Introduction
In general, habitual and daily physical activity has
been associated with a healthy status, prolonged life expectance
and reduced prevalence of chronic diseases (1,2). The
grade of physical activity is also an important determinant factor
of the clinical condition, progression and outcome in patients with
chronic obstructive pulmonary disease (COPD) (3–5).
Patients with COPD with reduced physical activity have been
demonstrated to have a rapid decline of pulmonary function,
weakened and reduced limb muscle mass, frequent hospitalizations,
poor quality of life and worse prognosis compared to patients with
an active lifestyle (3–5). Therefore, one of the main goals of COPD
therapy is to increase the physical participation during daily life
activity.
The long-acting muscarinic antagonists (LAMAs) and
the long-acting β2-agonists (LABAs) are bronchodilators that are
presently recommended in clinical practice (6,7). The
LAMA tiotropium and the LABA olodaterol, that require only
once-a-day administration, have been indicated to be very effective
at improving the clinical outcome in COPD (6,7). As
inhaled bronchodilators are currently the mainstay for long-term
maintenance therapy of patients with COPD with moderate to severe
disease and combination of bronchodilators with different
mechanisms of action may enhance efficacy and reduce the
possibility of adverse events, clinical trials with combination of
a fixed dose of tiotropium and olodaterol have been conducted, and
the results have revealed significant improvement in parameters of
pulmonary function tests and quality of life in comparison with
each mono-component drug (6–9). However, whether this combined therapy
may ameliorate the physical performance of patients with COPD
remains elusive.
In the present investigation, the physical activity
of patients with COPD before and after a combined therapy with
tiotropium and olodaterol under a smart watch-based encouragement
system was evaluated and compared.
Patients and methods
Patients
A total of 20 patients with COPD (18 males and 2
females; mean age, 70.3±6.4 years; age range, 58–83 years) with
stable disease followed up at the Respiratory Center of Matsusaka
Municipal Hospital (Matsusaka, Japan) from February-April 2016 were
enrolled in the present study. COPD was diagnosed following the
criteria of the American Thoracic Society (9). A volume-type spirometer (Super Spiro,
DISCOM-21FX III; Chest M.I., Inc., Tokyo, Japan) was used to
measure lung function parameters, including forced expiratory
volume in 1 sec (FEV1) and forced vital capacity (FVC). The
protocol and criteria for the 6-min walk test (6MWT) and the COPD
Assessment Test (CAT) were previously described (10,11). All
subjects provided informed consent prior to initiation of the
study. The protocol was approved by the Matsusaka Municipal
Hospital Ethics Board for Clinical Investigation (approval no.
150304-2-2) and the study was undertaken following the principles
of the Declaration of Helsinki (clinical registration no. UMIN
23583). Table I summarizes the
clinical and functional parameters of the subjects. All eligible
patients were ex-smokers and none of them were receiving any
smoking cessation pharmacotherapy, home oxygen therapy or pulmonary
rehabilitation.
 | Table I.Baseline demography data of patients
with chronic obstructive pulmonary disease. |
Table I.
Baseline demography data of patients
with chronic obstructive pulmonary disease.
| Variable | Value |
|---|
| Number of
patients | 20 |
| Age, years | 70.3±6.4
(58–83)a |
| Sex
(male/female) | 18/2 |
| Body mass index | 21.59±0.72 |
| Global Initiative for
Chronic Obstructive |
|
| Lung Disease scale
(n) |
|
| 2 | 11 |
| 3 | 9 |
| FVC, 1 | 3.12±0.88 |
| FEV1, 1 | 1.38±0.40 |
| FEV1 (%
predicted) | 5.14±12.8 |
| FEV1/FVC, % | 45.5±10.2 |
Study protocol
All patients with COPD carried a Lifecorder (Suzuken
Co., Ltd., Nagoya, Japan), a uniaxial accelerometer sensor, fixed
to their belts during the entire study period of 4 weeks in order
to record the daily physical activity, including the number of
steps, energy expenditure and the walking distance. A smart watch
(Apple, Inc., Cupertino, CA, USA) worn by the patients during the
4-week study was used to encourage exercise performance. A moderate
grade of physical activity (number of steps) was set up in the
smart watch based on the height and weight of the patients. When
the patient's physical activity was below or near the established
goal, the smart watch vibrated and provided real-time motivational
cues in the form of text messages, for example ‘time to stand up
and stay active for 1 min’ or ‘you are almost there.’ The patients
were instructed to take fixed-dose combination of tiotropium (5 µg)
+ olodaterol (5 µg) once daily using the Respimat inhaler
(Spiolto™, Respimat; Boehringer Ingelheim, am Rhein,
Germany) during the last 2 weeks of the study period. Rescue
therapy with short-acting β agonist was not used during the study.
All patients received sufficient instruction on how to fix and use
the devices in the morning when they woke up and to remove them at
night.
Statistical analysis
Data were expressed as the mean ± standard
deviation. The non-parametric Wilcoxon rank test was employed to
assess the difference among the means of two variables. Statistical
analyses were performed using the StatView 4.5 package for
Macintosh (Abacus Concepts, Piscataway, NJ, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Lung function and symptomatic
parameters
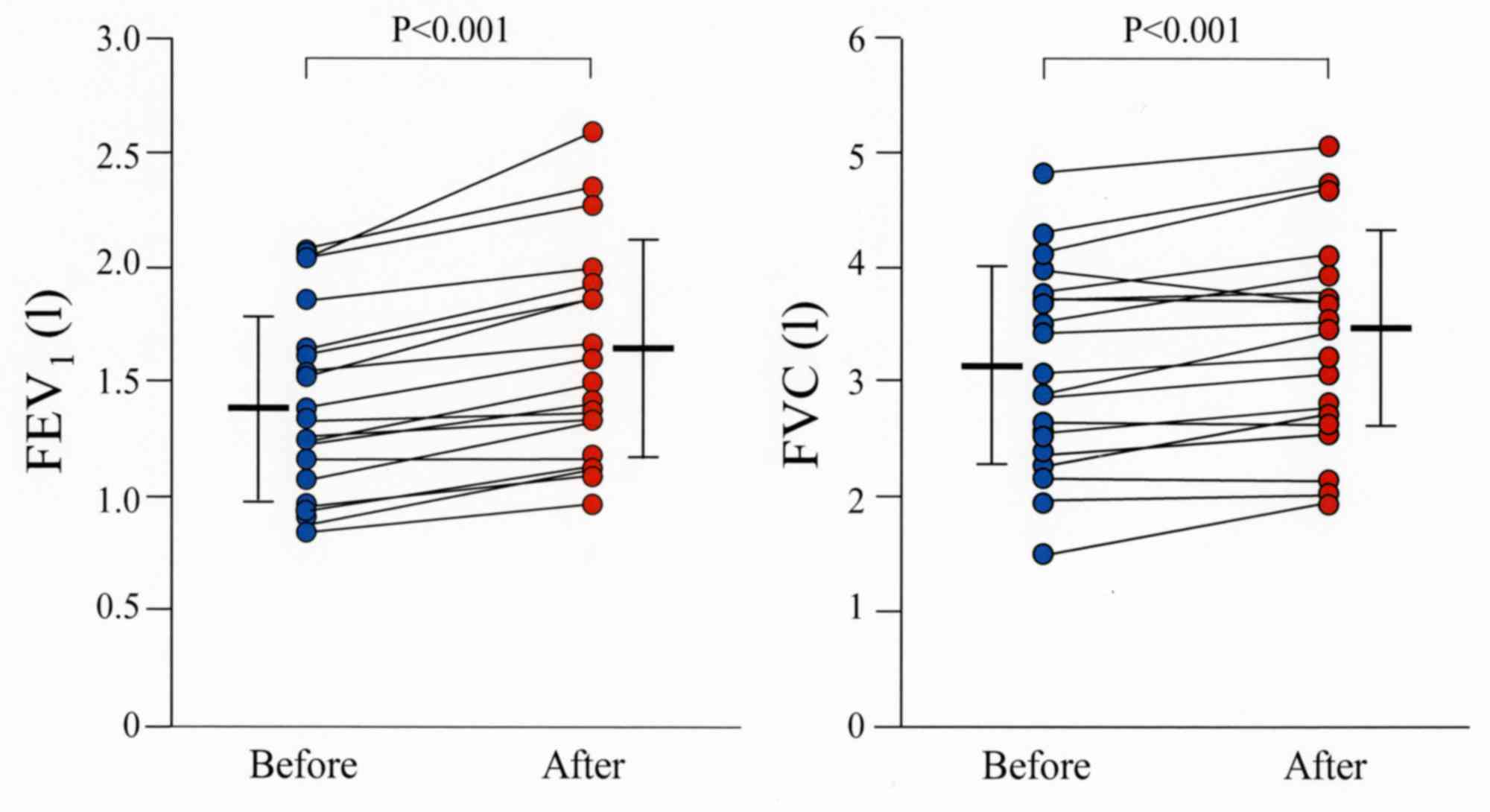
Comparative assessment of pulmonary function tests
before and after treatment with combination therapy demonstrated a
significant increase of FEV1 and FVC after 2 weeks (P<0.001;
Fig. 1) in all treated patients. In
addition to spirometric findings, symptom burden indicators, such
as CAT, have also been recently recommended by the Global
Initiative for COPD for follow-up and management of patients with
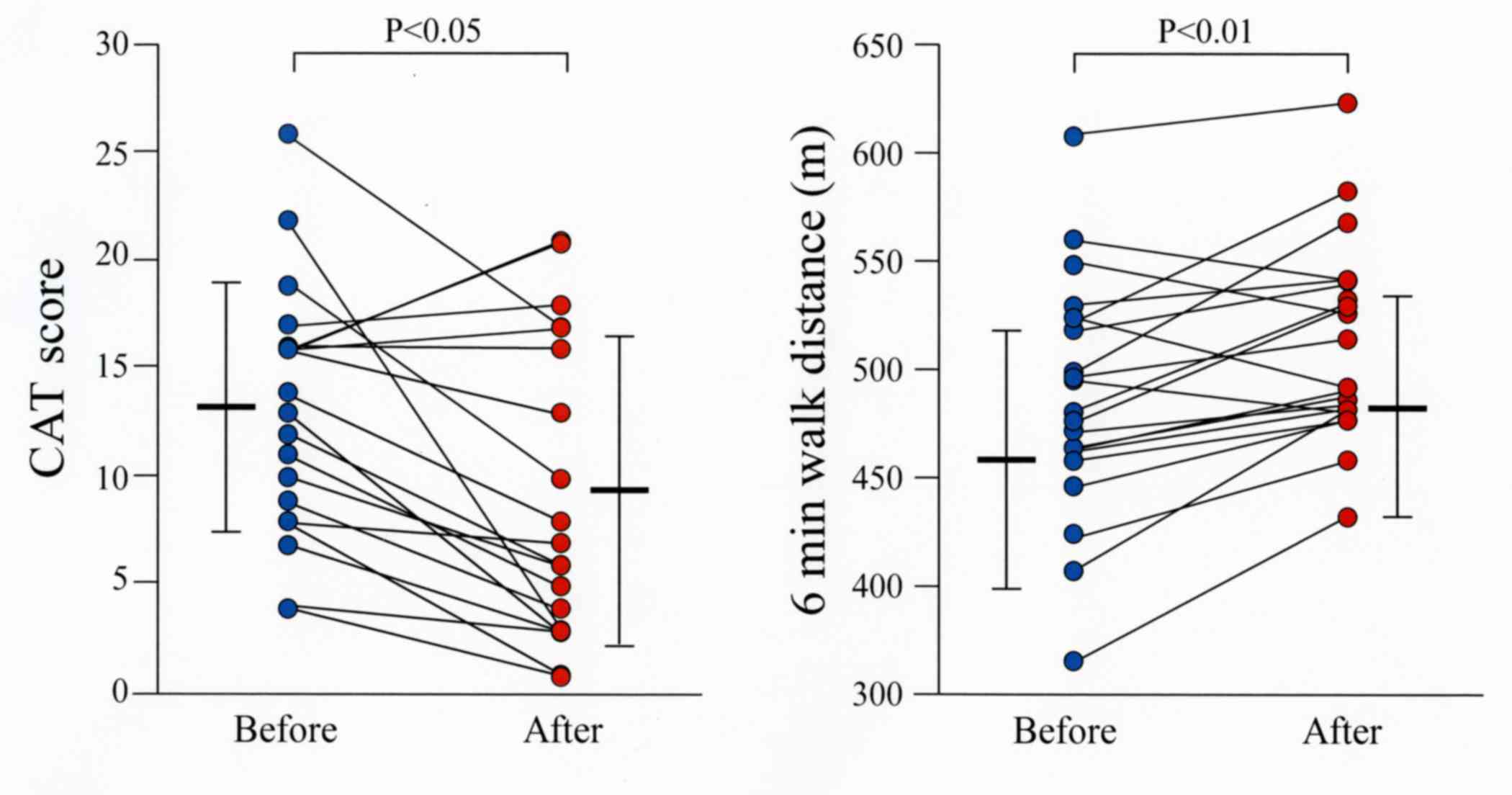
COPD (7). The CAT score was
significantly improved in all patients by combination therapy
compared to values before initiation of the treatment (P<0.05;
Fig. 2).
Physical activity parameters
The distance walked during the 6MWT was
significantly increased in all patients following treatment
compared to that before starting the combination therapy with
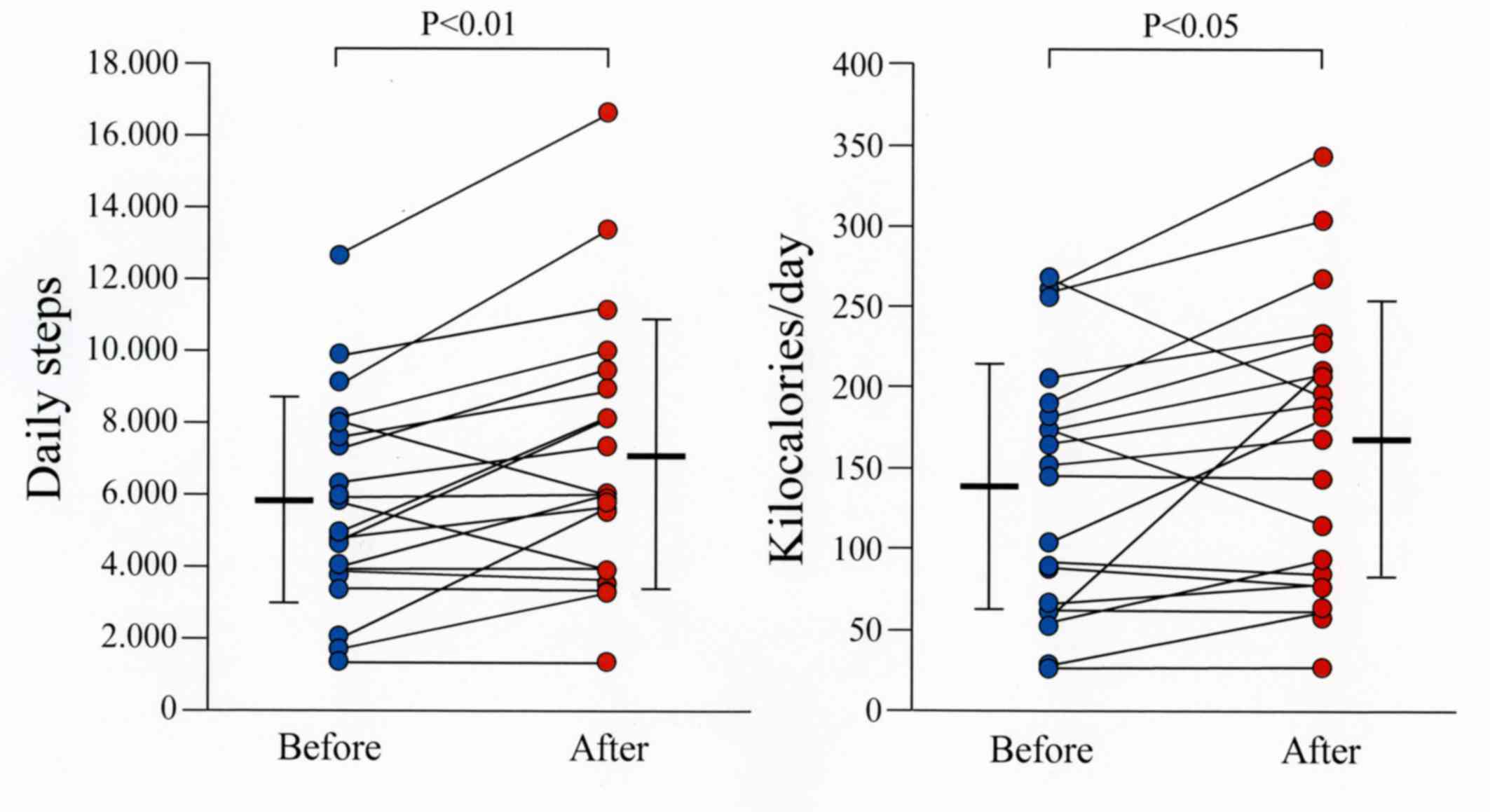
tiotropium and olodaterol (P<0.01; Fig. 2). The number of steps, energy
expenditure expressed in kilocalories and the metabolic equivalent
of task (MET) from 1–9 METs (total activity), 1–3 METs
(low-intensity activity), 4–6 METs (moderate-intensity activity)
and 7–9 METs (high-intensity activity), were measured using the
Lifecorder. The mean number of steps and the mean energy expended
in kilocalories for all patients were significantly increased
(P<0.01 and P<0.05, respectively) following treatment
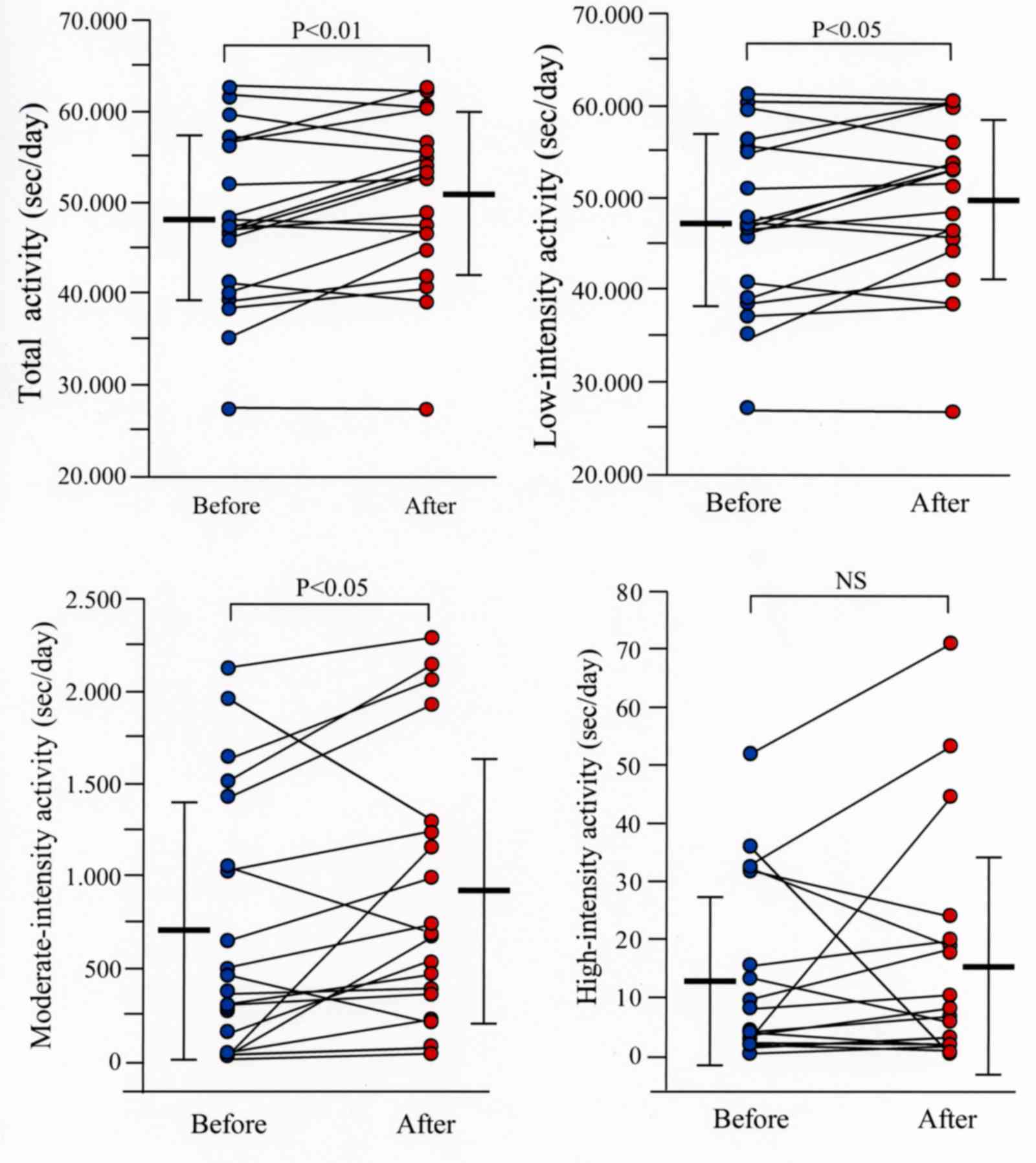
compared to the mean values before treatment (Fig. 3). The total, low-intensity and
moderate-intensity activities were significantly higher (P<0.01,
P<0.05 and P<0.05, respectively) following combination
therapy than values before therapy (Fig.
4). No significant difference was observed in physical activity
parameters when patients were under smart watch-based encouragement
but without receiving combination therapy with bronchodilators
(data not shown).
Discussion
To the best of our knowledge, the present study
demonstrated for the first time that combination therapy with
tiotropium + olodaterol administered once daily significantly
ameliorates physical activity parameters, including 6-min walking
distance, number of steps, energy expenditure and metabolic
equivalent of task, in patients with COPD.
Accumulating evidence supports the critical
importance of enhancing physical activity in COPD (12). One strong illustrative example is the
reported beneficial effects of the formal pulmonary rehabilitation
program on health status, exercise performance and life expectancy
of patients with COPD (13,14). Unfortunately, pulmonary
rehabilitation is not accessible to all patients as it is costly,
time-consuming and requires an interdisciplinary professional team
and special facilities (15–17). Therefore, alternative methods,
including pharmacological and non-pharmacological approaches, have
been undertaken to encourage physical activity in patients with
COPD (18). LABA and LAMA are
included among the pharmacological approaches. However, despite the
beneficial effects of LABA or LAMA on subjective symptoms,
pulmonary function and quality of life, the impact of LABA or LAMA
on daily life physical activity in patients with COPD is
controversial (19,20). Short-term studies have demonstrated
amelioration of physical activity by individual bronchodilators;
however, long-term clinical trials have shown no effect (19,20).
Non-pharmacological incentives, including the use of pedometers,
phone calls, web-based support or smartphone-based activity
stimulators, have also been trialled but without any conclusive
results (21–24).
In the present study, to further address this
question, a combined pharmacological and non-pharmacological
approach was used to motivate physical activity in patients with
COPD. In the present study, the patients were encouraged to be
physically active by a smart watch-based coaching system during the
period they were being treated with a combination of tiotropium +
olodaterol. Through this dual approach using a smart watch-based
alarm and a drug containing both LAMA + LABA, the patients
demonstrated significant improvement in daily life physical
activity.
In conclusion, the present study demonstrated that a
smart watch-based coaching system in combination with tiotropium
and olodaterol has a favorable impact on patients with stable COPD
by increasing their physical activity level.
Acknowledgements
OH has received honorarium for lectures from
Boehringer Ingelheim.
References
|
1
|
Kohl HW III, Craig CL, Lambert EV, Inoue
S, Alkandari JR, Leetongin G and Kahlmeier S: Lancet Physical
Activity Series Working Group: The pandemic of physical inactivity:
Global action for public health. Lancet. 380:294–305. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee IM, Shiroma EJ, Lobelo F, Puska P,
Blair SN and Katzmarzyk PT: Lancet Physical Activity Series Working
Group: Effect of physical inactivity on major non-communicable
diseases worldwide: An analysis of burden of disease and life
expectancy. Lancet. 380:219–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benzo RP, Chang CC, Farrell MH, Kaplan R,
Ries A, Martinez FJ, Wise R, Make B and Sciurba F: NETT Research
Group: Physical activity, health status and risk of hospitalization
in patients with severe chronic obstructive pulmonary disease.
Respiration. 80:10–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcia-Rio F, Rojo B, Casitas R, Lores V,
Madero R, Romero D, Galera R and Villasante C: Prognostic value of
the objective measurement of daily physical activity in patients
with COPD. Chest. 142:338–346. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pitta F, Troosters T, Probst VS, Spruit
MA, Decramer M and Gosselink R: Physical activity and
hospitalization for exacerbation of COPD. Chest. 129:536–544. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buhl R, Maltais F, Abrahams R, Bjermer L,
Derom E, Ferguson G, Fležar M, Hébert J, McGarvey L, Pizzichini E,
et al: Tiotropium and olodaterol fixed-dose combination versus
mono-components in COPD (GOLD 2–4). Eur Respir J. 45:969–979. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferguson GT, Fležar M, Korn S, Korducki L,
Grönke L, Abrahams R and Buhl R: Efficacy of tiotropium +
olodaterol in patients with chronic obstructive pulmonary disease
by initial disease severity and treatment intensity: A post hoc
analysis. Adv Ther. 32:523–536. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vestbo J, Edwards LD, Scanlon PD, Yates
JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, et
al: Changes in forced expiratory volume in 1 sec over time in COPD.
N Engl J Med. 365:1184–1192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rabe KF, Hurd S, Anzueto A, Barnes PJ,
Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R,
van Weel C, et al: Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease: GOLD
executive summary. Am J Respir Crit Care Med. 176:532–555. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
ATS Committee on Proficiency Standards for
Clinical Pulmonary Function Laboratories: ATS statement: Guidelines
for the six-minute walk test. Am J Respir Crit Care Med.
166:111–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mackay AJ, Donaldson GC, Patel AR, Jones
PW, Hurst JR and Wedzicha JA: Usefulness of the Chronic Obstructive
Pulmonary Disease Assessment Test to evaluate severity of COPD
exacerbations. Am J Respir Crit Care Med. 185:1218–1224. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vaes AW, Garcia-Aymerich J, Marott JL,
Benet M, Groenen MT, Schnohr P, Franssen FM, Vestbo J, Wouters EF,
Lange P and Spruit MA: Changes in physical activity and all-cause
mortality in COPD. Eur Respir J. 44:1199–1209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dodd JW, Hogg L, Nolan J, Jefford H, Grant
A, Lord VM, Falzon C, Garrod R, Lee C, Polkey MI, et al: The COPD
assessment test (CAT): Response to pulmonary rehabilitation. A
multicentre, prospective study. Thorax. 66:425–429. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lacasse Y, Goldstein R, Lasserson TJ and
Martin S: Pulmonary rehabilitation for chronic obstructive
pulmonary disease. Cochrane Database Syst Rev: CD003793. 2006.
View Article : Google Scholar
|
|
15
|
Casaburi R and ZuWallack R: Pulmonary
rehabilitation for management of chronic obstructive pulmonary
disease. N Engl J Med. 360:1329–1335. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nici L, Donner C, Wouters E, Zuwallack R,
Ambrosino N, Bourbeau J, Carone M, Celli B, Engelen M, Fahy B, et
al: American thoracic society/european respiratory society
statement on pulmonary rehabilitation. Am J Respir Crit Care Med.
173:1390–1413. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yohannes A, Stone R, Lowe D, Pursey N,
Buckingham R and Roberts C: Pulmonary rehabilitation in the United
Kingdom. Chron Respir Dis. 8:193–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davis AH: Exercise adherence in patients
with chronic obstructive pulmonary disease: An exploration of
motivation and goals. Rehabil Nurs. 32:104–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishijima Y, Minami S, Yamamoto S, Ogata
Y, Koba T, Futami S and Komuta K: Influence of indacaterol on daily
physical activity in patients with untreated chronic obstructive
pulmonary disease. Int J Chron Obstruct Pulmon Dis. 10:439–444.
2015.PubMed/NCBI
|
|
20
|
Watz H, Krippner F, Kirsten A, Magnussen H
and Vogelmeier C: Indacaterol improves lung hyperinflation and
physical activity in patients with moderate chronic obstructive
pulmonary disease-a randomized, multicenter, double-blind,
placebo-controlled study. BMC Pulm Med. 14:1582014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mendoza L, Horta P, Espinoza J, Aguilera
M, Balmaceda N, Castro A, Ruiz M, Díaz O and Hopkinson NS:
Pedometers to enhance physical activity in COPD: A randomised
controlled trial. Eur Respir J. 45:347–354. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moy ML, Weston NA, Wilson EJ, Hess ML and
Richardson CR: A pilot study of an Internet walking program and
pedometer in COPD. Respir Med. 106:1342–1350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tabak M, Brusse-Keizer M, van der Valk P,
Hermens H and Vollenbroek-Hutten M: A telehealth program for
self-management of COPD exacerbations and promotion of an active
lifestyle: A pilot randomized controlled trial. Int J Chron
Obstruct Pulmon Dis. 9:935–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wewel AR, Gellermann I, Schwertfeger I,
Morfeld M, Magnussen H and Jörres RA: Intervention by phone calls
raises domiciliary activity and exercise capacity in patients with
severe COPD. Respir Med. 102:20–26. 2008. View Article : Google Scholar : PubMed/NCBI
|