Introduction
MicroRNAs (miRs) are endogenous small non-coding
RNAs and exert their functions by binding to the 3′-untranslated
region (3′UTR) of the messenger RNAs of target genes, resulting in
inhibition of translation or mRNA degradation (1–4). miRs
are involved in numerous cellular processes, such as proliferation,
migration, cell cycle, cell apoptosis and energy metabolism.
Aberrant expression of miRs has been detected in numerous tumor
types and the dysregulation of miRs is likely to be associated with
the development of tumors (5).
The host gene of miR-155 is B cell integration
cluster, which is located in chromosome 21q21. miR-155 is a
well-known miR that is associated with the inflammatory system.
miR-155 is closely linked with proliferation, apoptosis and
differentiation of lymphocytes (6–8), and
regulates the balance of type 17 T-helper/T-regulatory cells, the
imbalance of which is a major cause of numerous auto-immune
diseases (9). miR-155 has been
demonstrated to be overexpressed in various types of tumor tissue,
such as renal carcinoma and hepatocellular carcinoma, compared with
that in the adjacent normal tissues, and is associated with the
malignant clinicopathological characteristics of tumors (10,11).
Furthermore, miR-155 has been reported to be associated with the
proliferation, invasion, apoptosis and cell cycle of tumor cells
in vivo and in vitro (10,11).
Casitas B-lineage lymphoma (CBL) is an E3 ubiquitin
ligase, which mediates the ubiquitinated degradation of activated
receptor tyrosine kinases (RTKs), resulting in a halt in
RTK-mediated signaling. CBL is associated with the proliferation,
apoptosis, invasion and migration and is linked to the development
of tumors (12–15). CBL also regulates the proliferation,
differentiation and survival of human mesenchymal-derived
osteoblasts (16). It has also been
reported that CBL acts as a tumor suppressor in colon cancer cells
(17,18).
miR-155 has been previously demonstrated to be
overexpressed in colon cancer tissues compared with that in
adjacent tissues (19,20). However, the biological functions and
downstream targets of miR-155 in colon cancer have remained
elusive. In the present study, the effects of miR-155 on colon
cancer cells were explored. The results demonstrated that miR-155
regulated the proliferation, cell cycle, apoptosis and migration of
colon cancer cells through targeting CBL. The present study
indicated that miR-155 may become a promising therapeutic target
for the treatment of colon cancer.
Materials and methods
Cell culture
The HCT-116 colon cancer cell line was obtained from
the American Type Culture Collection (Manassas, VA, USA) and
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) in a humidified atmosphere at 37°C
with 5% CO2.
Transfection
miR-155 mimics (5′-UUAAUGCUAAUCGUGAUAGGGGU-3′) and
inhibitor (5′-AAUUACGAUUAGCACUAUCCCCA-3′) or their corresponding
negative controls were purchased from Biomics Biotech (Nantong,
China). Cells were harvested and seeded in 6-well plates at a
density of 1×105 cells/well. After 24 h of incubation,
the cell medium was changed to serum-free medium. After additional
culture for 6 h, 4 µg DNA or 100 pmol RNA were transfected into
cells using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
At 48 h after transfection, cells in each group were
collected. Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The RNA was reverse-transcribed and the
levels of miR-155 were detected by RT-qPCR (SYBR Green method) with
a miR-155 detection kit (Biomics Biotech; catalog number, BK3100)
according to the manufacturer's instructions. The miR-155 levels
were normalized to U6 small hairpin RNA and the relative miR-155
levels were calculated using the 2−ΔΔCq method (21). Total RNA was reverse-transcribed into
complementary (c)DNA using Moloney murine leukemia virus reverse
transcriptase (Promega Corp., Madison, WI, USA) and random primer.
The mRNA levels of CBL were detected by RT-qPCR with cDNA as
templates and primers as follows: CBL forward,
5′-GGACCAGTGAGTTGGGAGTTATTACT-3′ and reverse, CBL,
5′-GGCAAGACTTCACTGTGAAGTCA-3′; GAPDH forward,
5′-AAGGTCGGAGTCACCGGATT-3′ and reverse,
5′-CTGGAAGATGGTGATGGGATT-3′. The PCR mixture contained the
following: 2 µl cDNA, 1 µl forward primer, 1 µl reverse primer, 10
µl 2X SYBR mix, and ddH2O up to 20 µl. The thermocycling
conditions were the following: 95°C for 10 min; 95°C for 10 sec,
62°C for 20 sec, 72°C for 30 sec for 40 cycles; then 4°C for 5 min.
The mRNA levels of CBL were normalized to GAPDH and relative mRNA
levels of CBL were calculated using the 2−ΔΔCq method
(21).
MTT assay
Cells were seeded in 96-well plates with 6,000 cells
in each well. The cells were then transfected with miR-155 mimics,
negative control of mimics, miR-155 inhibitor or negative control
of inhibitor. At 0, 24, 48, 72 and 96 h after transfection, 5 mg/ml
MTT was added to each well. After incubation for an additional 4 h,
the supernatant was removed and 200 µl dimethyl sulfoxide was added
to each well. The absorbance was measured using a microplate reader
at 490 nm.
Colony formation assay
After transfection with miR-155 mimics, miR-155
inhibitor or their negative controls, 200 cells were incubated in
6-well plates and cultured in an incubator containing 5%
CO2 at 37°C. At 7 days post-incubation, the cells were
stained with crystal violet for 30 min and the number of colonies
was counted after washing with PBS.
Cell cycle analysis
Cells were harvested after transfection with miR-155
mimics, miR-155 inhibitor or their corresponding negative controls
and fixed in ice-cold 70% ethanol at 4°C overnight. Cells were
washed with PBS and stained with a cell cycle detection kit
(Beyotime Institute of Biotechnology, Haimen, China) in the dark
for 30 min. The cell cycle distribution was then analyzed with a
FACScalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ,
USA).
Apoptosis assay
Cell apoptosis was detected with an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection kit (KeyGEN Biotech, Jiangsu, China) according to the
manufacturer's protocol. After transfection with miR-155 mimics,
miR-155 inhibitor or their corresponding negative controls, the
cells were washed with PBS and re-suspended in 500 µl binding
buffer. After addition of 5 µl Annexin V-FITC and 5 µl PI, the cell
suspension was incubated for 15 min in the dark. Cells were then
analyzed with a FACScalibur flow cytometer.
Wound healing assay
Cells were seeded in a 6-well plate and transfected
with miR-155 mimics, miR-155 inhibitor or their negative controls.
When cells had grown 90% confluent, scratches were made to the
monolayer surfaces of cells with sterile micropipette 200 µl tips
(Beyotime Institute of Biotechnology). After washing with
serum-free medium for several times to remove cell debris, cells
were cultured in serum-free medium to eliminate the influence of
cell proliferation and images of scratches were captured at 0 and
24 h. The relative migration rate was calculated as follows:
Relative migration rate=(gap between the edges at 0 h-gap between
the edges at 24 h)/gap between the edges at 0 h.
Western blot analysis
At 48 h after transfection, cells in each group were
collected. Total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology). Protein concentrations were determined with an
enhanced bicinchoninic acid protein assay kit (Beyotime Institute
of Biotechnology). A total of 40 µl of protein was loaded and
subjected to 10% SDS-PAGE. The separated protein was then
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). After blocking with 5% skimmed milk
at 37°C for 1 h, the PVDF membranes were incubated with primary
antibodies against CBL (1:1,000 dilution; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA; catalog number, sc-170) and β-actin (1:2,000
dilution; Santa Cruz Biotechnology, Inc.; catalog number, 4970) at
4°C overnight. After washing with Tris-buffered saline containing
Tween-20, the membranes were incubated with horseradish
peroxidase-labeled secondary antibodies (1:5,000 dilution; Beyotime
Institute of Biotechnology; catalog number: A0208) at room
temperature for 60 min. The target bands were visualized using an
enhanced chemiluminescence detection kit (Beyotime Institute of
Biotechnology) according to the manufacturer's instructions.
Relative protein levels of CBL were calculated using β-actin as an
internal reference.
Luciferase reporter assay
A luciferase reporter assay was employed to detect
whether miR-155 bound to the 3′UTR of CBL directly. As predicted by
TargetScan (http://www.targetscan.org/), fragments of CBL mRNA
containing wild type (WT) or mutant (MUT) miR-155 binding sites or
lacking the miR-155 binding site (DEL) were respectively cloned
into the pMIR-REPORT luciferase reporter vector (Ambion, San Diego,
CA, USA). HCT-116 cells were seeded into 24-well plates and 0.2 µg
luciferase reporter plasmid WT, MUT or DEL, together with 0.2 µg
β-galactosidase control plasmid (Ambion) plus either 20 pmol
miR-155 mimics or negative control of mimics were co-transfected
into HCT-116 cells using Lipofectamine 2000 reagent. At 48 h after
transfection, luciferase activity was measured using a Luciferase
Assay System (Promega Corp.) according to the manufacturer's
protocols.
Statistical analysis
All experiments were performed three times. Values
are expressed as the mean ± standard deviation. Student's t-test
was performed for comparisons between two groups using Graphpad
Prism 5.0 (Graphpad Software, Inc., San Diego, CA, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
Upregulation of miR-155 promotes the
proliferation, cell cycle and migration, while inhibiting apoptosis
of colon cancer cells
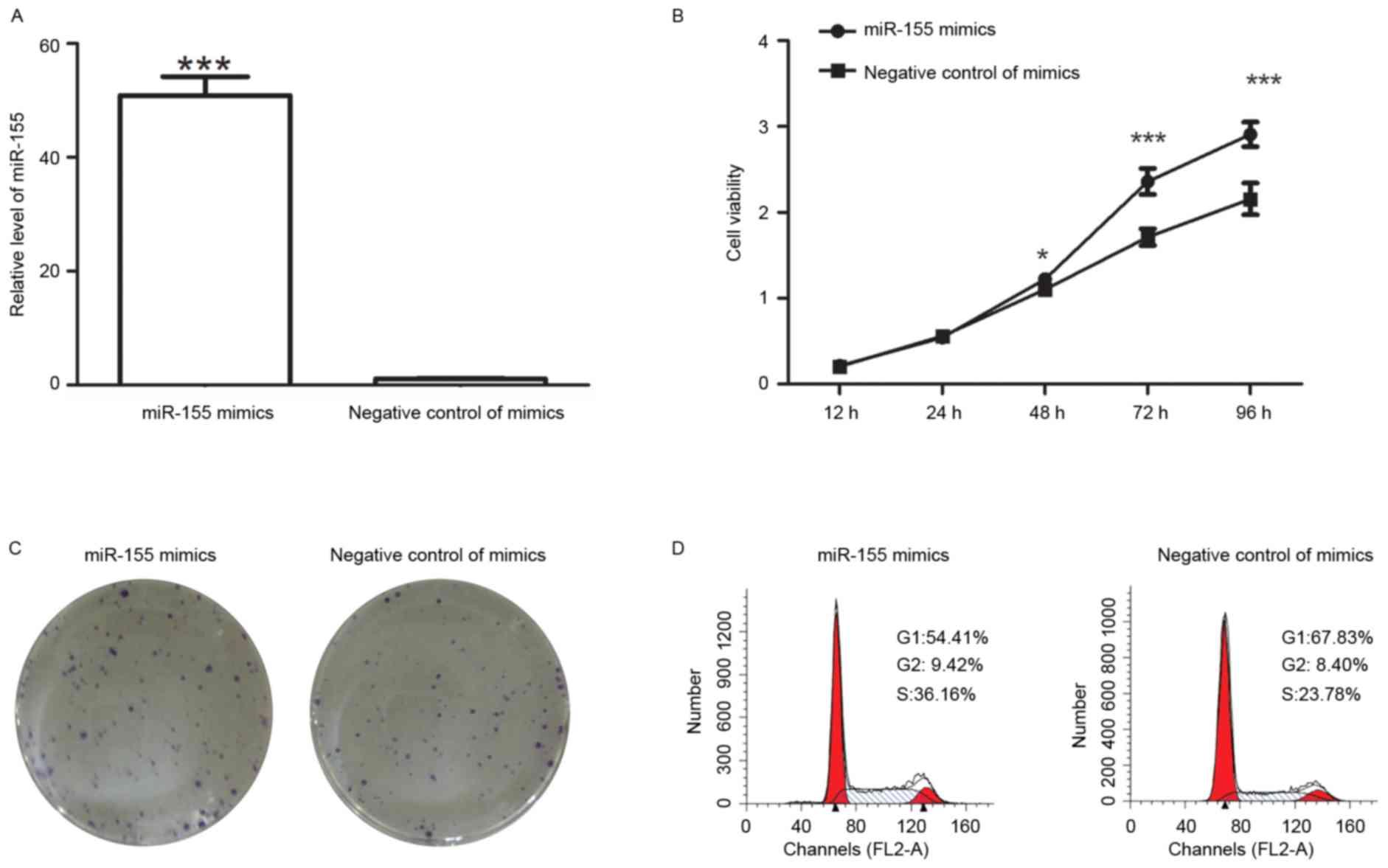
After transfection with miR-155 mimics, the levels
of miR-155 were detected by RT-qPCR. The results indicated that
after transfection with miR-155 mimics, the miR-155 levels were
increased to 48±3.6-fold of those in cells transfected with
negative control of mimics (Fig.
1A).
After transfection with miR-155 mimics, the
viability of colon cancer cells was detected by an MTT assay. The
results demonstrated that the cell viability was enhanced after
transfection with miR-155 mimics (Fig.
1B). The colony formation capability of colon cancer cells was
also measured after transfection. The results of the colony
formation assay revealed that after transfection with miR-155
mimics, the number of colonies was 90±2.6, which was significantly
higher than that of cells transfected with negative control of
mimics (60.6±5.1; P<0.001; Fig.
1C). It was therefore demonstrated that miR-155 mimics promoted
the proliferation of colon cancer cells.
As an important factor affecting the growth of
cells, the cell cycle distribution was detected after transfection
with miR-155 mimics or their corresponding negative control. Flow
cytometric analysis revealed that the percentage of cells in G1
phase was decreased from 67.83 to 54.41% after transfection with
miR-155 mimics, but the percentage of cells in S phase was
increased from 23.78 to 36.16% (Fig.
1D). These results demonstrated that miR-155 mimics increased
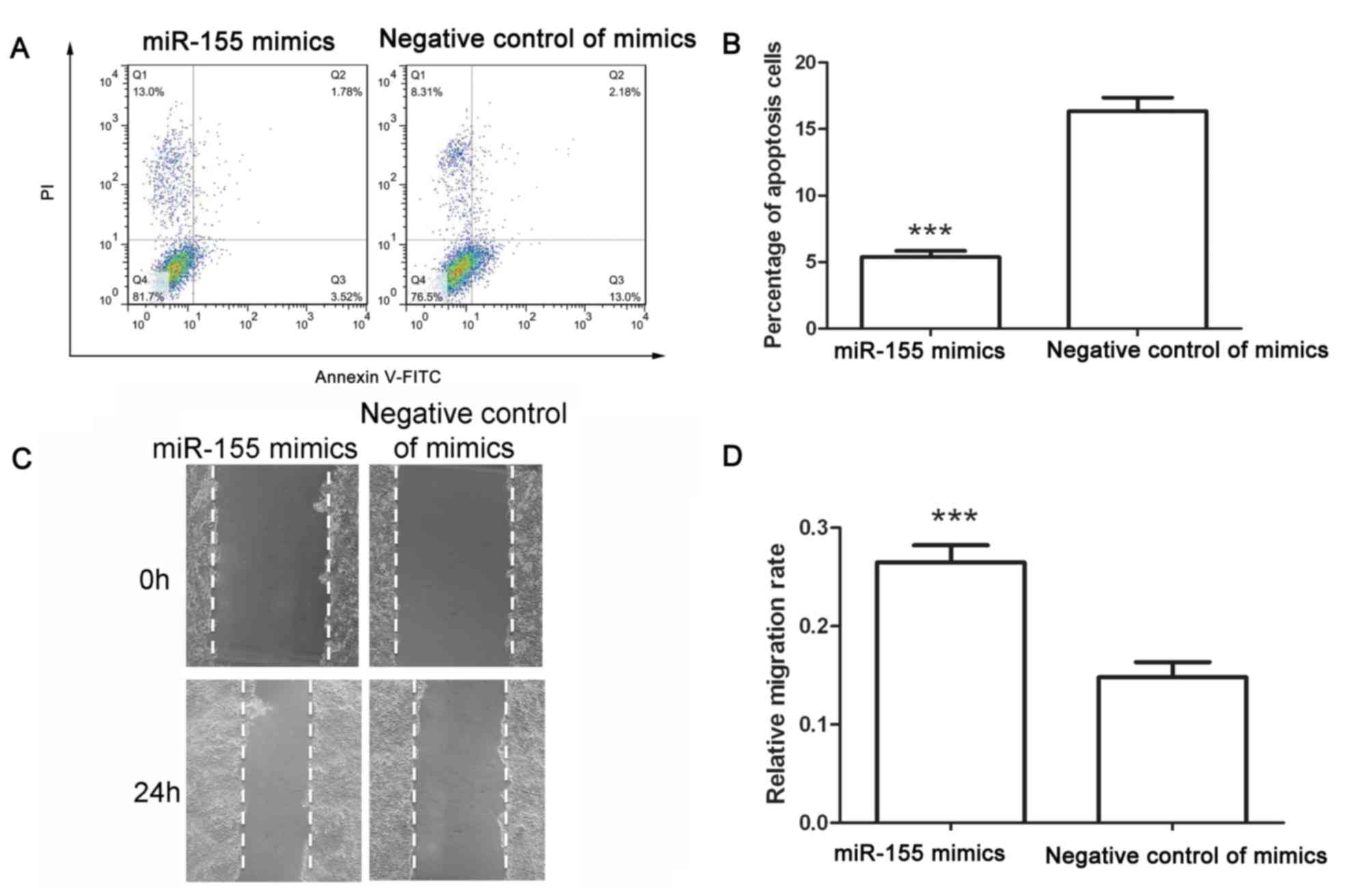
the percentage of cells in S phase. After transfection, an
apoptosis assay was performed. Following transfection with negative
control of miR-155 mimics, the early apoptotic rate was 13.83±0.76%
and the late apoptotic rate was 2.51±0.31%. However, after
transfection with miR-155 mimics, the early apoptotic rate
decreased to 3.72±0.59% and the late apoptotic rate decreased to
1.65±0.14% (Fig. 2A and B). These
results demonstrated that miR-155 mimics inhibited apoptosis of
colon cancer cells.
The migration capability of colon cancer cells after
transfection was assessed using a wound healing assay. As presented
in Fig. 2C and D, the migration rate
of cells transfected with negative control of mimics was
0.148±0.015, whereas that of cells transfected with miR-155 mimics
was 0.265±0.18 (Fig. 2C and D).
These results demonstrated that miR-155 mimics promoted the
migration of colon cancer cells.
Downregulation of miR-155 inhibits the
proliferation, cell cycle and migration, while promoting apoptosis
of colon cancer cells
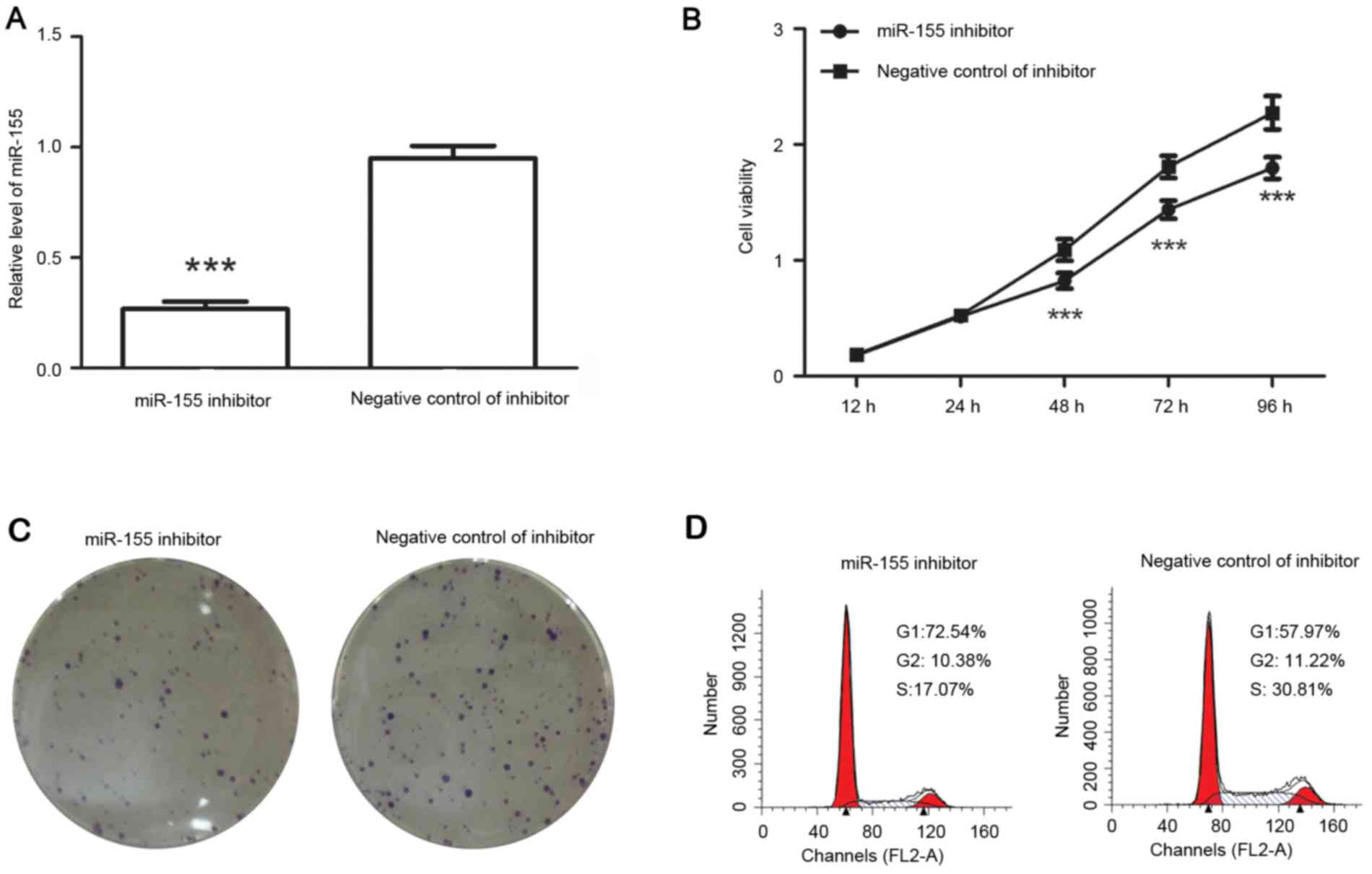
After transfection with miR-155 inhibitor, the
levels of miR-155 were detected by RT-qPCR. The results indicated
that after transfection with miR-155 inhibitor, the relative
miR-155 levels were decreased to 28±2% of those in negative
control-transfected cells (Fig.
3A).
The cell viability and colony formation capability
of colon cancer cells were then detected. The results demonstrated
that, compared with that of cells transfected with negative control
of inhibitor, the viability of colon cancer cells was inhibited
after transfection with miR-155 inhibitor (Fig. 3B) and the number of colonies was
decreased from 73.67±4.16 to 48.33±3.05 (P<0.01; Fig. 3C). It was therefore suggested that
miR-155 inhibitor reduced the proliferation of colon cancer
cells.
The cell cycle distribution was detected after
transfection with miR-155 inhibitor or its corresponding negative
control. The results demonstrated that the percentage of cells in
G1 phase was increased from 57.97 to 72.54% after transfection with
miR-155 inhibitor, but the percentage of cells in S phase was
decreased from 30.81 to 17.07% (Fig.
3D). It was therefore indicated that miR-155 inhibitor
increased the percentage of cells in G1 phase.
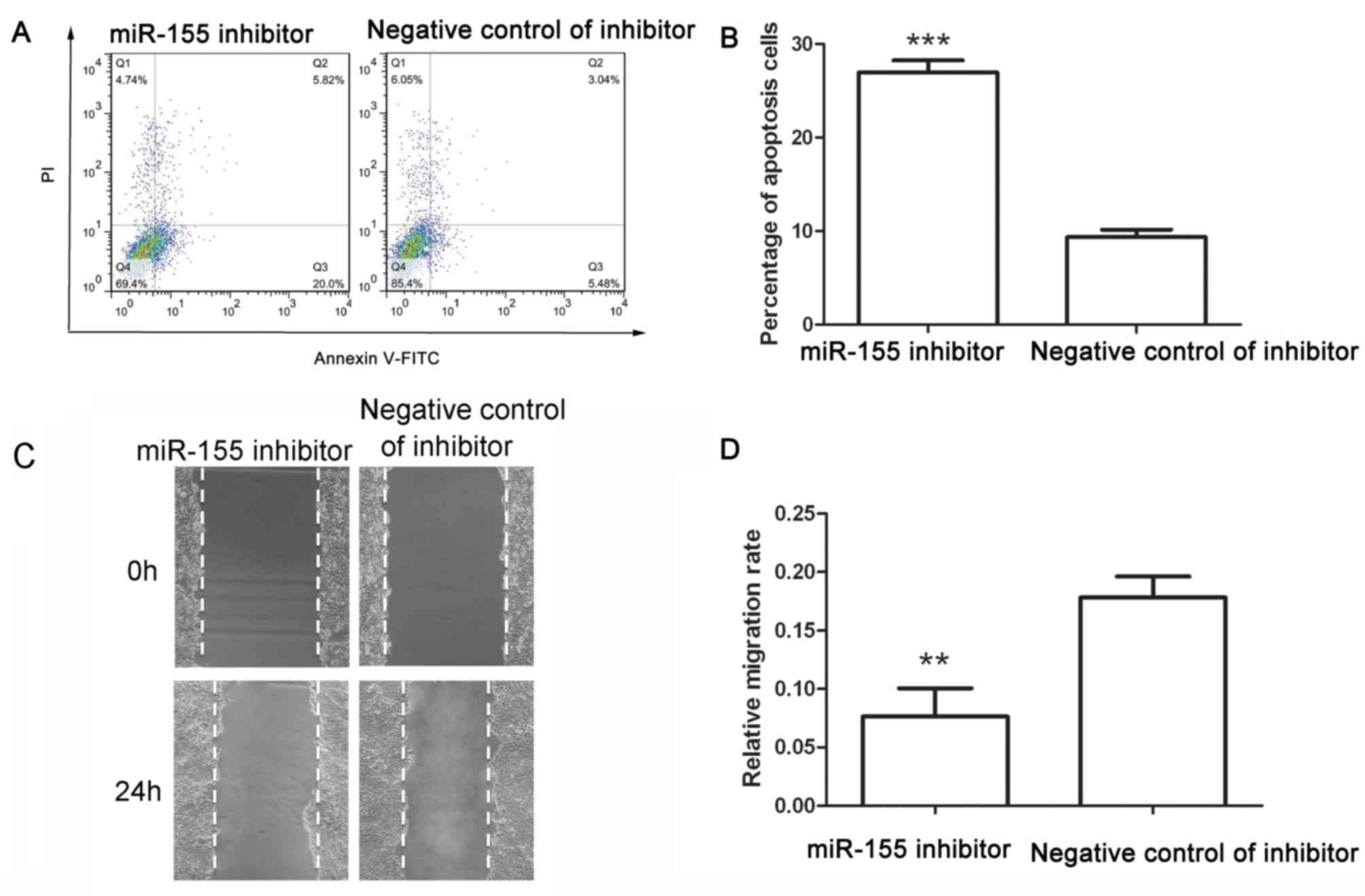
An apoptosis assay was performed after transfection
with miR-155 inhibitor or its corresponding negative control. After
transfection with negative control of miR-155 inhibitor, the early
apoptotic rate was 6.49±0.90% and the late apoptotic rate was
2.88±0.15%, whereas after transfection with miR-155 inhibitor, the
early apoptotic rate was increased to 21.03±1.17% and the late
apoptotic rate was increased to 5.92±0.29% (Fig. 4A and B). These results demonstrated
that miR-155 inhibitor induced apoptosis of colon cancer cells.
The migration capability of colon cancer cells was
detected after transfection with miR-155 inhibitor. As presented in
Fig. 4C and D, the migration rate of
cells transfected with negative control of inhibitor was
0.178±0.018, whereas that of cells transfected with miR-155
inhibitor was 0.076±0.025. The wound healing assay demonstrated
that miR-155 inhibitor reduced the migration of colon cancer
cells.
CBL is a direct target of miR-155
CBL was predicted as a target of miR-155 by
TargetScan. The levels of CBL were detected by RT-qPCR and western
blot analysis. The RT-qPCR results demonstrated that the mRNA
levels of CBL were decreased to 25.33±3.05% after transfection with
miR-155 mimics, and increased to 3.51±0.27-fold of those of the
negative control group after transfection with miR-155 inhibitor
(Fig. 5A). Western blot analysis
provided similar results to those of RT-qPCR. The protein levels of
CBL were decreased to 14±5.29% after transfection with miR-155
mimics and increased to 1.69±0.27-fold of those of the negative
control group after transfection with miR-155 inhibitor (Fig. 5B and C). These results demonstrated
that CBL was regulated by miR-155.
 | Figure 5.CBL is a direct target of miR-155.
(A) After transfection with miR-155 mimics, miR-155 inhibitor or
their corresponding negative control, the mRNA levels of CBL were
detected by reverse-transcription quantitative polymerase chain
reaction. (B and C) The protein levels of CBL were detected by
western blot analysis after transfection with miR-155 mimics,
miR-155 inhibitor or their corresponding negative control. (D) A
luciferase reporter assay was performed to identify whether CBL is
a direct target of miR-155. Values are expressed as the mean ±
standard deviation (n=3). *P<0.05, **P<0.01, ***P<0.001 as
indicated. miR, microRNA; CBL, casitas B-lineage lymphoma; hsa,
Homo sapiens; DEL, deletion; WT, wild-type; Mut, mutated;
155M, miR-155 mimics; MNC, negative control miR. |
To identify whether CBL is a direct target of
miR-155, a luciferase reporter assay was performed. Plasmids
containing WT, MUT or DEL were constructed as illustrated in
Fig. 5D, and a luciferase reporter
assay was then performed. As presented in Fig. 5D, co-transfection with WT plus
miR-155 mimics resulted in a significant decreased in relative
luciferase activity to 61±17% of that of the WT plus negative
control of mimics group. However, there was no significant
difference between the relative luciferase activities of cells
transfected with MUT plus miR-155 mimics and MUT plus negative
control of mimics. There was also no significant difference between
the relative luciferase activities of cells transfected with DEL
plus miR-155 mimics and DEL plus negative control of mimics. These
results demonstrated that miR-155 bound to the 3′UTR of CBL and
that CBL was a direct target of miR-155.
Discussion
In the present study, the effects of miR-155 on
colon cancer cells were explored. Upregulation of miR-155 was found
to promote the proliferation and migration of colon cancer cells,
while promoting cell cycle progression and inhibiting apoptosis.
Conversely, downregulation of miR-155 was found to inhibit the
proliferation and migration of colon cancer cells, cause cell cycle
arrest and induce apoptosis. Further study demonstrated that CBL
was a direct target of miR-155, through which miR-155 may exert its
effects on the proliferation, migration, cell cycle and
apoptosis.
Changes of miR expression frequently occur in cancer
cells and are usually associated with tumorigenesis and the
development of cancer. In numerous types of cancer, miR-155
promotes proliferation, migration and differentiation, and inhibits
apoptosis (11,19,22–27).
However, in certain types of cancer, miR-155 represses
proliferation and migration, and promotes apoptosis (28,29).
miR-155 therefore acts as either an oncomiR or tumor suppressor in
different cancer cell types. In the present study, miR-155 was
found to act as an oncomiR in colon cancer cells.
miR-155 has been reported to be overexpressed in
colon cancer (19,20); however, the roles of miR-155 in colon
cancer have remained to be fully elucidated. In the present study,
miR-155 was found to act as an oncomiR in colon cancer cells to
promote the proliferation, cell cycle and migration, but inhibit
apoptosis. Consistent with the results of the present study, Qu
et al (19), Li et al
(30) and Zhang et al
(31) report that miR-155 is
associated with the proliferation, migration and invasion of colon
cancer cells. As an oncomiR, miR-155 may be a promising therapeutic
target for colon cancer therapy. Nanoparticle-based anti-miR-155
therapy has been reported to have excellent therapeutic effects in
an miR-155-dependent mouse model of lymphoma (32). miR-155 is frequently correlated with
poor prognosis and may become a key target of colorectal carcinoma
therapy.
In the present study, CBL was identified as a novel
target of miR-155 using RT-qPCR, western blot analysis and a
luciferase reporter assay. Moreover, Jablonska et al
(33) also mentioned that CBL may be
a target of miR-155. CBL is an E3 ubiquitin ligase, which has
important roles in cell adhesion and migration (34). Migration is a multistep process
involving extension of lamellipodia, formation of focal adhesions
at the leading edge, translocation of the cell body, release of
adhesive contacts and finally retraction at the cell rear (35). CBL is known to regulate the actin
cytoskeleton, to ubiquitinate mDab1 and WAVE2 and to inhibit actin
polymerization (34). CBL also binds
to tubulin and microtubules through its tyrosine kinase-binding
domain and regulates the microtubular network (36). CBL facilitates cytoskeletal
rearrangements and matrix deposition via regulation of Ras-related
C3 botulinum toxin substrate 1 (35), which is required for lamellipodia
extension. CBL also regulates Ras homolog gene family, member A
(35), which in turn regulates
actomyosin contractility and is necessary for cells to move forward
(37). In a different manner, CBL
regulates migration by downregulation of growth factor receptors
(38). Growth factors induce
directional migration by promoting cell polarization, and CBL
modulates the spatial distribution of growth factor receptors in
cells through ubiquitinating the receptors. However, cells
deficient of CBL were found to have severe migration defects,
suggesting that CBL is required for cell migration (34).
CBL is also involved in the growth of cancer cells.
Ectopic expression of CBL inhibited the proliferation of cancer
cells and induced cell cycle arrest at G1 phase (39,40).
Knockdown of CBL was reported to impair colony formation capability
(41) and induce apoptosis (42). Phosphatidylinositol 3 kinase (PI3K)
has important roles in the regulation of proliferation and
apoptosis, and CBL also interacts with PI3K and regulates
proliferation and apoptosis (43).
Increasing CBL expression resulted in decreased cell growth,
increased cell apoptosis and inhibition of tumor development in a
mouse model (44). In addition, CBL
was reported to have a tumor suppressor role in colon cancer cells
(17,18), which was opposite to the effects of
miR-155. This provided further evidence for the present hypothesis
that CBL is a target of miR-155.
In the present study, miR-155 was indicated to
promote the proliferation, arrest cell cycle at S phase, promote
migration of colon cancer cells, and inhibit their apoptosis. CBL,
whose biological functions are opposite to those of miR-155, was
identified as a target of miR-155. The results of the present study
demonstrated that miR-155 acted as an oncomiR in colon cancer cells
through targeting CBL, and miR-155 may become a promising
therapeutic target for colon cancer.
Acknowledgements
The authors would like to thank Dr Min Wang, Dr
Hanrong Liu and Dr Song Han in The Branch of Shanghai First
People's Hospital (Shanghai, China) for their guidance and help
with the study design, experimental operation and data analysis as
well as writing and revision of the manuscript.
References
|
1
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meltzer PS: Cancer genomics: Small RNAs
with big impacts. Nature. 435:745–746. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foshay KM and Gallicano GI: Small RNAs,
big potential: The role of MicroRNAs in stem cell function. Curr
Stem Cell Res Ther. 2:264–271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fabian MR, Sonenberg N and Filipowicz W:
Regulation of mRNA translation and stability by microRNAs. Annu Rev
Biochem. 79:351–379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Connell RM, Kahn D, Gibson WS, Round JL,
Scholz RL, Chaudhuri AA, Kahn ME, Rao DS and Baltimore D:
MicroRNA-155 promotes autoimmune inflammation by enhancing
inflammatory T cell development. Immunity. 33:607–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calame K: MicroRNA-155 function in B
Cells. Immunity. 27:825–827. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vigorito E, Perks KL, Abreu-Goodger C,
Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A,
Bradley A, et al: microRNA-155 regulates the generation of
immunoglobulin class-switched plasma cells. Immunity. 27:847–859.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan L, Hu F, Yan X, Wei Y, Ma W, Wang Y,
Lu S and Wang Z: Inhibition of microRNA-155 ameliorates
experimental autoimmune myocarditis by modulating Th17/Treg immune
response. J Mol Med (Berl). 94:1063–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao Y, Ma X, Yao Y, Li H, Fan Y, Zhang Y,
Zhao C, Wang L, Ma M, Lei Z and Zhang X: miR-155 regulates the
proliferation and invasion of clear cell renal cell carcinoma cells
by targeting E2F2. Oncotarget. 7:20324–20337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Wang W, Li X, He S, Yao J, Wang
X, Zhang D and Sun X: MicroRNA-155 promotes tumor growth of human
hepatocellular carcinoma by targeting ARID2. Int J Oncol.
48:2425–2434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sumara I, Maerki S and Peter M: E3
ubiquitin ligases and mitosis: Embracing the complexity. Trends
Cell Biol. 18:84–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HG, Wang J, Yang X, Hsu HC and
Mountz JD: Regulation of apoptosis proteins in cancer cells by
ubiquitin. Oncogene. 23:2009–2015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bernassola F, Karin M, Ciechanover A and
Melino G: The HECT family of E3 ubiquitin ligases: Multiple players
in cancer development. Cancer Cell. 14:10–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jing Z, Li L, Wang X, Wang M, Cai Y, Jin
ZI and Zhang YE: High c-Cbl expression in gliomas is associated
with tumor progression and poor prognosis. Oncol Lett.
11:2787–2791. 2016.PubMed/NCBI
|
|
16
|
Severe N, Miraoui H and Marie PJ: The
casitas B lineage lymphoma (Cbl) mutant G306E enhances osteogenic
differentiation in human mesenchymal stromal cells in part by
decreased Cbl-mediated platelet-derived growth factor receptor
alpha and fibroblast growth factor receptor 2 ubiquitination. J
Biol Chem. 286:24443–24450. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shashar M, Siwak J, Tapan U, Lee SY, Meyer
RD, Parrack P, Tan J, Khatami F, Francis J, Zhao Q, et al: c-Cbl
mediates the degradation of tumorigenic nuclear β-catenin
contributing to the heterogeneity in Wnt activity in colorectal
tumors. Oncotarget. 7:71136–71150. 2016.PubMed/NCBI
|
|
18
|
Wang L, Cao H, Lu N, Liu L, Wang B, Hu T,
Israel DA, Peek RM Jr, Polk DB and Yan F: Berberine inhibits
proliferation and down-regulates epidermal growth factor receptor
through activation of Cbl in colon tumor cells. PLoS One.
8:e566662013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu YL, Wang HF, Sun ZQ, Tang Y, Han XN, Yu
XB and Liu K: Up-regulated miR-155-5p promotes cell proliferation,
invasion and metastasis in colorectal carcinoma. Int J Clin Exp
Pathol. 8:6988–6994. 2015.PubMed/NCBI
|
|
20
|
Velazquez KT, Enos RT, McClellan JL,
Cranford TL, Chatzistamou I, Singh UP, Nagarkatti M, Nagarkatti PS,
Fan D and Murphy EA: MicroRNA-155 deletion promotes tumorigenesis
in the azoxymethane-dextran sulfate sodium model of colon cancer.
Am J Physiol Gastrointest Liver Physiol. 310:G347–G358. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mattiske S, Suetani RJ, Neilsen PM and
Callen DF: The oncogenic role of miR-155 in breast cancer. Cancer
Epidemiol Biomarkers Prev. 21:1236–1243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu
H, Liu MF and Wang ED: MicroRNA-155 functions as an OncomiR in
breast cancer by targeting the suppressor of cytokine signaling 1
gene. Cancer Res. 70:3119–3127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y, Wang M, Lin G, Sun S, Li X, Qi J
and Li J: Serum microRNA-155 as a potential biomarker to track
disease in breast cancer. PLoS One. 7:e470032012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie Q, Chen X, Lu F, Zhang T, Hao M, Wang
Y, Zhao J, McCrae MA and Zhuang H: Aberrant expression of microRNA
155 may accelerate cell proliferation by targeting sex-determining
region Y box 6 in hepatocellular carcinoma. Cancer. 118:2431–2442.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tiago DM, Conceição N, Caiado H, Laizé V
and Cancela ML: Matrix Gla protein repression by miR-155 promotes
oncogenic signals in breast cancer MCF-7 cells. FEBS Lett.
590:1234–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li S, Chen T, Zhong Z, Wang Y, Li Y and
Zhao X: microRNA-155 silencing inhibits proliferation and migration
and induces apoptosis by upregulating BACH1 in renal cancer cells.
Mol Med Rep. 5:949–954. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Z, Ma Y, Xia Q, Li Y, Li R, Chang W,
Chen J, Leng Z and Tao K: MicroRNA-155 expression inversely
correlates with pathologic stage of gastric cancer and it inhibits
gastric cancer cell growth by targeting cyclin D1. J Cancer Res
Clin Oncol. 142:1201–1212. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai Y, Qiu Z, Diao Z, Shen L, Xue P, Sun H
and Hu Y: MicroRNA-155 inhibits proliferation and migration of
human extravillous trophoblast derived HTR-8/SVneo cells via
down-regulating cyclin D1. Placenta. 33:824–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li T, Yang J, Lv X, Liu K, Gao C, Xing Y
and Xi T: miR-155 regulates the proliferation and cell cycle of
colorectal carcinoma cells by targeting E2F2. Biotechnol Lett.
36:1743–1752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang GJ, Xiao HX, Tian HP, Liu ZL, Xia SS
and Zhou T: Upregulation of microRNA-155 promotes the migration and
invasion of colorectal cancer cells through the regulation of
claudin-1 expression. Int J Mol Med. 31:1375–1380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Babar IA, Cheng CJ, Booth CJ, Liang X,
Weidhaas JB, Saltzman WM and Slack FJ: Nanoparticle-based therapy
in an in vivo microRNA-155 (miR-155)-dependent mouse model of
lymphoma. Proc Natl Acad Sci USA. 109:E1695–E1704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jablonska E, Gorniak P, Prusisz W,
Kiliszek P, Szydlowski M, Sewastianik T, Bialopiotrowicz E, Polak
A, Prochorec-Sobieszek M, Szumera-Cieckiewicz A, et al:
Downregulation of deptor By miR-155 promotes cell survival through
activation of PI3K/AKT and NFkB signaling in ABC-type diffuse large
B-cell Lymphomas. Blood. 128:17612016.
|
|
34
|
Huang C: Roles of E3 ubiquitin ligases in
cell adhesion and migration. Cell Adh Migr. 4:10–18. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Teckchandani AM, Panetti TS and Tsygankov
AY: c-Cbl regulates migration of v-Abl-transformed NIH 3T3
fibroblasts via Rac1. Exp Cell Res. 307:247–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teckchandani AM, Birukova AA, Tar K, Verin
AD and Tsygankov AY: The multidomain protooncogenic protein c-Cbl
binds to tubulin and stabilizes microtubules. Exp Cell Res.
306:114–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mitchison TJ and Cramer LP: Actin-based
cell motility and cell locomotion. Cell. 84:371–379. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jekely G, Sung HH, Luque CM and Rørth P:
Regulators of endocytosis maintain localized receptor tyrosine
kinase signaling in guided migration. Dev Cell. 9:197–207. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shen M and Yen A: c-Cbl tyrosine
kinase-binding domain mutant G306E abolishes the interaction of
c-Cbl with CD38 and fails to promote retinoic acid-induced cell
differentiation and G0 arrest. J Biol Chem. 284:25664–25677. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lo FY, Tan YH, Cheng HC, Salgia R and Wang
YC: An E3 ubiquitin ligase: c-Cbl: A new therapeutic target of lung
cancer. Cancer. 117:5344–5350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
An W, Nadeau SA, Mohapatra BC, Feng D,
Zutshi N, Storck MD, Arya P, Talmadge JE, Meza JL, Band V and Band
H: Loss of Cbl and Cbl-b ubiquitin ligases abrogates hematopoietic
stem cell quiescence and sensitizes leukemic disease to
chemotherapy. Oncotarget. 6:10498–10509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim SY, Kim JH and Song JJ: c-Cbl
shRNA-expressing adenovirus sensitizes TRAIL-induced apoptosis in
prostate cancer DU-145 through increases of DR4/5. Cancer Gene
Ther. 20:82–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brennan T, Adapala NS, Barbe MF, Yingling
V and Sanjay A: Abrogation of Cbl-PI3K interaction increases bone
formation and osteoblast proliferation. Calcif Tissue Int.
89:396–410. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Severe N, Dieudonné FX, Marty C, Modrowski
D, Patiño-García A, Lecanda F, Fromigué O and Marie PJ: Targeting
the E3 ubiquitin casitas B-lineage lymphoma decreases osteosarcoma
cell growth and survival and reduces tumorigenesis. J Bone Miner
Res. 27:2108–2117. 2012. View Article : Google Scholar : PubMed/NCBI
|