Introduction
Cerebral venous sinus thrombosis (CVST) is a
potentially life-threatening disease due to cerebral venous
thrombosis or sinus thrombosis. Approximately 3 to 4 cases of CVST
per million adults and 7 cases of CVST per million neonates occur
annually (1,2). CVST is typically associated with
pregnancy, neoplasm, systemic diseases, puerperium, dehydration,
oral contraceptives and coagulopathies (3). However, in 30% of cases, no underlying
etiology can be identified (3).
Timely detection of CVST is vital for patients as prompt management
prevent fatalities and also averts further disabilities (2). The confirmed diagnosis of CVST relies
on the demonstration of thrombus in the sinuses and/or cortical
veins by neuroimaging (4). Computed
tomography (CT) is useful to rule out tumor or subdural hematoma,
particularly for patients suspected of CVST or presenting with
subacute headache (4). Direct signs
of CVST include the cord sign, the dense triangle sign and the
empty delta sign which may be identified in ~1/3 of cases by CT
(5). Magnetic resonance venography
(MRV) is currently the best noninvasive method to confirm the
diagnosis of CVST (6). An abnormal
signal in a sinus and the corresponding absence of flow on MRV
supports the diagnosis of CVST (7).
Compared with MRV, digital subtraction angiography (DSA) not only
indicates the thrombosis in sinuses perfectly, but also displays
the occlusion in cortical veins precisely, thus this method has
become the gold standard of CVST (8).
Systemic anticoagulation is the first-line treatment
and the majority of patients with CVST will respond to systemic
anticoagulation (9). CVST has a
favorable prognosis; however, the condition may be associated with
neurologic deterioration, despite reasonable treatment with
anticoagulation (10). With novel
endovascular techniques and devices developed, direct thrombolysis
and mechanical thrombectomy without a craniotomy is becoming
increasingly general (7,11–13).
Endovascular treatment of CVST has the potential advantages of
direct dissolution or asportation of clots, allowing recanalization
of blood flow, decreased intracranial pressure and rapid
improvement of severe symptoms (9).
Recently, mechanical thrombectomy was used as an
aggressive therapeutic method for the treatment of CVST,
particularly for patients exhibiting clinical deterioration
(14). Stent retrievers to treat
CVST have previously been indicated in a small sample report
(15). However, the therapeutic
effect of these devices requires further verification with an
abundant number of samples. Local thrombolytic therapy is able to
effectively recanalize the thrombosed cortical veins (16). Therefore, individual treatment of
mechanical thrombectomy combined with local thrombolytic therapy
may achieve an improved curative effect.
In the present study, the medical records of 29
patients with CVST were retrospectively reviewed. From these
patients, 14 patients were successfully treated with stent
retriever thrombectomy combined with local thrombolytic therapy.
The present study identified that, despite anticoagulant treatment
failure, the majority of CVST patients with evident cortical venous
outflow stasis, rapid worsening of consciousness or neurological
deficits had a favorable prognosis following treatment with stent
retriever thrombectomy combined with local thrombolytic
infusion.
Materials and methods
Patients
Before the study, ethical approval was received from
the Ethics Committee of Zhujiang Hospital (Guangzhou, China) and
patients and (or) relatives were informed about the risks and
benefits of the procedure and written consent was obtained. We
initiated a retrospective on 29 patients (17 women:12 men) who were
diagnosed with CVST by magnetic resonance venography (MRV) or
digital subtraction angiography (DSA) in Zhujiang Hospital,
Southern Medical University, Guangzhou, China between January 2011
and November 2015. The following information was recorded: Age,
symptoms, etiological factors, onset, Glasgow Coma Scale (GCS)
applied as described by Okamura et al (17), location of the thrombus,
recanalization, outcome and complications. Systemic anticoagulation
was the initial treatment for all patients following admission.
Endovascular treatment was administered to patients presenting
with: i) Anticoagulation therapy failure; ii) rapid worsening of
consciousness or neurological deficits (GCS ≤10); and iii) evident
cortical venous outflow stasis (arteriovenous circulation time
>11 sec; venous phase >5 sec). The following exclusion
criteria for receiving endovascular treatment was followed: i)
Clinical symptoms were controlled when regular anticoagulant
treatment was initiated; ii) no obvious cortical venous outflow
stasis was present (arteriovenous circulation time <11 sec;
venous phase <5 sec). In the present study, 14 patients (group
A) received endovascular treatment whereas 15 patients (group B)
were given systemic anticoagulant therapy.
Treatment
Patients with CVST in group A underwent stent
retriever thrombectomy combined with continued partial
thrombolysis. A 6-Fr sheath was placed into the right common
femoral artery to allow a diagnostic cerebral angiogram.
Subsequently, a 6-Fr guiding catheter was selectively placed in the
internal jugular vein through the left femoral vein. A 0.027-inch
microcatheter (Rebar-27; eV3 Neurovascular, Irvine, CA, USA) was
introduced via the internal jugular vein into the position of the
thrombus. A microwire was used to break down thrombi and facilitate
the advancement of the microcatheter. Subsequently, the microwire
was withdrawn and a 6×30 mm stent (Solitaire AB; eV3 Neurovascular)
was semi-deployed at the distal part of the thrombus via the
microcatheter. After 5 sec, the stent, which covered the clot, and
the microcatheter were slowly pulled into the guiding catheter and
removed from the body. This procedure was performed 3 to 6 times to
retrieve the clot until recanalization of the venous sinus was
achieved. Subsequently, the stent was removed from the body and the
microcatheter was retained in the superior sagittal sinus for
continued partial thrombolysis. Urokinase was infused into the
thrombosis site using microinjection techniques at the speed of 5.0
U/min for 3 to 5 days. The total concentration of urokinase did not
exceed 1,000,000 units. Throughout the procedure, the activated
clotting time was maintained at 2 to 3 times above the normal value
(80 to 160 sec). When a patient presented with venous sinus
stenosis, a 6×30 mm stent (Solitaire AB, Medtronic, Inc.,
Minneapolis, MN, USA) was advanced and deployed at the site of
stenosis for angioplasty in order to dilate the venous sinus.
Following admission, group B patients received
systemic anticoagulation. During the first 3 days, low molecular
weight heparin was infused intravenously at 2,500 to 3,000 IU/d.
From the fourth day following treatment, 5 mg/day warfarin was
administered orally for 3 to 12 months. The international
normalized ratio (INR) was carefully monitored 2 to 4 times in the
first two weeks and once in the following four weeks, while
patients received anticoagulant therapy.
Follow-up
Follow-up visits were performed at 3, 6 and 12
months via outpatient clinics or over the telephone. MRV or DSA
were performed to monitor whether the thrombus recurred in the
sinus. Prognosis of patients with CVST was classified according to
the modified Ranking Scale (mRS): 0, normal; 1, no significant
disability; 2, slight disability (look after own affairs without
assistance); 3, moderate disability (required help, able to walk
assisted); 4, moderately severe disability (unable to walk
assisted); 5, severe disability (unable to ambulate, altered
mentation); and 6, mortality.
Statistical analysis
Data are presented as the mean ± standard deviation.
Groups were compared using the Student's t-test or a one-way
analysis of variance, followed by the least significant difference
test for pairwise comparisons. Discrete data were given as counts
and percentages. P<0.05 was considered to indicate a
statistically significant difference. All calculations were
evaluated using the SPSS 20.0 statistical package (IBM SPSS,
Armonk, NY, USA).
Results
Baseline characteristics
From 29 patients, the mean age was 34 years (range,
17–60 years). A total of 17 patients (58.6%) were women and 12
patients (41.4%) were men. A total of 16 patients (55.2%) exhibited
acute onset (within 7 days), 4 patients (13.8%) were included into
sub-acute onset (7 to 14 days) and 9 patients (31.0%) were included
into chronic onset (>14 days). Common risk factors of CVST were
identified in 15 patients (51.7%), puerperium in 7 patients
(24.1%), head injury in 2 patients (6.9%), 4 patients (17.2%)
presented with oral contraceptives and 1 patient (3.4%) had
vasculitis disease. No risk factors were identified in 5 patients
(48.3%; Tables I and II). The predominant clinical presentations
were headache, epilepsy, neurologic deficits, nausea, emesis,
dizziness, pyrexia and papilledema. The condition of 7/29 patients
(24.1%) deteriorated following admission. Superior sagittal sinus
(alone or in combination with other venous sinus) was the primary
thrombosis-affected sinus. The affected sinus of each case is
summarized in Tables I and II, which was evaluated by DSA or MRV.
 | Table I.Baseline characteristics and outcomes
of 14 patients who underwent endovascular treatment. |
Table I.
Baseline characteristics and outcomes
of 14 patients who underwent endovascular treatment.
| Case no. | Age (year) | Symptom | Etiological
factors | Onset | GCS on
admission | ICH or infarct | Condition after
admission | Affected venous
sinus | Recanalization of
venous sinus | GCS after admission
before discharge | Outcome and
complication |
|---|
| 1 | 24 | Headache,
epilepsy | Puerperium | Acute | 5 | Y | Stable | SSS | Complete | 11 | Asymptomatic |
| 2 | 55 | Headache,
hemiparesis | Unexplained | Sub-acute | 6 | Y | Stable | SSS+TS+SigS | Partial | 12 | Asymptomatic |
| 3 | 31 | Headache,
dizzy | Puerperium | Acute | 4 | Y | Deteriorate | SSS | Partial | 10 | Rehaemorrhagia was
found after operation. Residual neurologic deficit. |
| 4 | 23 | Headache,
epilepsy | Puerperium | Acute | 7 | Y | Stable | TS+StrS+SigS | Complete | 14 | Asymptomatic |
| 5 | 24 | Headache,
emesis | Puerperium | Acute | 5 | Y | Deteriorate | SSS+TS+SigS | Partial | 11 | Rehaemorrhagia was
found after operation. Asymptomatic |
| 6 | 57 | hemiparesis | Unexplained | Acute | 4 | N | Stable | SSS+TS | Complete | 12 | Asymptomatic |
| 7 | 24 | Epilepsy | Unexplained | Sub-acute | 6 | Y | Deteriorate | SSS+TS | Complete | 14 | Asymptomatic |
| 8 | 36 | Epilepsy,
hemiparesis | Puerperium | Acute | 6 | N | Stable | SSS | Complete | 14 | Asymptomatic |
| 9 | 46 | Headache | Unexplained | Chronic | 8 | N | Stable | TS+SigS | Complete | 13 | Asymptomatic |
| 10 | 38 | Epilepsy,
hemiparesis | Unexplained | Chronic | 9 | N | Stable | SSS+TS+SigS | Complete | 15 | Asymptomatic |
| 11 | 23 | Epilepsy,
emesis | Oral
contraceptive | Acute | 7 | Y | Deteriorate | SSS | Complete | 13 | Asymptomatic |
| 12 | 17 | Headache, epilepsy,
emesis, hemiparesis | Trauma | Acute | 7 | Y | Deteriorate |
SSS+TS+StrS+SigS | Partial | 11 | Residual neurologic
deficit |
| 13 | 31 | Headache, emesis,
epilepsy | Puerperium | Acute | 7 | Y | Stable | SSS | Complete | 12 | Residual neurologic
deficit |
| 14 | 30 | Headache,
emesis | Puerperium | Acute | 8 | Y | Stable | SSS | Complete | 13 | Asymptomatic |
 | Table II.Baseline characteristics and outcomes
of 15 patients who did not undergo endovascular treatment. |
Table II.
Baseline characteristics and outcomes
of 15 patients who did not undergo endovascular treatment.
| Case no. | Age (year) | Symptom | Etiological
factors | Onset | GCS on
admission | ICH or infarct | Condition after
admission | Affected venous
sinus | Recanalization of
venous sinus | GCS after admission
before discharge | Outcome and
complication |
|---|
| 15 | 23 | Headache | Oral
contraceptive | Acute | 14 | N | Stable | TS+SigS | Partial | 15 | Asymptomatic |
| 16 | 50 | Headache,
hemiparesis | Unexplained | Acute | 12 | Y | Stable | SSS+TS | Partial | 14 | Epilepsy |
| 17 | 20 | Headache, coma | Oral
contraceptive | Chronic | 10 | Y | Deteriorate | SSS+TS+SigS | Partial | 5 | Herniation was
found after admission, and decompressive craniectomy was performed.
Residual neurologic deficit. |
| 18 | 34 | Headache,
epilepsy | Unexplained | Sub-acute | 13 | Y | Stable | SSS | Complete | 14 | Asymptomatic |
| 19 | 47 | Headache,
hemiparesis, emesis | Vasculitis
disease | Acute | 12 | Y | Deteriorate | TS | Partial | 13 | Residual neurologic
deficit |
| 20 | 31 | Headache | Unexplained | Acute | 13 | Y | Stable | SSS+TS+SigS | Partial | 13 | Asymptomatic |
| 21 | 64 | Headache, epilepsy,
emesis, pyrexia | Unexplained | Acute | 14 | Y | Stable | SSS+TS+SigS | Partial | 15 | Asymptomatic |
| 22 | 24 | Headache,
epilepsy | Unexplained | Acute | 12 | Y | Stable | SSS+TS | Partial | 13 | Asymptomatic |
| 23 | 43 | Papilledema | Unexplained | Chronic | 11 | Y | Stable | CS | Partial | 13 | Asymptomatic |
| 24 | 29 | Blurred vision,
headache | Oral
contraceptive | Chronic | 13 | N | Stable | SSS+TS | Partial | 14 | Asymptomatic |
| 25 | 17 | Epilepsy,
hemiparesis | Unexplained | Chronic | 13 | Y | Stable | SSS+TS+SigS | Partial | 13 | Asymptomatic |
| 26 | 30 | Headache, pyrexia,
emesis | Trauma | Chronic | 14 | Y | Stable | SSS+TS+SigS | Partial | 14 | Headache |
| 27 | 38 | Headache, dizzy,
emesis | Unexplained | Sub-acute | 12 | N | Stable | SSS+TS | Partial | 13 | Asymptomatic |
| 28 | 43 | Headache | Oral
contraceptive | Chronic | 12 | N | Stable | TS+SigS | Partial | 13 | Asymptomatic |
| 29 | 31 | Hemiparesis,
epilepsy, | Unexplained | Chronic | 12 | Y | Stable | SSS+TS | Partial | 14 | Asymptomatic |
Outcome
In the present study, 14/29 patients (48.3%)
received endovascular therapy whereas 15/29 patients (51.7%)
received systemic anticoagulant treatment. Additionally, in group
A, 2/14 patients (14.3%) underwent stent-assisted angioplasty.
Following the procedure, in group A, the clinical symptoms in 12
patients (85.7%) had improved immediately; however, 2 patients
(6.9%) suffered from intracranial hemorrhage. In group B, the
clinical symptoms in 14/15 patients (93.3%) had improved; however,
1 patient (6.7%) suffered from herniation.
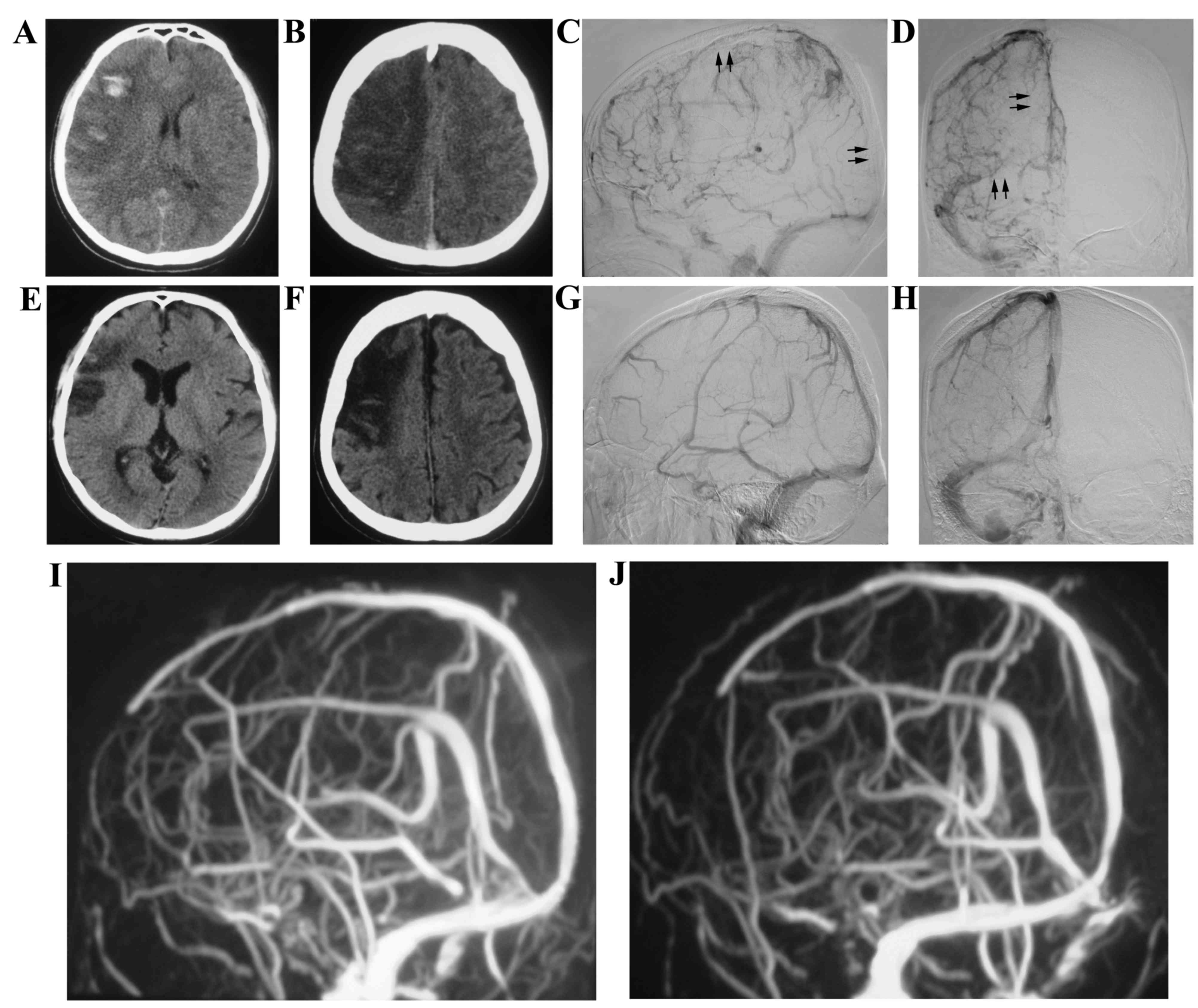
A 38-year-old male with no identified underlying
etiology presented with epilepsy and hemiparesis. The patient was
admitted to the hospital in May 2011 and was diagnosed with CVST by
MRV. Anticoagulation Treatment with heparin was initiated for 3
days; however, there was no improvement in his epilepsy and
hemiparesis. DSA revealed thrombose of the superior sagittal sinus,
right transverse sinus and right sigmoid sinus. Subsequently, stent
retriever thrombectomy combined with local thrombolytic therapy was
performed on the patient (Fig. 1).
The symptoms subsequently resolved in the following days and the
patient was discharged 15 days following admission. The patient
presented with no symptoms and there was no recurrence of CVST at
the 12-month follow-up.
A 24-year-old male presented with epilepsy for 3
days was admitted to the hospital in June 2012. The patient was
diagnosed with CVST and right frontal lobe hemorrhage accompanied
with ischemia by MRV and CT. DSA revealed thrombosis occurred in
the superior sagittal sinus and left transverse sinus (Fig. 2). Endovascular treatment was
subsequently performed on the patient. His symptom was relieved
with no thrombosis; however, recurrence of thrombosis in the
sinuses at the 12-month follow-up was indicated.
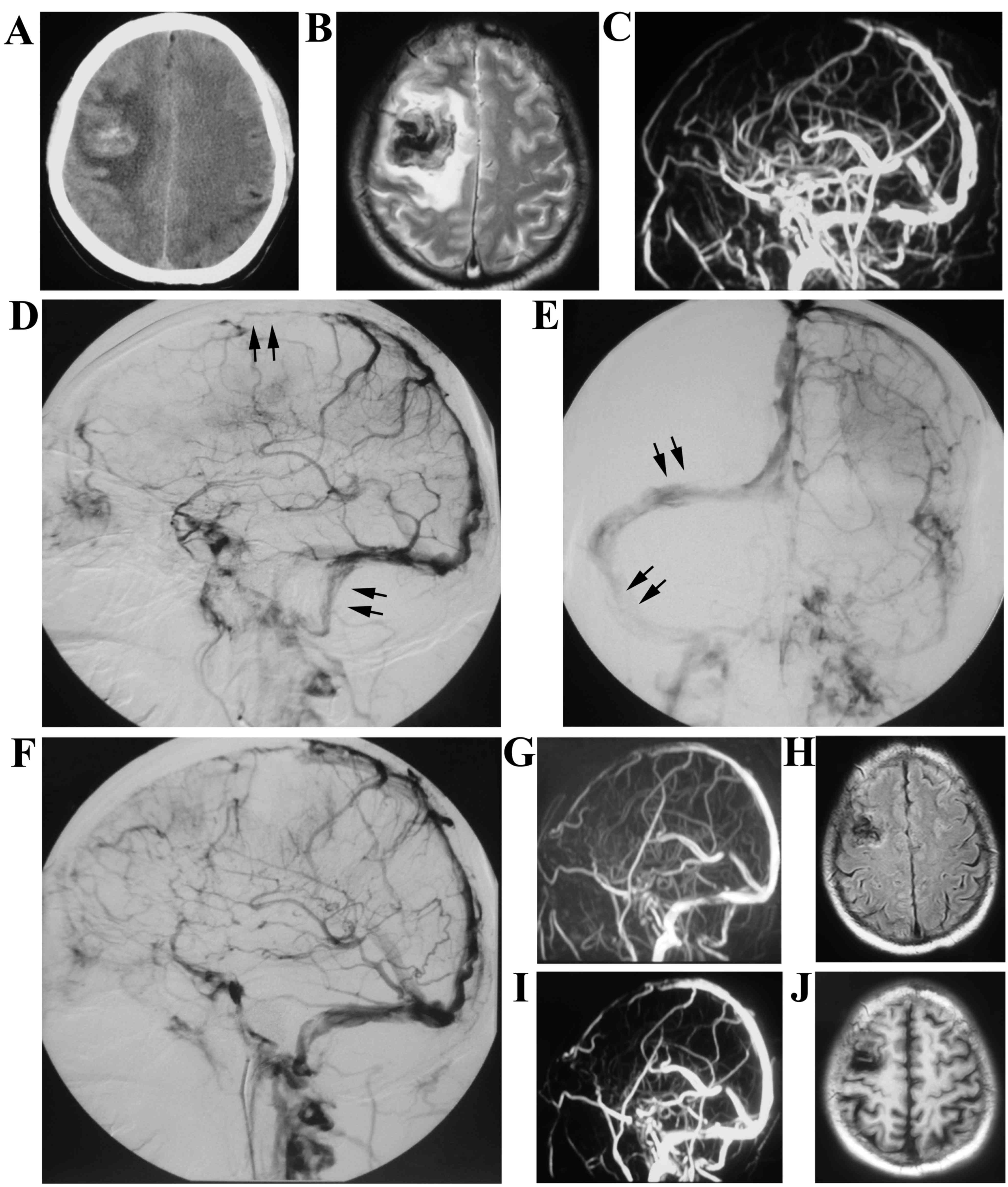
A 55-year-old male presented, with a chief complaint
of headache and hemiparesis for 4 days, was admitted to the
hospital in October 2013. A CT scan revealed right frontal lobe
intracranial hemorrhage and ischemia. Meanwhile, MRV demonstrated
the thrombosis in the superior sagittal sinus, left transverse
sinus and sigmoid sinus. Stent retriever thrombectomy was performed
and urokinase was infused into the thrombosis site using
microinjection techniques at the speed of 5.0 U/min for 3 days
(Fig. 3). The patient continued to
take oral anticoagulants for 12 months, and there was no recurrence
of headaches or hemiparesis at the 12-month follow-up.
A 23-year-old female, who was in puerperium,
presented with acute onset of severe headaches, vomiting and
generalized seizures. She was immediately treated with systemic
anticoagulation therapy for 2 days. However, the patient continued
to experience neurologic deficits and fell into a mild coma the
following day. Stent retriever thrombectomy combined with local
thrombolytic therapy was therefore undertaken. At 3 months, the
involved sinuses were completely recanalized and no clinical
symptoms were observed. Another 24-year-old female deteriorated on
the first postoperative day and intracranial hemorrhage was
diagnosed. Endovascular treatment was immediately stopped and
anticoagulation therapy with oral warfarin was administered two
weeks later. One month later, neurologic deficits were notably
improved and the patient was discharged.
A-17-year-old male presented to the hospital with
epilepsy and hemiparesis for 15 days. MRI indicated left frontal
lobe intracranial hemorrhage and DSA revealed that thrombus
occurred in the superior sagittal sinus, left transverse sinus and
sigmoid sinus but without cortical venous flow stasis. He received
systemic anticoagulant therapy during the stay of hospital. His
symptoms were relieved and he was subsequently discharged after
receiving treatment of low molecular weight heparin that was
intravenously infused at 3,000 IU/day for the first 3 days and
administered orally with warfarin at 5 mg/day for the next 27 days.
MRV indicated no obvious recanalization in sinuses and he presented
with no symptoms on the 12-month follow-up (Fig. 4).
Recanalization of the venous sinus is demonstrated
in Tables I and II. In group A, complete venous flow
restoration was obtained in 10/14 patients (71.4%), whereas partial
venous flow restoration was exhibited in 4/14 patients (28.6%)
following therapy. Angioplasty was performed in 2/14 patients
(14.3%) who suffered from sinus stenosis; 11/14 patients (78.6%)
resulted in a positive neurological outcome, whereas residual
neurological deficits persisted in 3/14 patients (21.4%). In group
B, 1/15 patients (6.7%) obtained complete venous flow restoration,
whereas 14/15 patients exhibited partial venous flow restoration
following treatment. A total of 11/15 patients presented with no
symptoms and 4/15 patients exhibited residual neurological deficits
following treatment. Neurological function was evaluated by mRS.
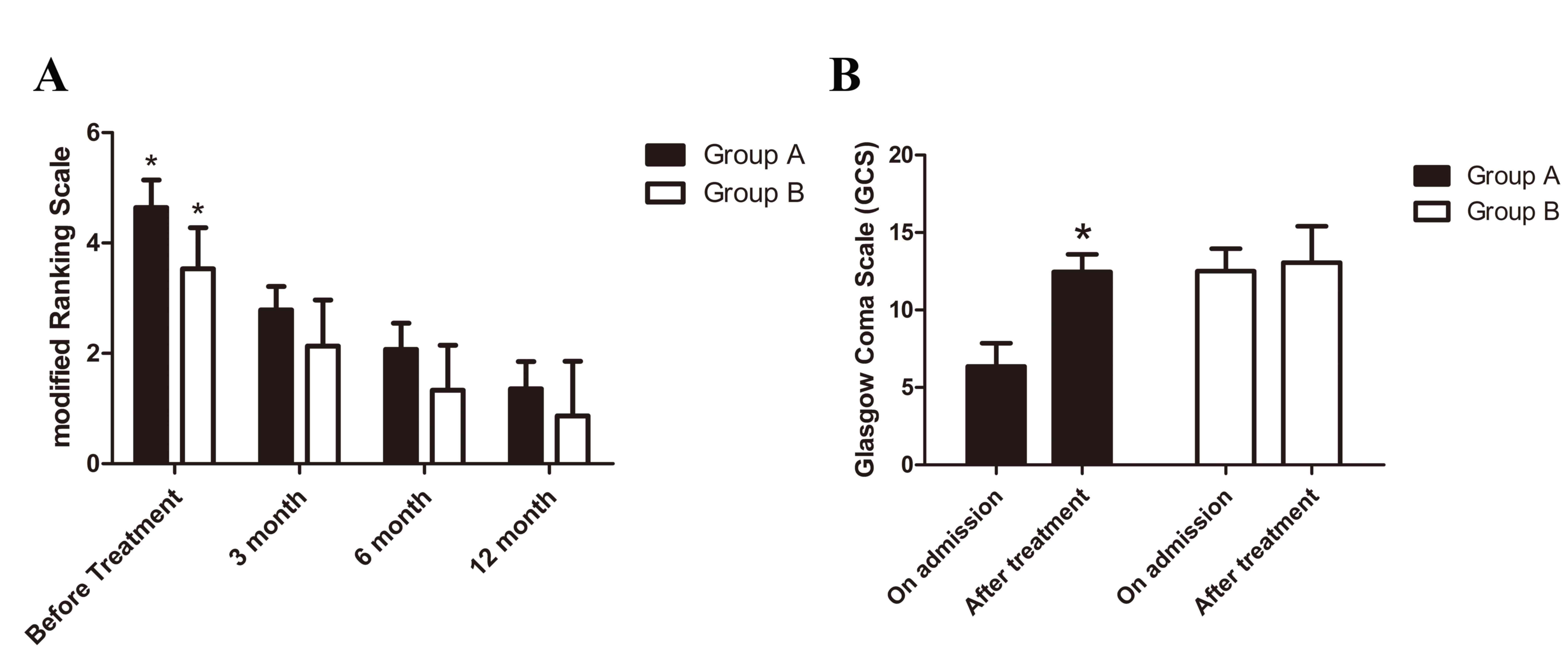
The outcome at the different time points is demonstrated in
Fig. 5. In group A, stent retriever
thrombectomy combined with local thrombolytic infusion
significantly improved mRS (at 3 months, 2.79±0.43; at 6 months,
2.07±0.47; and at 12 months, 1.36±0.50) when compared with mRS
prior to treatment (4.64±0.50; P<0.05; Fig. 5A). In group B, mRS (at 3 months,
2.13±0.83; at 6 months, 1.33±0.82; and at 12 months, 0.87±0.99) was
also significantly improved by systemic anticoagulation when
compared with the mRS prior to treatment (3.54±0.74; P<0.05;
Fig. 5A). The average GCS increased
significantly following endovascular treatment (group A) when
compared with that on admission (P<0.05; Fig. 5B). However, there was no obvious
improvement in the average GCS following anticoagulant therapy
(group B).
Discussion
Patients with CVST may present with a variety of
symptoms, including headaches, hemiparesis, seizures, sensory
disturbances, and other focal deficits (18); however, headaches are the most common
clinical symptom (4,19). In the present study, intracerebral
hemorrhage infarct, which is typically associated with a severe
clinical presentation at onset and results in a poor outcome
(20), occurred in ~2/3 of patients
with CVST.
There was a female predominance in CVST in the
present study, which is in agreement with previous findings
(3,21). Common risk factors for CVST include:
Hypercoagulable disorders due to, for example, oral contraceptive
use, puerperium, or hematological malignancies; head trauma;
intracranial hypotension; and systemic infections (3). In the present study, 12 patients were
considered to be in a hypercoagulable state. A total of 7 patients
were in puerperium and 4 patients were using oral contraception. In
a previous report, underlying etiology could not be identified in
30% of patients who involved with CVST (3). In the present study, no known risk
factor was identified in 12/29 patients (41.4%), which was higher
than the previous report (3).
Treating CVST with mechanical thrombectomy has been
reported previously (14). Although
no relevant randomized trials have been performed, data from
previous cases have revealed that mechanical thrombectomy has
notable advantages as a treatment approach for CVST and promotes a
high recanalization rate and favorable prognosis (4,12,14,15,22,23).
Therefore, mechanical thrombectomy has been the first-line tool for
neurologists in treating patients with worsening clinical
deterioration following anticoagulant therapy.
A series of different mechanical endovascular
treatments have been reported previously. These include rheolytic
thrombectomy, the Penumbra system, the Merci device, Solitaire AB
or FR (4,12,14,15,22,23). The
AngioJet system is the most commonly used rheolytic thrombectomy
device. In a retrospective study by Dashti et al (24), 13 patients with CVST were treated
with rheolytic thrombectomy and thrombosed intracranial sinuses
were recanalized in all patients following treatment. Although the
AngioJet device has an advantage in recanalizing the occluded
sinuses immediately due to the application of the Bernoulli
principle (25), it is hard to
deliver the device to the target sinuses due to stiffness of the
delivery system (26). Therefore,
the AngioJet system may not be an optimal option in treating
thrombus in tortuous sinuses.
The Penumbra system allows for continuous aspiration
of the thrombotic fragments the fragment of thrombus. In the
meantime, the sinus wall is protected by a separator. The flexible
delivery system allows the device to reach the thrombosed sinuses
effortlessly. Choulakian and Alexander (27) reported that 4 adult patients with
CVST who underwent mechanical thrombectomy using the 0.041-inch
Penumbra system achieved satisfactory sinus patency. Furthermore,
Velat et al (28) reported
the successful treatment of female treated with direct thrombectomy
using 0.054-inch Penumbra thromboaspiration catheter. The patient
improved symptomatically following the intervention and no thrombus
recurred at the 6-month follow-up.
The Merci clot retriever is a snare-type device that
removes thrombus using a corkscrew-shaped wire (11). Repeatedly pulling and pushing of the
microsnare in the clot can increase the surface of the thrombolytic
agents; however, this also increases the risk of endothelium damage
(29). The Merci device offers a
prompt resolution and can be used in combination with other
mechanical thrombectomy methods or thrombolytic agents (30). Khan et al (11) successfully used the device in
combination with local recombinant tissue plasminogen activator
infusion to restore flow in the superior sagittal sinus of a
middle-aged woman.
Mechanical thrombectomy approaches have been
utilized individually or in various combinations (31). For example, in some cases, balloon
angioplasty combined with mechanical thrombectomy is a sufficient
alternative for treating CVST with sinus stenosis (7). Stent retrievers (solitaire AB or FR),
approved by the Food and Drug Administration for the treatment of
ischemic stroke caused by large vessel occlusion, have become the
first-line tools for endovascular treatment of stroke (32). In a previous case report, Froehler
was the first to utilize a large-sized stent retriever (6×30 mm) to
treat CVST (5). The self-expanding
design of stent retrievers provide flexibility and the device is
able to be effectively deployed in the thrombosis sigmoid (15). Later case reports have indicated
promising treatment results using stent retrievers (22,32);
however, due to the limited sample size it is difficult to draw any
conclusion.
In the present study, within the 29 cases, stent
retriever thrombectomy combined with local thrombolytic therapy was
performed in 14 cases. In a previous study, in order to dissolve
intravascular thrombus, rapidly metabolized thrombolytic agents,
such as urokinase and recombinant tissue plasminogen activator
(rt-PA), were delivered to the thrombosis site using a
microcatheter (31). A number of
studies claim that local thrombolytic therapy is reasonably safe
for acute deteriorating patients (9,33);
however, its efficacy cannot be assessed from the available data
due to the lack of randomized controlled trials. Furthermore, local
thrombolytic therapy, which may increase the local drug
concentration in the thrombosis sinus and reduce the total dosage
of thrombolytic agents when compared with systemic thrombolysis
(34,35), may lower the risk of hemorrhage
(13). Thrombosis in cortical veins
has been indicated to be a critical factor in the development of
irreversible brain tissue damage in animal models of CVST (36,37).
Moreover, evident cortical venous outflow stasis results in the
increased risk of hemorrhagic infarct and brain edema (38). Hence, recanalization in thrombosed
cortical veins is a key factor to rescue brain tissue damage.
Furthermore, unobstructed cortical venous drainage is able to
reduce the risk of hemorrhage (39).
Thrombolytic agents are able to contact the surface of the thrombus
in cortical veins that mechanical techniques cannot reach and
prevent vessel angiolysis (31).
Therefore, the present study suggested that mechanical thrombectomy
in combination with local continuous infusion of urokinase provides
promising recanalization rates in sinuses and cortical veins.
CVST likely leads to an increase in venous pressure
of the sinus and cortical veins, which causes venous congestion
that results in hemorrhagic infarction and localized vasogenic
edema (40). Furthermore, venous
congestion likely reduces arterial perfusion, causing ischemia,
which leads to neuronal cell death (41). Thrombolytic therapy may continue for
several days and may increase the risk of hemorrhagic
complications, particularly in patients with pre-existing
hemorrhagic venous infarction. However, those who present with
clinical worsening may still benefit from intravascular
thrombolytic therapy (9,33,42). In
the present study, 10/14 (71.4%) patients with pre-existing
hemorrhage received local continuous infusion of urokinase
following stent retriever thrombectomy. Only 2/10 (20%) patients
experienced intracranial hemorrhage once intrasinus thrombolytic
therapy was initated. A young female suffered from intracranial
hemorrhage two days following intrasinus thrombolytic therapy and
fell into a deep coma. Her symptoms were relieved following
decompressive craniectomy and the patient gradually recovered over
the course of three months.
CVST patients with mild symptoms, GCS≥10 and fluent
cortical venous drainage, received systemic anticoagulant treatment
at the time of hospitalization. Only 1 patient achieved complete
recanalization of the venous sinus; however, the majority of
patients had a favorable prognosis, despite the low recanalization
rate of venous sinus. This may be explained by the compensation of
cortical venous flow and neuronal function restoration, which has
been demonstrated in a novel CVST animal model (43). Therefore, systemic anticoagulant
therapy is still an optimal option for treating CVST patients who
present with superior neurological status.
CVST has been demonstrated to have a good prognosis,
dependent on early diagnosis and prompt anticoagulant therapy
treatment (2). In the present study,
the majority of CVST patients that presented with evident cortical
venous outflow stasis, rapid worsening of consciousness or
neurological deficits treated with stent retriever thrombectomy
combined with local thrombolytic infusion had a favorable
prognosis. Dosages of the thrombolytic agents used range from
single bolus injections to continuous infusion (9,31,33). In
the present study, we indicated that prolonged infusion with a
small dosage of thrombolytic agents may establish an adequate
recanalization rate and may also avoid the recurrence of
hemorrhage. MRV and DSA are the optimal approaches used for
confirming the patency of the venous sinuses and cerebral veins,
and subsequently determining recanalization (20,21). In
the present study, oral anticoagulant treatment with warfarin
effectively avoided the recurrence of thrombus when evaluated by
MRV or DSA at follow-ups.
In conclusion, the present findings suggest that
favorable outcomes may be achieved using stent retriever
thrombectomy combined with local thrombolytic therapy in patients
with CVST who exhibit cortical venous outflow stasis or severe
neurological deterioration, despite systemic anticoagulation.
However, despite the favorable therapeutic benefits, the risk of
hemorrhagic complications may be an important end point during the
treatment process. Therefore, large scale randomized controlled
trials are required to establish and compare mechanical
thrombectomy alone to mechanical thrombectomy in combination with
local thrombolytic therapy in the future.
Acknowledgements
The Guangdong Provincial Department of Science and
technology provided financial support in the form of Guangdong
Provincial Clinical Medical Centre for Neurosurgery funding (grant
no. 2013B020400005) and the Special Program of Guangdong Province,
China (grant no. 00136530154773042). Haizhuqu District Department
of Science and Technology provided financial support in the form of
Science and Technology Program of Haizhu District, Guangzhou,
Guangdong Province, China (grant no. 2013-cg-28). The sponsors had
no role in the design or conduct of this research.
References
|
1
|
Martinelli I, Bucciarelli P, Passamonti
SM, Battaglioli T, Previtali E and Mannucci PM: Long-term
evaluation of the risk of recurrence after cerebral sinus-venous
thrombosis. Circulation. 121:2740–2746. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferro JM, Canhão P, Stam J, Bousser MG and
Barinagarrementeria F and ISCVT Investigators: Prognosis of
cerebral vein and dural sinus thrombosis: Results of the
international study on cerebral vein and dural sinus thrombosis
(ISCVT). Stroke. 35:664–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saadatnia M, Fatehi F, Basiri K, Mousavi
SA and Mehr GK: Cerebral venous sinus thrombosis risk factors. Int
J Stroke. 4:111–123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferro JM and Canhão P: Cerebral venous
sinus thrombosis: Update on diagnosis and management. Curr Cardiol
Rep. 16:5232014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Buonanno FS, Moody DM, Ball MR and Laster
DW: Computed cranial tomographic findings in cerebral sinovenous
occlusion. J Comput Assist Tomogr. 2:281–290. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klingebiel R, Bauknecht HC, Bohner G,
Kirsch R, Berger J and Masuhr F: Comparative evaluation of 2D
time-of-flight and 3D elliptic centric contrast-enhanced MR
venography in patients with presumptive cerebral venous and sinus
thrombosis. Eur J Neurol. 14:139–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chow K, Gobin YP, Saver J, Kidwell C, Dong
P and Viñuela F: Endovascular treatment of dural sinus thrombosis
with rheolytic thrombectomy and intra-arterial thrombolysis.
Stroke. 31:1420–1425. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qu H and Yang M: Early imaging
characteristics of 62 cases of cerebral venous sinus thrombosis.
Exp Ther Med. 5:233–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahman M, Velat GJ, Hoh BL and Mocco J:
Direct thrombolysis for cerebral venous sinus thrombosis. Neurosurg
Focus. 27:E72009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mortimer AM, Bradley MD, O'Leary S and
Renowden SA: Endovascular treatment of children with cerebral
venous sinus thrombosis : A case series. Pediatr Neurol.
49:305–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan SH, Adeoye O, Abruzzo TA, Shutter LA
and Ringer AJ: Intracranial dural sinus thrombosis: Novel use of a
mechanical thrombectomy catheter and review of management
strategies. Clin Med Res. 7:157–165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raychev R, Tateshima S, Rastogi S, Balgude
A, Yafeh B, Saver JL, Vespa PM, Buitrago M and Duckwiler G:
Successful treatment of extensive cerebral venous sinus thrombosis
using a combined approach with Penumbra aspiration system and
Solitaire FR retrieval device. BMJ Case Rep. 2013:1–5. 2013.
View Article : Google Scholar
|
|
13
|
Yue X, Xi G, Zhou Z, Xu G and Liu X:
Combined intraarterial and intravenous thrombolysis for severe
cerebral venous sinus thrombosis. J Thromb Thrombolysis.
29:361–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siddiqui FM, Dandapat S, Banerjee C,
Zuurbier SM, Johnson M, Stam J and Coutinho JM: Mechanical
thrombectomy in cerebral venous thrombosis: Systematic review of
185 cases. Stroke. 46:1263–1268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Froehler MT: Successful treatment of
cerebral venous sinus thrombosis with the Solitaire FR thrombectomy
device. J Neurointerv Surg. 5:e452013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wasay M, Bakshi R, Kojan S, Bobustuc G,
Dubey N and Unwin DH: Nonrandomized comparison of local urokinase
thrombolysis versus systemic heparin anticoagulation for superior
sagittal sinus thrombosis. Stroke. 32:2310–2317. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okamura K: Glasgow coma scale flow chart:
A beginner's guide. Br J Nurs. 23:1068–1073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang JW, Li JP, Song YL, Tan K, Wang Y, Li
T, Guo P, Li X, Wang Y and Zhao QH: Clinical characteristics of
cerebral venous sinus thrombosis. Neurosciences (Riyadh).
20:292–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alberti A, Venti M and Biagini S: Headache
and cerebral vein and sinus thrombosis. Front Neurol Neurosci.
23:89–95. 2008.PubMed/NCBI
|
|
20
|
Stam J: Thrombosis of the cerebral veins
and sinuses. N Engl J Med. 352:1791–1798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferro J, Lopes M and Rosas M: Long-term
prognosis of cerebral vein and dural sinus thrombosis. Stroke.
35:664–670. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shaikh H, Pukenas BA, Mcintosh A, Licht D
and Hurst RW: Combined use of solitaire FR and penumbra devices for
endovascular treatment of cerebral venous sinus thrombosis in a
child. J Neurointerv Surg. 7:e102015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nimjee SM, Powers CJ, Kolls BJ, Smith T,
Britz GW and Zomorodi AR: Endovascular treatment of venous sinus
thrombosis: A case report and review of the literature. J
Neurointerv Surg. 3:30–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dashti SR, Hu YC, Yao T, Fiorella D, Mitha
AP, Albuquerque FC and McDougall CG: Mechanical thrombectomy as
first-line treatment for venous sinus thrombosis: Technical
considerations and preliminary results using the AngioJet device. J
Neurointerv Surg. 5:49–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haghighi Borhani A, Mahmoodi M, Edgell RC,
Cruz-Flores S, Ghanaati H, Jamshidi M and Zaidat OO: Mechanical
thrombectomy for cerebral venous sinus thrombosis: A comprehensive
literature review. Clin Appl Thromb Hemost. 20:507–515. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang A, Collinson RL, Hurst RW and
Weigele JB: Rheolytic thrombectomy for cerebral sinus thrombosis.
Neurocrit Care. 9:17–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Choulakian A and Alexander MJ: Mechanical
thrombectomy with the penumbra system for treatment of venous sinus
thrombosis. J Neurointerv Surg. 2:153–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Velat GJ, Skowlund CJ, Waters MF, Mocco J
and Hoh BL: Direct thrombectomy using the Penumbra
thromboaspiration catheter for the treatment of cerebral venous
sinus thrombosis. World Neurosurg. 77:591.e15–18. 2012. View Article : Google Scholar
|
|
29
|
Kirsch J, Rasmussen PA, Masaryk TJ, Perl J
II and Fiorella D: Adjunctive rheolytic thrombectomy for central
venous sinus thrombosis: Technical case report. Neurosurgery.
60:E577–E578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Philips MF, Bagley LJ, Sinson GP, Raps EC,
Galetta SL, Zager EL and Hurst RW: Endovascular thrombolysis for
symptomatic cerebral venous thrombosis. J Neurosurg. 90:65–71.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qiu Z, Sang H, Dai Q and Xu G:
Endovascular treatments for cerebral venous sinus thrombosis. J
Thromb Thrombolysis. 40:353–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mokin M, Lopes DK, Binning MJ,
Veznedaroglu E, Liebman KM, Arthur AS, Doss VT, Levy EI and
Siddiqui AH: Endovascular treatment of cerebral venous thrombosis:
Contemporary multicenter experience. Interv Neuroradiol.
21:520–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kamal AK: Thrombolytic therapy in cerebral
venous sinus thrombosis. J Pak Med Assoc. 56:538–40.
2006.PubMed/NCBI
|
|
34
|
Li G, Zeng X, Hussain M, Meng R, Liu Y,
Yuan K, Sikharam C, Ding Y, Ling F and Ji X: Safety and validity of
mechanical thrombectomy and thrombolysis on severe cerebral venous
sinus thrombosis. Neurosurgery. 72:730–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bagley LJ, Hurst RW, Galetta S, Teener J
and Sinson GP: Use of a microsnare to aid direct thrombolytic
therapy of dural sinus thrombosis. AJR Am J Roentgenol.
170:784–786. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakase H, Kakizaki T, Miyamoto K,
Hiramatsu K and Sakaki T: Use of local cerebral blood flow
monitoring to predict brain damage after disturbance to the venous
circulation: Cortical vein occlusion model by photochemical dye.
Neurosurgery. 37:285–286. 1995. View Article : Google Scholar
|
|
37
|
Fries G, Wallenfang T, Hennen J, Velthaus
M, Heimann A, Schild H, Perneczky A and Kempski O: Occlusion of the
pig superior sagittal sinus, bridging and cortical veins: Multistep
evolution of sinus-vein thrombosis. J Neurosurg. 77:127–133. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frerichs KU, Deckert M, Kempski O, Schürer
L, Einhäupl K and Baethmann A: Cerebral sinus and venous thrombosis
in rats induces long-term deficits in brain function and
morphology-evidence for a cytotoxic genesis. J Cereb Blood Flow
Metab. 14:289–300. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Akins PT, Axelrod YK, Ji C, Ciporen JN,
Arshad ST, Hawk MW and Guppy KH: Cerebral venous sinus thrombosis
complicated by subdural hematomas: Case series and literature
review. Surg Neurol Int. 4:852013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ito K, Tsugane R, Ikeda A, Suzuki Y and
Sato K: Cerebral hemodynamics and histological changes following
acute cerebral venous occlusion in cats. Tokai J Exp Clin Med.
22:83–93. 1997.PubMed/NCBI
|
|
41
|
Singh R, Cope WP, Zhou Z, De Witt ME,
Boockvar JA and Tsiouris AJ: Isolated cortical vein thrombosis:
Case series. J Neurosurg. 427–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Christo PP, Carvalho GM and Neto Gomes AP:
Cerebral venous thrombosis: Study of fifteen cases and review of
literature. Rev Assoc Med Bras. 56:288–292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen C, Wang Q, Gao Y, Lu Z, Cui X, Zheng
T, Liu Y, Li X, He X, Zhang X, et al: Photothrombosis combined with
thrombin injection establishes a rat model of cerebral venous sinus
thrombosis. Neuroscience. 306:39–49. 2015. View Article : Google Scholar : PubMed/NCBI
|