiliated to Xiamen
University (Xiamen, China). Written informed consent was obtained
from all participants who were involved in the study. All
procedures involving experimental animals were performed in
accordance with the protocols that were approved by the Committee
for Animal Research of Xiamen University and complied with the
Guideline for the Care and Use of Laboratory Animals.
Study population and administration of
anesthesia
All patients were aged 20–45 years and had been
admitted to The First Affiliated Hospital of Xiamen University
between July 2013 and December 2014. Patients were randomly split
into two equal groups. The following inclusion criteria was applied
to all patients in the present study: i) Patients with ongoing
myocardial damage (LV dysfunction, electrocardiographic
abnormalities or elevated troponin); and ii) patients exhibtied new
onset or persistent symptoms suggestive of myocarditis. The
following exclusion criteria was followed: i) exclusion for history
of systemic viral disease; and ii) exclusion for relevant coronary
artery disease (CAD) on angiography. One group received isoflurane
+ N2O inhalation (n=30) and the other group received
propofol + fentanyl anesthesia (n=30). In the isoflurane +
N2O inhalation group, patients were given 1.5%
isoflurane + NO2/O2 (50/50%) after induction
with thiopental sodium (5 mg/kg) + fentanyl (1 µg/kg). In the
propofol + fentanyl anesthesia group, patients were given propofol
(0.2 mg/kg/min) and fentanyl (0.2 µg/kg/min), and ventilated with
50% O2 and 50% air, after induction with propofol (3
mg/kg) and fentanyl (5 µg/kg).
Animals and experimental
procedures
A total of 20 male Sprague-Dawley rats (aged 8
weeks) were housed at 22–24°C, 12-h light/dark cycle and 50–60%
humidity with free access to food and water. All Sprague-Dawley
rats were randomly assigned into two groups: One group received
isoflurane + N2O inhalation (n=10) and the other group
received propofol + fentanyl anesthesia (n=10). In the isoflurane +
N2O inhalation group, rats were performed with 1.5%
isoflurane + NO2/O2 (50%/50%) in the buffer
for 30 min after induction with thiopental sodium (5 mg/kg) +
fentanyl (1 µ/kg). In the propofol + fentanyl anesthesia group,
rats were administered propofol (0.2 mg/kg/min) and fentanyl (0.2
µg/kg/min), and ventilated with 50% O2 and 50% air in
the buffer for 30 min after induction with propofol (3 mg/kg) and
fentanyl (5 µg/kg).
To establish MI in rats, all the rats were
anesthetized with 35 mg/kg of pentobarbital and carefully
catheterized with a stump needle. An incision was cut along the
left side of sternum, the thorax was opened and the pericardium was
exposed. Left coronary artery (LCA) was ligated via using a 6–0
prolene suture and the chest was closed.
Hematoxylin and eosin (H&E)
staining
Hearts were harvested from the experiment rats and
perfusion-fixed with 4% buffered formalin (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) for 24 h at room temperature. Tissue
samples were horizontally sectioned and embedded in paraffin.
Tissue sections were cut into 5-µm thick slices and subsequently
stained with H&E (Beyotime Institute of Biotechnology,
Shanghai, China) for 5–10 min at room temperature and analyzed
using Image Pro-Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA).
Detection index
Blood samples (500 µl) were obtained from rats after
treatment with isoflurane + N2O inhalation and propofol
+ fentanyl anesthesia, and transferred to sterile tubes without
EDTA and heparin prior to centrifugation at 3,000 × g for 10 min at
4°C, and serum was collected and storage at −20°C. Nuclear factor
(NF)-κB of p65 (ml003404), interleukin (IL)-6 (ml102828),
superoxide dismutase (SOD; ml540172), glutathione (GSH; ml531010),
glutathione peroxidase (GSH-PX; ml097316) and malondialdehyde (MDA;
ml022446) activities were measured using ELISA kits (Shanghai
Enzyme-linked Biotechnology Co, Shanghai, China). Caspase-3 and −9
activity was measured using Ac-DEVD-pNA/LEHD-pNA (C1115 and C1157;
Beyotime Institute of Biotechnology; Nanjing, China) and incubated
for 2 h at 37°C. Activities were determined using an ELISA plate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
Total protein from rat hippocampi was extracted
using protein lysis buffer containing protease inhibitor (PMSF;
Beyotime Institute of Biotechnology; Nanjing, China) at 4°C for 30
min and centrifuged for 10 min at 4°C at 12,000 × g. Total cellular
proteins (50 µg) were separated by 12% SDS-PAGE and
electrotransferred to polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.). The membrane was blocked with 5% skimmed milk
powder in Tris-buffered saline with Tween-20 (TBST) for 1 h at 37°C
and incubated with the following primary antibodies:
Anti-cyclooxygenase-2 (sc-7951; COX-2; 1:3,000), anti-inducible
nitric oxide synthase (sc-649; iNOS; 1:3,000) and anti-β-actin
(sc-7210; 1:5,000; all Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) at 4°C overnight. Subsequently, the membrane was washed with
TBST three times for 15 min and incubated with an anti-mouse or
anti-rabbit IgG secondary antibody conjugated to horseradish
peroxidase (sc-2004, 1:5,000; Santa Cruz Biotechnology, Inc.) for 1
h at 37°C and visualized using enhanced chemiluminescence reagent
(Beyotime Institute of Biotechnology, Jiangsu, China). Protein
bands were analyzed using Bio-Rad Laboratories Quantity One
software 3.0 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using SPSS 13.0 for Windows (SPSS, Inc., Chicago,
IL, USA). One-way analysis of variance tests were performed
followed by the Student-Newman-Keuls test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Basic characteristics of patients
In the isoflurane + N2O inhalation group,
the mean age of patients was 37.81±6.71 years, their body weight
was 65.59±2.12 kg and the female:male ratio was 20:0 (Table I). In the propofol + fentanyl
anesthesia group, the mean age of patients was 38.33±6.21 years,
their body weight was 66.48±1.89 kg and the female:male ratio was
20:0 (Table I).
 | Table I.Basic characteristics of the patients
in the two groups. |
Table I.
Basic characteristics of the patients
in the two groups.
| Group | Control group | Intervention
group | P-value |
|---|
| Age (years) | 37.81±6.71 | 38.33±6.21 | 0.812 |
| Body weight (kg) | 65.59±2.12 | 66.48±1.89 | 0.871 |
| Sex (F/M) | 20/0 | 20/0 |
|
Hemodynamic parameters in
patients
Prior to induction, as shown in Table II, the preanesthetic and anesthetic
hemodynamic parameters of patients in the isoflurane +
N2O inhalation group were similar to those of patients
in the propofol + fentanyl anesthesia group (P>0.05). However,
the pulse rates of those in the isoflurane + N2O
inhalation group were lower than that of the propofol + fentanyl
anesthesia group (P<0.05; Table
II).
 | Table II.Hemodynamic parameters of the patients
in the two groups. |
Table II.
Hemodynamic parameters of the patients
in the two groups.
| Parameters | Control group | Intervention
group | P-value |
|---|
| Before
induction |
| SBP
(mm/Hg) | 126.28±4.71 | 125.91±5.21 | 0.871 |
| DBP
(mm/Hg) | 76.12±2.33 | 76.72±2.67 | 0.912 |
| Pulse
(per min) | 80.91±2.47 | 79.87±2.03 | 0.638 |
|
O2 saturation
(%) | 96.14±0.51 | 96.21±0.57 | 0.899 |
| After
induction |
| SBP
(mm/Hg) | 127.58±5.26 | 132.91±4.94 | 0.661 |
| DBP
(mm/Hg) | 83.07±1.93 | 79.67±2.38 | 0.697 |
| Pulse
(per min) | 89.51±3.08 | 76.92±3.18 | 0.001 |
|
O2 saturation
(%) | 97.93±0.39 | 97.01±0.49 | 0.812 |
Cardiac troponin T (cTnT) levels in
patients
Levels of cTnT and troponin from the two patient
groups are presented in Tables III
and IV. There was no significant
inter-group difference between the isoflurane + N2O
inhalation group and propofol + fentanyl anesthesia group when
assessing the level of cTnT and troponin (P>0.05; Tables III and IV).
 | Table III.Cardiac troponin T levels of the
patients in the two groups. |
Table III.
Cardiac troponin T levels of the
patients in the two groups.
| Variable
(ng/ml) | Control group | Intervention
group | P-value |
|---|
| Preanesthetic | 0.22±0.02 | 0.23±0.03 | 0.276 |
| Anesthetic | 0.24±0.03 | 0.21±0.01 | 0.179 |
| Postanesthetic | 0.23±0.03 | 0.22±0.03 | 0.233 |
 | Table IV.Troponin levels of the patients in
the two groups. |
Table IV.
Troponin levels of the patients in
the two groups.
| Group | Control group
(P-value) | Intervention group
(P-value) |
|---|
|
Preanesthetic/anesthetic | 0.731 | 0.392 |
|
Anesthetic/postanesthetic | 0.512 | 0.478 |
H&E staining in rat models of
myocardial infarction (MI)
To examine the effect of isoflurane + N2O
inhalation and propofol + fentanyl anesthesia on myocardial
function in rats, a model of MI was induced using male
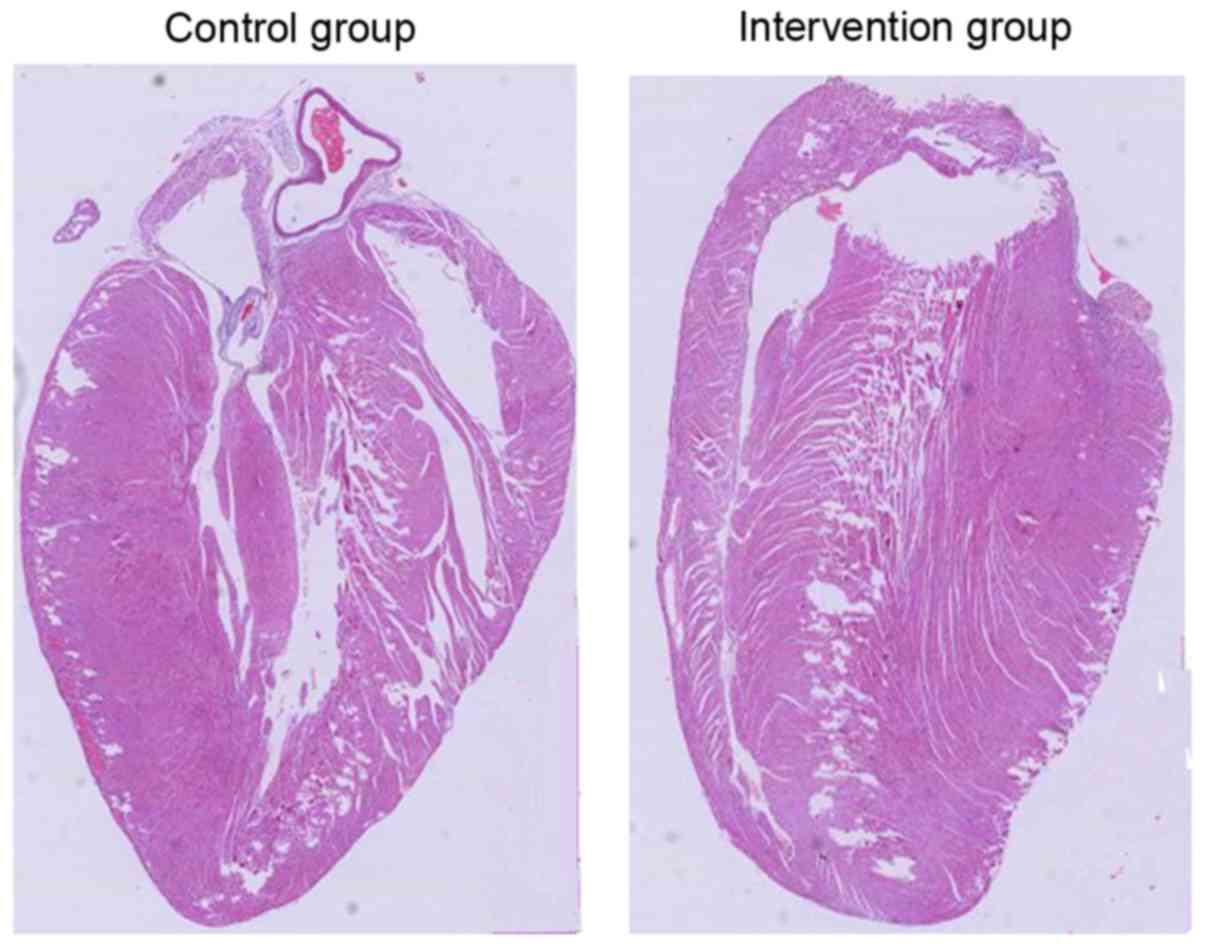
Sprague-Dawley rats. As shown in Fig.
1, there was no notable difference between these two
experimental groups in terms of myocardial cell damage.
Inflammation levels in rat models of
MI
To examine whether the effect of isoflurane +
N2O inhalation and propofol + fentanyl anesthesia on
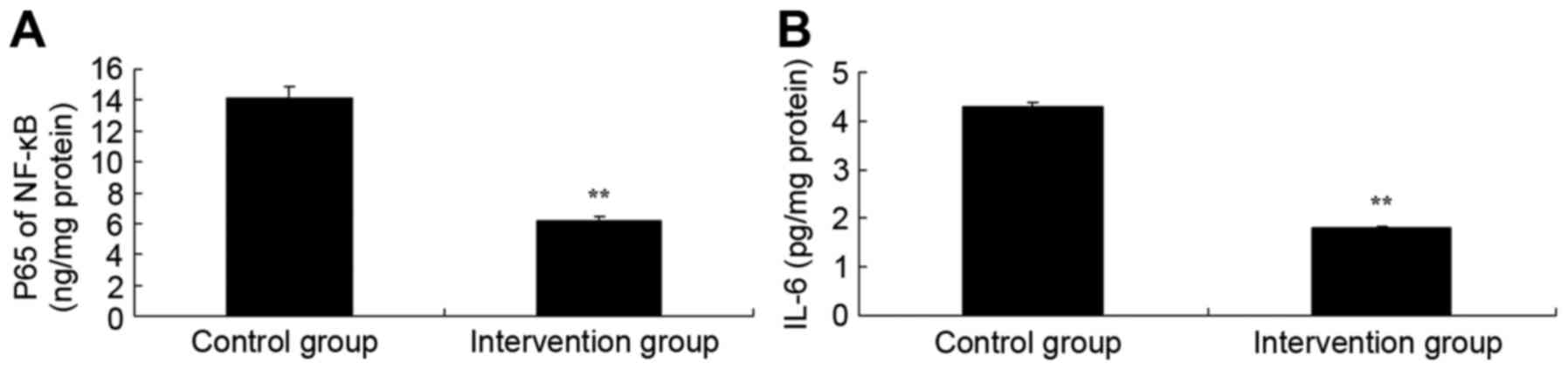
inflammation levels in rats, NF-κB of p65 and IL-6 activities were
measured. As shown in Fig. 2, NF-κB
of p65 and IL-6 activities of isoflurane + N2O
inhalation group were significantly lower than those of the
propofol + fentanyl anesthesia group (P<0.05). These findings
demonstrated that propofol + fentanyl anesthesia possesses
anti-inflammation effects in rats.
Oxidative stress levels in rat models
of MI
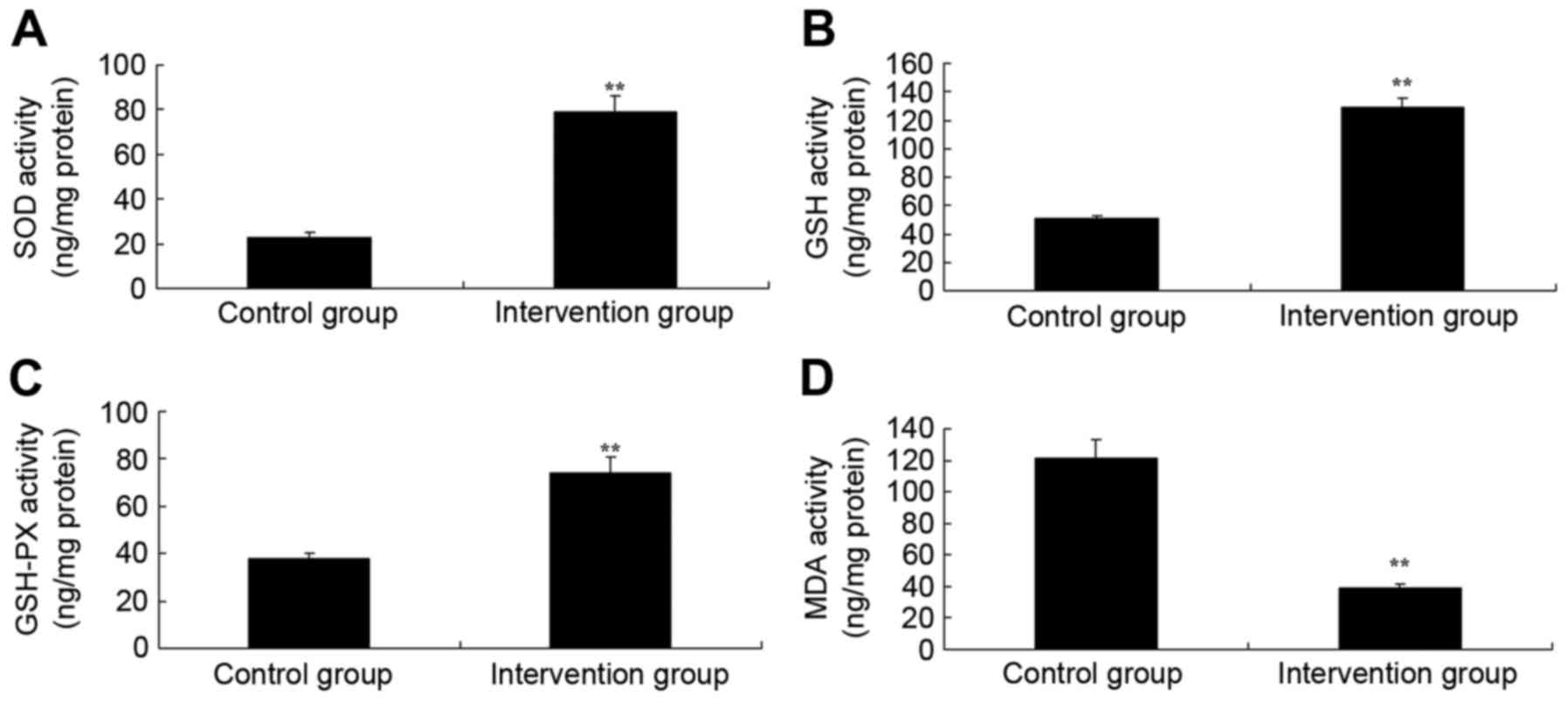
To further investigate the effect of isoflurane +
N2O inhalation and propofol + fentanyl anesthesia on
oxidative stress levels in rats, the activities of SOD, GSH, GSH-PX
and MDA were measured using ELISA kits. As shown in Fig. 3, the activities of SOD, GSH and
GSH-PX of isoflurane + N2O inhalation group were lower
and those of propofol + fentanyl anesthesia group; whereas the
activity of MDA in the isoflurane + N2O inhalation group
was significantly lower than that of the propofol + fentanyl
anesthesia group. These findings demonstrated that propofol +
fentanyl anesthesia also possesses anti-oxidative effects in
rats.
Caspase-3 and −9 activity
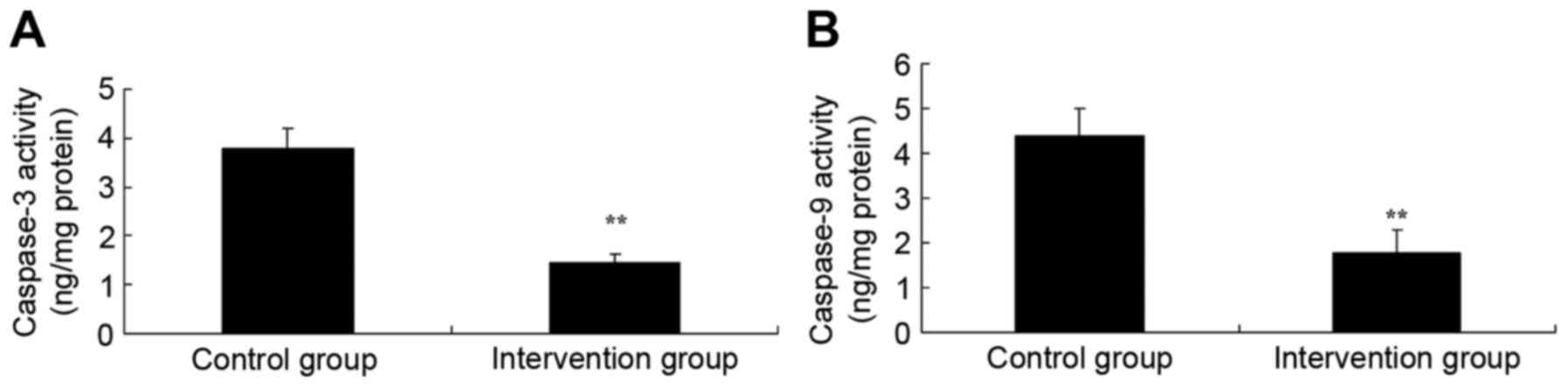
Using ELISA kits, the effects of isoflurane +
N2O inhalation and propofol + fentanyl anesthesia on
caspase-3 and −9 activity were determined in the rat models of MI.
As shown in Fig. 4, caspase-3 and −9
activities in the isoflurane + N2O inhalation group were
significantly suppressed, as compared with the propofol + fentanyl
anesthesia group (P<0.05). The study indicated that propofol +
fentanyl anesthesia inhibited caspase-3/9 activity to reduce heart
cell apoptosis in in rats of MI.
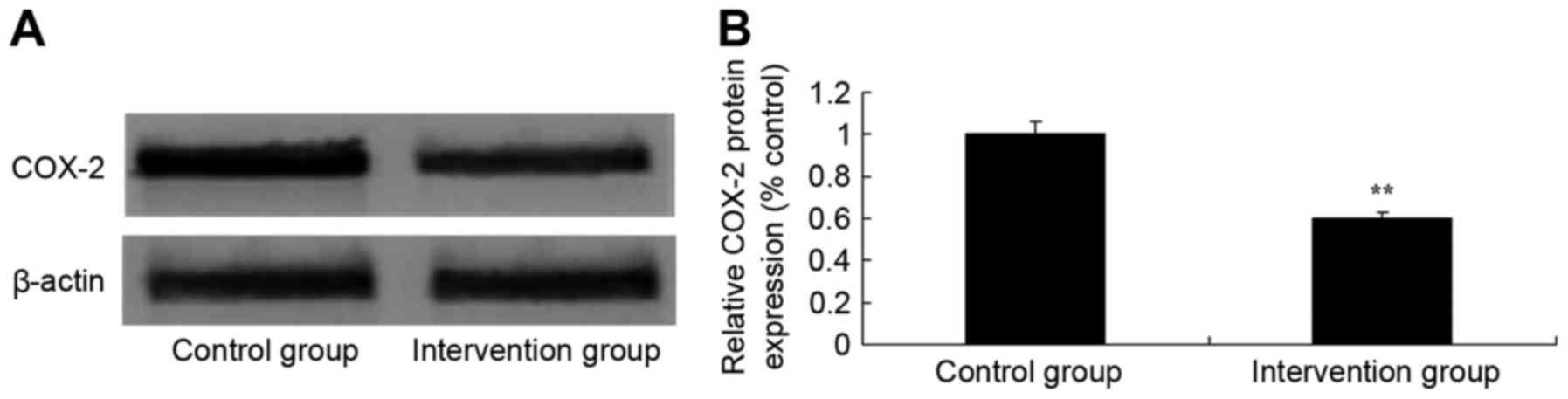
COX-2 protein expression
Using western blot analysis, the effect of
isoflurane + v inhalation and propofol + fentanyl anesthesia on
COX-2 protein expression levels was determined. Western blot
analysis demonstrated that COX-2 protein expression levels in the
isoflurane + N2O inhalation group were significantly
inhibited, as compared with the propofol + fentanyl anesthesia
group, which suggested that propofol + fentanyl anesthesia weakened
COX-2 protein expression and prevented inflammation in rats of MI
(P<0.05; Fig. 5).
iNOS protein expression
To further confirm the effect of isoflurane +
N2O inhalation and propofol + fentanyl anesthesia on
iNOS protein expression, iNOS protein expression levels were
detected using western blot analysis. As shown in Fig. 6, iNOS protein expression in the
isoflurane + N2O inhalation group was significantly
higher than that of the propofol + fentanyl anesthesia group
(P<0.05). These findings demonstrated that propofol + fentanyl
anesthesia also promoted iNOS protein expression in rats of MI.
Discussion
MI is an ischemic heart disease. When the coronary
artery or branches have lesions, stenosis or blocking occurs, which
causes myocardial ischemic-anoxic injury or necrosis (12). Neither traditional therapy nor newer
intervention therapy has been able to achieve satisfactory effects
for diffuse injuries of coronary arterioles (13). This is mainly as blood
micro-circulation at the ischemic region cannot be improved.
In this study, the pulse rates of the isoflurane +
N2O inhalation group were lower than that of the
propofol + fentanyl anesthesia group. Clinically, the judgment of
anesthesia depth for patients is based on heart rate, blood
pressure and body movement (14).
However, the dose of propofol was decreased in anesthetic depth
monitoring, which suggests that blood pressure and heart rate are
inadequate for judging anesthesia depth, which may lead to
excessive dosages of anesthetic drugs (6). Our study showed that no significant
inter-group difference existed between the isoflurane +
N2O inhalation group and the propofol + fentanyl
anesthesia group in terms of the levels of cTnT and troponin.
N2O, which is colorless, odorless and
non-irritating, is an inorganic gas with a low blood/gas
distribution coefficient (5). In
addition to rapid induction, quick waking and non-irritation of the
respiratory tract, N2O does not damage important organs,
such as the heart, lung, liver and kidneys (15). It does not participate in
bio-conversion or degradation in vivo, thus it is
predominantly discharged when breathing (16). There is minimal evaporation through
skin without accumulation; therefore, N2O is an ideal
inhaled anesthetic (17). As
anesthesia performance of N2Ois low and weak, a high
concentration of single use may cause anoxia; thus, its
concentration should not be higher than 60% (18). We found that NF-κB of p65 and IL-6,
caspase-3 and 9 activities in the isoflurane + N2O
inhalation group were suppressed, compared with the propofol +
fentanyl anesthesia group.
Fentanyl-propofol is a common intravenous anesthesia
(19). However, respiratory
depression of fentanyl during surgery is rather common and
hyperpathia after surgery occurs frequently (19). Propofol is an
alkylphenolsedative-hypnotic drug and its advantages include quick
effects, short hold time and fast waking (20). It is eliminated through the liver
without accumulation. It also has weak antiemetic and analgesic
functions (21). The greatest
disadvantage of propofol is dose-dependent respiratory inhibition
and the fact that it is influenced by injection rates (22). Large dosages induce notable decreases
in blood pressure, heart rate, hyoxemia, bradypnea or apnea. With
short-term effect, fentanyl is a new opium analgesics (23). It can be degraded quickly through
non-specific esterase in blood and tissues and its elimination rate
is not affected by hepatorenal functions (24). Characterized by taking effect
quickly, strong abirritation, quick recovery times and a lack of
accumulation, it rarely exhibits side effects, such as decreased
blood pressure, declined heart rate and breath inhibition, which
are related with dosage and injection rates (25). COX-2 and iNOS protein expression
levels in the isoflurane + N2O inhalation group were
inhibited and activated, respectively, as compared with the
propofol + fentanyl anesthesia group. In conclusion, isoflurane +
N2O inhalation and propofol + fentanyl anesthesia were
demonstrated to not affect myocardial function.
References
|
1
|
Bayir H, Kapralov AA, Jiang J, Huang Z,
Tyurina YY, Tyurin VA, Zhao Q, Belikova NA, Vlasova II, Maeda A, et
al: Peroxidase mechanism of lipid-dependent cross-linking of
synuclein with cytochrome C: Protection against apoptosis versus
delayed oxidative stress in Parkinson disease. J Biol Chem.
284:15951–15969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huerta C, Sánchez-Ferrero E, Coto E,
Blázquez M, Ribacoba R, Guisasola LM, Salvador C and Alvarez V: No
association between Parkinson's disease and three polymorphisms in
the eNOS, nNOS, and iNOS genes. Neurosci Lett. 413:202–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Przedborski S, Jackson-Lewis V, Yokoyama
R, Shibata T, Dawson VL and Dawson TM: Role of neuronal nitric
oxide in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
(MPTP)-induced dopaminergic neurotoxicity. Proc Natl Acad Sci USA.
93:4565–4571. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pontone GM, Palanci J, Williams JR and
Bassett SS: Screening for DSM-IV-TR cognitive disorder NOS in
Parkinson's disease using the Mattis Dementia Rating Scale. Int J
Geriatr Psychiatry. 28:364–371. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manosroi A, Kitdamrongtham W, Ishii K,
Shinozaki T, Tachi Y, Takagi M, Ebina K, Zhang J, Manosroi J,
Akihisa R and Akihisa T: Limonoids from Azadirachta indica
var. Siamensis extracts and their cytotoxic and
melanogenesis-inhibitory activities. Chem Biodivers. 11:505–531.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martins VL, Caley MP, Moore K, Szentpetery
Z, Marsh ST, Murrell DF, Kim MH, Avari M, McGrath JA, Cerio R, et
al: Suppression of TGFβ and Angiogenesis by Type VII Collagen in
Cutaneous SCC. J Natl Cancer Inst. 108:pii: djv293. 2015.PubMed/NCBI
|
|
7
|
Lindholm EE, Aune E, Noren CB, Seljeflot
I, Hayes T, Otterstad JE and Kirkeboen KA: The anesthesia in
abdominal aortic surgery (ABSENT) study: A prospective, randomized,
controlled trial comparing troponin T release with
fentanyl-sevoflurane and propofol-remifentanil anesthesia in major
vascular surgery. Anesthesiology. 119:802–812. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klein BY, Tamir H, Hirschberg DL, Ludwig
RJ, Glickstein SB, Myers MM and Welch MG: Oxytocin opposes effects
of bacterial endotoxin on ER-stress signaling in Caco2BB gut cells.
Biochim Biophys Acta. 1860:402–411. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoneshima E, Okamoto K, Sakai E,
Nishishita K, Yoshida N and Tsukuba T: The transcription factor EB
(TFEB) regulates osteoblast differentiation through
ATF4/CHOP-dependent pathway. J Cell Physiol. 23:1321–1333. 2016.
View Article : Google Scholar
|
|
10
|
Restuccia A, Tian YF, Collier JH and
Hudalla GA: Self-assembled glycopeptide nanofibers as modulators of
galectin-1 bioactivity. Cell Mol Bioeng. 8:471–487. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng LJ, Peng QL, Wang LH, Xu J, Liu JS,
Li YJ, Zhuo ZJ, Bai LL, Hu LP, Chen WM, et al: Arenobufagin
intercalates with DNA leading to G2 cell cycle arrest via ATM/ATR
pathway. Oncotarget. 6:34258–34275. 2015.PubMed/NCBI
|
|
12
|
Shrivastava P, Vaibhav K, Tabassum R, Khan
A, Ishrat T, Khan MM, Ahmad A and Islam F, Safhi MM and Islam F:
Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA
induced Parkinson's rat model. J Nutr Biochem. 24:680–687. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yasuda T, Hayakawa H, Nihira T, Ren YR,
Nakata Y, Nagai M, Hattori N, Miyake K, Takada M, Shimada T, et al:
Parkin-mediated protection of dopaminergic neurons in a chronic
MPTP-minipump mouse model of Parkinson disease. J Neuropathol Exp
Neurol. 70:686–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Wang D, Xu T, Liu P, Cao Y, Wang
Y, Yang X, Xu X, Wang X and Niu H: Bladder cancer cells re-educate
TAMs through lactate shuttling in the microfluidic cancer
microenvironment. Oncotarget. 6:39196–39210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soares DG, Godin AM, Menezes RR, Nogueira
RD, Brito AM, Melo IS, Coura GM, Souza DG, Amaral FA, Paulino TP,
et al: Anti-inflammatory and antinociceptive activities of
azadirachtin in mice. Planta Med. 80:630–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim WH, Song HO, Jin CM, Hur JM, Lee HS,
Jin HY, Kim SY and Park H: The methanol extract of Azadirachta
indica A. Juss leaf protects mice against lethal endotoxemia
and sepsis. Biomol Ther (Seoul). 20:96–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Omobowale TO, Oyagbemi AA, Oyewunmi OA and
Adejumobi OA: Chemopreventive effect of methanolic extract of
Azadirachta indica on experimental Trypanosoma brucei
induced oxidative stress in dogs. Pharmacognosy Res. 7:249–258.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dkhil MA, Al-Quraishy S, Moneim Abdel AE
and Delic D: Protective effect of Azadirachta indica extract
against Eimeria papillata-induced coccidiosis. Parasitol
Res. 112:101–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rodriguez DAO, de Lima RF, Campos MS,
Costa JR, Biancardi MF, Marques MR, Taboga SR and Santos FCA:
Intrauterine exposure to bisphenol A promotes different effects in
both neonatal and adult prostate of male and female gerbils
(Meriones unguiculatus). Environ Toxicol. 31:1740–1750.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yue K, Trujillo-de Santiago G, Alvarez MM,
Tamayol A, Annabi N and Khademhosseini A: Synthesis, properties and
biomedical applications of gelatin methacryloyl (GelMA) hydrogels.
Biomaterials. 73:254–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan Y, Martin LM, Bosco DB, Bundy JL,
Nowakowski RS, Sang QX and Li Y: Differential effects of acellular
embryonic matrices on pluripotent stem cell expansion and neural
differentiation. Biomaterials. 73:231–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koyano-Nakagawa N, Shi X, Rasmussen TL,
Das S, Walter CA and Garry DJ: Feedback mechanisms regulate Ets
variant 2 (Etv2) gene expression and hematoendothelial lineages. J
Biol Chem. 290:28107–28119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Warsinske HC, Ashley SL, Linderman JJ,
Moore BB and Kirschner DE: Identifying mechanisms of homeostatic
signaling in fibroblast differentiation. Bull Math Biol.
77:1556–1582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyamoto DT, Zheng Y, Wittner BS, Lee RJ,
Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, et
al: RNA-Seq of single prostate CTCs implicates noncanonical Wnt
signaling in antiandrogen resistance. Science. 349:1351–1356. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khoo NK, Cantu-Medellin N, St Croix C and
Kelley EE: In vivo immuno-spin trapping: Imaging the footprints of
oxidative stress. Curr Protoc Cytom. 74:12.42.1–11. 2015.
View Article : Google Scholar
|