Introduction
A contaminated atmospheric environment, including
that in numerous Chinese regions suffering from fog and haze, has a
serious impact on human health. The increasing levels of mortality
and morbidity due to lung infection and respiratory diseases are
attributed to elevated levels of particulate matter (PM),
particularly small inhalable particles like PM2.5
(1).
PM2.5 is a type of PM that is ≤2.5 µm in
diameter. The physical damage caused by PM is associated with its
size; the smaller the size, the greater damage it causes. In
addition, PM2.5 accumulates toxic heavy metals, acid
oxides, organic pollutants, bacteria and viruses in the air,
PM2.5 can also remain in the air for a long time and be
deposited in the lungs through inhalation, so it is a major threat
to human health (2,3). Numerous previous studies have suggested
that PM2.5 can stimulate the production of reactive
oxygen species (ROS) and certain inflammatory mediators, resulting
in changes to vascular permeability, airway constriction and tissue
injury (4–6). The majority of previous studies
investigating PM2.5 have histopathologically examined
lung sections (7,8).
Chronic obstructive pulmonary disease (COPD) is
characterized by airway obstruction due to the destruction of lung
parenchyma, structural alterations of the small airways and
systemic inflammation (9). COPD is a
major cause of morbidity and mortality globally, and knowledge
about its pathogenesis has increased substantially over the past
decade (9,10). The primary risk factor for COPD is
prolonged cigarette smoking (11).
Another risk factor for COPD is chronic environmental exposure to
toxic atmospheric pollutants, including PM (2–4). Several
mechanisms of action have been proposed for the anti-inflammatory
efficacy of antibiotics and traditional Chinese medicines (TCMs) on
respiratory diseases, including COPD, house dust mite-induced
allergic asthma, resistance of Klebsiella pneumoniae, and
ventilator-associated pneumonia (12–15).
However, the efficacy of such antibiotics was limited by their side
effects in clinical trials, which included vomiting, diarrhea,
weight loss and headaches (16–18). At
present, no effective control measures have been developed for the
treatment of PM2.5-induced respiratory diseases apart
from reducing PM2.5 emissions, wearing a dust respirator
and increasing the number of plants. Therefore, novel medicines
with fewer side effects and a high efficacy for treating
PM2.5-induced respiratory diseases are required.
A TCM extracted from Stemona tuberosa,
stemonine, has been applied for insecticidal and medicinal purposes
(19). S. tuberosa is found
in certain regions of Japan and China, and its root can be used to
obtain stemonine. In TCM it is believed that the external use of
stemonine deters mosquitoes and that its oral administration can
relieve a cough. In previous studies, several types of stemonine
were used to treat chronic lung diseases, including chronic
bronchitis, pneumonia, asthma and COPD, through antibacterial
action, resolving phlegm and relaxing bronchial smooth muscle
(20–22).
In the present study, the effects of stemonine and
its mechanism of action were investigated in mice with
PM2.5-induced COPD. The results revealed that stemonine
attenuated acute PM2.5-induced lung inflammation by
inhibiting the infiltration of inflammatory cells. These results
suggest that stemonine is a potential candidate for the treatment
of respiratory diseases.
Materials and methods
Animals and reagents
A total of 50 adult male Kunming mice aged 6–8 weeks
and weighing 20–22 g were housed in a pathogen-free environment
with a 12 h light/dark cycle at room temperature (20±2°C) with a
relative humidity of 50–70%. All animal protocols were conducted in
accordance with the Declaration of Helsinki and the Guide for the
Care and Use of Laboratory Animals. The present study was approved
by the Ethics Committee of Yantai Hospital of Traditional Chinese
Medicine (Yantai, China).
All chemicals and solvents used were of analytical
grade. The lactate dehydrogenase (LDH, ml002267), alkaline
phosphatase (AKP, ml002235), acidic phosphatase (ACP, ml037464),
albumin (ALB, ml037889), nitric oxide (NO, ml022390), nitric oxide
synthase (NOS, ml001884), malonyldialdehyde (MDA, ml016824) and
superoxide dismutase (SOD, ml001998) ELISA kits were obtained from
Shanghai Enzyme-Linked Biotechnology Co., Ltd. (Shanghai, China)
and used according to the manufacturer's protocol.
Source and separation of
PM2.5
Urban airborne PM2.5 was collected with a
Thermo Anderson sampler (PDR-1500; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in Yantai, China for 2 consecutive weeks in
January 2016. Subsequently, the sampling filter membrane was cut
into 3×2 cm sections and immersed in ultrapure water for ultrasonic
oscillation (room temperature; 100 kHz; four times, 30 min each).
After filtration with gauze, the filtrate was centrifuged at 16,060
× g for 20 min at room temperature. After centrifugation, the
supernatant was removed and the precipitation was collected into
physiological saline (PS), autoclave-sterilized, freeze-dried and
stored at 4°C until required.
COPD model establishment and
administration
The model mice (n=40) received intranasal
instillation of 20 µl of PM2.5 suspension (40 mg/kg)
once a day for 7 consecutive days, whereas mice in the control
group (n=10) received the same amount of PS. The model mice were
then randomly divided into the following four groups (n=10/group):
Model group (40 mg/kg PM2.5), low-dose stemonine (LS)
treatment group (45 mg/kg stemonine), moderate-dose stemonine (MS)
treatment group (90 mg/kg stemonine) and the high-dose stemonine
(HS) treatment group (180 mg/kg stemonine). Stemonine was obtained
from Beijing Kangrentang Pharmaceutical Co. (Beijing, China; batch
no. 15009731). The treatment period lasted for 21 days, with
treatment occurring once daily. All of the mice were checked daily
for their general condition including physical appearance and
behavior of mice, including hair condition, liveliness, sensitivity
and respiratory murmur. A total of 24 h after the last intranasal
instillation, the mice were sacrificed. The left upper lobe of the
lungs was removed for hematoxylin and eosin (H&E) staining, and
bronchoalveolar lavage fluid (BALF) and lung specimens were
collected for further analysis.
BALF analysis
BALF was obtained by injecting 1 ml of 1X PBS and
withdrawing as much fluid as possible according to the procedure
used by Chen et al (13).
BALF was then centrifuged at 200 × g for 10 min at 4°C. The
obtained supernatants were used to detect the levels of LDH, AKP,
ACP, ALB, NO, NOS, MDA and SOD with the aforementioned kits
according to the manufacturer's protocol. Levels of the cytokines
tumor necrosis factor (TNF)-α and interleukin (IL)-6 in the BALF
were analyzed using ELISA kits according to the manufacturer's
protocol (H052 and H007; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China).
Lung histology
The lung samples (left upper lobe of the lungs) from
each group were fixed with 10% buffered formalin solution at room
temperature for 24 h. The fixed lung tissues were dehydrated,
embedded in paraffin and sectioned (5 µm). H&E staining was
performed at room temperature for 5–10 min each to determine
inflammatory changes to the lung tissue. The specimens were then
examined using light microscopy for the effects of inflammation,
including infiltrates, thickened alveolar septae, pus and cell
hyperplasia.
Statistical analysis
Comparisons between groups were analyzed by one-way
analysis of the variance followed by Fisher's least significant
difference test. Results are expressed as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
Stemonine improves the physical
appearance and behavior of mice with PM2.5-induced
COPD
Throughout the experimental period, mice in the
control group had shiny hair, and were lively and sensitive,
whereas the COPD model mice exhibited shaggy hair, and appeared
listlessness and unresponsive, in addition to having a respiratory
murmur (data not shown). Furthermore, the mean body weight of the
LS, MS and HS groups was higher compared with that of the model
group (data not shown).
Stemonine decreases pulmonary
inflammation in mice with PM2.5-induced COPD
To evaluate the effect of stemonine on the
biomembrane and parenchyma in the lungs of the mice, biochemical
markers in the BALF were measured, including LDH, ACP, AKP and ALB
(Fig. 1 and Table I). Compared with the control group
mice that received PS, the model mice that received
PM2.5 had significantly higher levels of LDH, ACP, AKP
and ALB (all P<0.05), indicating more pulmonary inflammation.
However, the groups treated with stemonine (45, 90 and 180 mg/kg)
exhibited a significant decrease in LDH, ACP, AKP and ALB compared
with the model group (all P<0.05). These results suggest that
stemonine has a dose-dependent negative effect on the levels of
LDH, ACP, AKP and ALB.
 | Figure 1.Stemonine decreases the levels of
LDH, ACP, AKP and ALB in mice with particulate matter 2.5-induced
chronic obstructive pulmonary disease. Levels of (A) LDH, (B) ACP,
(C) AKP and (D) ALB in the bronchoalveolar fluid. LDH, lactate
dehydrogenase; ACP, acidic phosphatase; AKP, alkaline phosphatase;
ALB, albumin; LS, low-dose stemonine; MS, moderate-dose stemonine;
HS, high-dose stemonine. *P<0.05 vs. the control group;
#P<0.05 vs. the model group. |
 | Table I.Stemonine decreases the levels of
LDH, ACP, AKP and ALB in mice with particulate matter 2.5-induced
chronic obstructive pulmonary disease. |
Table I.
Stemonine decreases the levels of
LDH, ACP, AKP and ALB in mice with particulate matter 2.5-induced
chronic obstructive pulmonary disease.
|
| Enzyme/protein
investigated |
|---|
|
|
|
|---|
| Group | LDH (U/l) | ACP (U/l) | AKP (U/l) | ALB (mg/ml) |
|---|
| Control | 112.975±6.562 | 1.642±0.136 | 4.653±0.247 | 0.064±0.023 |
| Model |
405.634±18.693a |
3.854±0623a |
8.795±0.312a |
0.198±0.264a |
| LS |
314.564±8.126a,b |
3.101±0282a,b |
7.582±0.196a,b |
0.159±0.132a,b |
| MS |
221.721±9.124a,b |
2.551±0362a,b |
6.538±0.264a,b |
0.105±0.067a,b |
| HS |
125.623±8.643a,b |
2.002±0328a,b |
5.315±0.169a,b |
0.079±0.153a,b |
Stemonine decreases the levels of
oxidative stress markers in mice with PM2.5-induced
COPD
The activity of SOD and NOS, and the amount of NO
and MDA were measured in the BALF of the different groups using
ELISA kits (Fig. 2 and Table II). SOD activity was measured
through the reduction of xanthine oxidase to uric acid and
H2O2, which reduces nitroblue tetrazolium
(NBT) to NBT-formazan (23). The
activity of NOS, and the levels of NO and MDA in the LS, MS and HS
groups was significantly decreased compared with the model group
(all P<0.05). In addition, the activity of SOD was significantly
increased in the LS, MS and HS groups compared with the model group
(P<0.05). These data indicate that stemonine inhibits the
oxidative stress induced by PM2.5 in the lungs of mice
in a dose-dependent manner, thus reducing oxidative
stress-associated lung damage.
 | Figure 2.Stemonine decreases the levels of
MDA. NO and NOS, and increases the level of SOD, in mice with
particulate matter 2.5-induced chronic obstructive pulmonary
disease. Levels of (A) MDA, (B) NO, (C) NOS and (D) SOD in the
bronchoalveolar fluid. MDA, malonyldialdehyde; NO, nitric oxide;
NOS, NO synthase; SOD, superoxide dismutase; LS, low-dose
stemonine; MS, moderate-dose stemonine; HS, high-dose stemonine.
*P<0.05 vs. the control group; #P<0.05 vs. the
model group. |
 | Table II.Stemonine decreases the levels of
MDA. NO and NOS, and increases the level of SOD, in mice with
particulate matter 2.5-induced chronic obstructive pulmonary
disease. |
Table II.
Stemonine decreases the levels of
MDA. NO and NOS, and increases the level of SOD, in mice with
particulate matter 2.5-induced chronic obstructive pulmonary
disease.
|
| Marker of cellular
oxidation state |
|---|
|
|
|
|---|
| Group | MDA (nmol/ml) | NO (µmol/l) | NOS (U/ml) | SOD (U/ml) |
|---|
| Control | 0.729±0.098 |
4.219±0.494 | 2.783±0.147 | 26.895±0.891 |
| Model |
1.791±0.524a |
13.712±0.628a |
5.962±0.135a |
13.246±0.628a |
| LS |
1.568±0.319a,b |
11.597±0.352a,b |
4.983±0.103a,b |
16.163±0.52a,b |
| MS |
1.335±0.208a,b |
8.846±0.523a,b |
3.893±0.136a,b |
19.682±0.424a,b |
| HS |
1.165±0.128a,b |
7.471±0.493a,b |
3.013±0.114a,b |
23.936±0.431a,b |
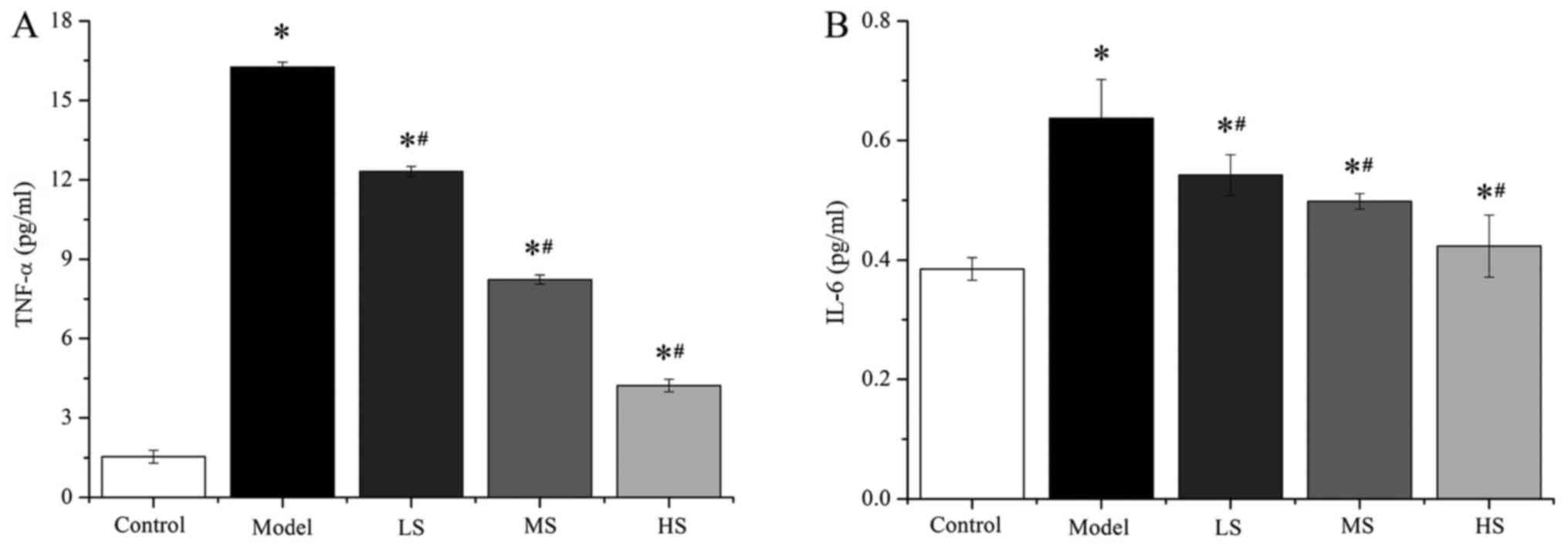
Stemonine alleviates lung inflammation
in mice with PM2.5-induced COPD
Previous studies have demonstrated that acute
cigarette smoke exposure depletes the antioxidant capacity of the
lungs (24,25). Thus, the effects of stemonine on the
levels of the cytokines TNF-α and IL-6 in the BALF of the different
groups were measured using ELISA kits in the present study
(Fig. 3 and Table III). The levels of TNF-α and IL-6
in the groups treated with stemonine (45, 90 and 180 mg/kg) were
significantly higher compared with those in the control group (all
P<0.05), whereas they were significantly lower compared with the
model group (all P<0.05). These results suggest that stemonine
alleviates PM2.5-induced lung inflammation in mice.
 | Table III.Stemonine decreases of the levels of
TNF-α and IL-6 in mice with particulate matter 2.5-induced chronic
obstructive pulmonary disease. |
Table III.
Stemonine decreases of the levels of
TNF-α and IL-6 in mice with particulate matter 2.5-induced chronic
obstructive pulmonary disease.
|
| Cytokine |
|---|
|
|
|
|---|
| Group | TNF-α (pg/ml) | IL-6 (pg/ml) |
|---|
| Control |
1.54±0.24 | 0.385±0.019 |
| Model |
16.25±0.19a |
0.637±0.065a |
| LS |
12.31±0.19a,b |
0.542±0.034a,b |
| MS |
8.23±0.17a,b |
0.498±0.013a,b |
| HS |
4.23±0.24a,b |
0.423±0.052a,b |
Stemonine reduces lung inflammation
and damage in mice with PM2.5-induced COPD
Histology analysis was performed and evaluated as
previously described (23,24). Pathological changes and the effects
of inflammation, including infiltrates, thickened alveolar septae,
pus and cell hyperplasia, were investigated in the lung tissues of
the different groups using H&E staining (Fig. 4). This revealed marked infiltration
of inflammatory cells and exudative changes in the lungs of the
model group (Fig. 4B), whereas no
inflammation was observed in the control mice (Fig. 4A). However, there was a notable
improvement in inflammation in the mice treated with stemonine
(Fig. 4C-E) in a dose-dependent
manner. These results indicate that stemonine reduces
PM2.5-induced lung inflammation and damage, suggesting
that it may have a therapeutic effect on the pathological effects
of PM2.5.
Discussion
In recent decades, much research has been focused on
the direct and indirect toxic effects of PM2.5 in China,
the USA and certain European countries. A previous study reported
that PM in the air, particularly PM2.5, can cause
bronchial wall thickening and the production of ROS (26). Long-term exposure to high levels of
PM is associated with respiratory diseases. A previous study
reported that PM2.5 exacerbates chronic inflammatory
conditions of the lungs, including COPD, pulmonary hypertension and
autoimmune diseases (25). With
increasing PM2.5 in the air, the incidence of
respiratory diseases, including pneumonia, asthma and COPD, will
gradually increase.
Recently, several types of TCMs have been used to
treat PM2.5-induced respiratory diseases (27–29). A
recent study reported that tuberostemonine could attenuate acute
cigarette smoke-induced inflammation of the lung via inhibiting the
infiltration of inflammatory cells through decreasing chemokine
expression (30). Chen et al
(13) reported that resveratrol
exhibits potent antiallergic activities on allergic airway
inflammation in a house dust mite-induced mouse model of asthma.
Qin et al (31) identified
that Guben Zhike granules could reduce inflammatory cell
infiltration and improve lung function in a
PM2.5-induced mouse model of lung injury. Other studies
have explored the underlying mechanisms of allergic airway
inflammation and pulmonary hypertension associated with
environmental toxins, for example, nasal inoculation of particulate
matter (PM2.5) could induce allergic airway inflammation in NC/Nga
mice (4), and Park et al
(32) identified B cells and antigen
specific IgG1 as potential therapeutic targets for pulmonary
hypertension associated with immune dysfunction and environmental
exposures. The present study demonstrated that stemonine, which is
derived from S. tuberose, exhibits therapeutic effects on
mice with PM2.5-induced COPD. An influx of inflammatory
cells, and changes to the lung tissue and alveolar structure, were
observed when the mice were exposed to PM2.5. However,
when these mice were treated with stemonine it inhibited the influx
of inflammatory cells.
Several previous studies have demonstrated that
PM2.5 exposure directly activates innate immune cells
and epithelial cells, and induces chemokine, proinflammatory
cytokine, growth factor, protease and antibacterial protein
expression (28,33,34). To
investigate the underlying molecular mechanisms of the effects of
PM2.5, the present study analyzed the levels of specific
enzymes, oxidative stress markers and cytokines in the BALF, in
addition to performing histology analysis. This revealed
significantly increased levels of LDH, ACP, AKP, ALB, MDA, NO, NOS,
TNF-α and IL-6, and significantly decreased levels of SOD, in the
BALF after exposure to PM2.5. This indicates that
PM2.5 exerts its effects through altering the expression
of cytokines and increasing ROS production in the lungs. In
addition, stemonine treatment significantly decreased the levels of
LDH, AKP, ACP, ALB, NO, NOS, MDA, TNF-α and IL-6, and significantly
increased the level of SOD, in a dose-dependent manner. These data
indicate that stemonine attenuates acute PM2.5-induced
lung inflammation via inhibiting the infiltration and cytotoxicity
of inflammatory cells. Therefore, stemonine is a potential
therapeutic drug for the treatment of respiratory diseases.
Since PM2.5 is a complex mixture of
various components, and the composition of PM2.5
collected at different time points and in different locations
varies, the mechanisms by which PM2.5 functions in the
body is likely to be complex. The present study was a preliminary
investigation into the therapeutic effects of stemonine on
immunoreactivity and oxidative stress in a mouse model of
PM2.5-induced lung injury. Future research should
explore other mechanisms of PM2.5 in lung injury, and
the interaction and mutual associations between these mechanisms.
In addition, the specific regulation of the mechanism by which
PM2.5 functions remains to be elucidated. Furthermore,
studies should aim to identify the essential targets of stemonine,
which could lead to the pharmacological development of treatments
for respiratory diseases induced by PM2.5.
In conclusion, stemonine is able to inhibit the
development of PM2.5-induced lung inflammation through
inhibition of the cytokine response, thus making it a therapeutic
candidate for the treatment of respiratory diseases, including
COPD. At present, no effective control measures have been developed
for the treatment of PM2.5-induced respiratory diseases
apart from reducing PM2.5 emissions, wearing a dust
respirator and increasing the number of plants. The results of the
present study highlight novel areas for the prevention and
treatment of respiratory diseases associated with environmental
pollution.
References
|
1
|
Kaiser J: Air pollution: Evidence mounts
that tiny particles can kill. Science. 289:22–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao Q, He KB, Ma YL, Jia YT, Cheng Y, Liu
H and Wang SW: Regional PM pollution in Beijing and surrounding
area during summertime. Huan Jing Ke Xue. 30:1873–1880. 2009.(In
Chinese). PubMed/NCBI
|
|
3
|
Pope CA III, Burnett RT, Thun MJ, Calle
EE, Krewski D, Ito K and Thurston GD: Lung cancer, cardiopulmonary
mortality and long-term exposure to fine particulate air pollution.
JAMA. 287:1132–1141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kodavanti UP, Jaskot RH, Su WY, Costa DL,
Ghio AJ and Dreher KL: Genetic variability in combustion
particle-induced chronic lung injury. Am J Physiol. 272:L521–L532.
1997.PubMed/NCBI
|
|
5
|
Tong Y, Ni X, Zhang Y, Chen F, Zhang G and
Ye S: Study of toxicological mechanism of acidified aerosols. Biol
Trace Elem Res. 85:149–156. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogino K, Zhang R, Takahashi H, Takemoto K,
Kubo M, Murakami I, Wang DH and Fujikura Y: Allergic airway
inflammation by nasal inoculation of particulate matter (PM2.5) in
NC/Nga mice. PloS One. 9:e927102014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dye JA, Lehmann JR, McGee JK, Winsett DW,
Ledbetter AD, Everitt JI, Ghio AJ and Costa DL: Acute pulmonary
toxicity of particulate matter filter extracts in rats: Conherence
with epidemiologic studies in Utah valley residents. Environ Health
Perspect. 109 Suppl 3:S395–S403. 2001. View Article : Google Scholar
|
|
8
|
Greenwell LL, Moreno T, Jones TP and
Richards RJ: Particle-induced oxidative damage is ameliorated by
pulmonary antioxidants. Free Radic Biol Med. 32:898–905. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cazzola M, Donner CF and Hanania NA: One
hundred years of chronic obstructive pulmonary disease (COPD).
Respir Med. 101:1049–1065. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bozarth AL, Covey A, Gohar A and Salzman
G: Chronic obstructive pulmonary disease: Clinical review and
update on consensus guidelines. Hosp Pract. 42:79–91. 2014.
View Article : Google Scholar
|
|
11
|
Kammerl IE, Dann A, Mossina A, Brech D,
Lukas C, Vosyka O, Nathan P, Conlon TM, Wagner DE, Overkleeft HS,
et al: Impairment of immunoproteasome function by cigarette smoke
and in chronic obstructive pulmonary disease. Am J Respir Crit Care
Med. 193:1230–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stefan MS, Rothberg MB, Shieh MS, Pekow PS
and Lindenauer PK: Association between antibiotic treatment and
outcomes in patients hospitalized with acute exacerbation of COPD
treated with systemic steroids. Chest. 143:82–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Zhou H, Wang J, Zhang B, Liu F,
Huang J, Li J, Lin J, Bai J and Liu R: Therapeutic effects of
resveratrol in a mouse model of HDM-induced allergic asthma. Int
Immunopharmacol. 25:43–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao XQ, Lai J, Luo YL, Wang HB, Fang YL
and Huang SF: Study on reversal of effect of double coptis in vivo
on the resistance of Klebsiella pneumoniae. J Gannan Med
Univ. 36:190–192. 2016.(In Chinese).
|
|
15
|
Zhang F: Clinical observation on treating
ventilator-associated pneumonia with the Qingjin Huatan decoction.
Clin J Chin Med. 8:71–72. 2016.(In Chinese).
|
|
16
|
Wright J and Paauw DS: Complications of
antibiotic therapy. Med Clin North Am. 97:667–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Blumenthal KG, Shenoy ES, Hurwitz S,
Varughese CA, Hooper DC and Banerji A: Effect of a drug allergy
educational program and antibiotic prescribing guideline on
inpatient clinical providers' antibiotic prescribing knowledge. J
Allergy Clin Immunol Pract. 2:407–413. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rolain JM, Abat C, Jimeno MT, Fournier PE
and Raoult D: Do we need new antibiotics? Clin Microbiol Infect.
22:408–415. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu YT, Shaw PC, Jiang RW, Hon PM, Chan YM
and But PP: Antitussive and central respiratory depressant effects
of Stemona tuberosa. J Ethnopharmacol. 128:679–684. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao JF and Zhang XY: Experimental
observation on germicidal efficacy of a herbal compound
disinfectant solution. Chin J Disinfection. 22:305–306. 2005.
|
|
21
|
Chung HS, Hon PM, Lin G, But PP and Dong
H: Antitussive activity of Stemona alkaloids from Stemona
tuberose. Planta Med. 69:914–920. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao JF, Shi CC, Chen SY, Fu YT and Chen
CF: Spasmolytic effect of walter extract of Stemonae radix on the
guinea-pig tracheal smooth muscle in vitro. J Ethnopharmacol.
57:57–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Hai CX, Liang X, Yu SX, Zhang W
and Li YL: The protective effects of acanthopanax senticosus, harms
aqueous extracts against oxidative stress: Role of Nrf2 and
antioxidant enzymes. J Ethnopharmacol. 127:424–432. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Onizawa S, Aoshiba K, Kajita M, Miyamoto Y
and Nagai A: Platinum nanoparticle antioxidants inhibit pulmonary
inflammation in mice exposed to cigarette smoke. Pulm Pharmacol
Ther. 22:340–349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rahman I, Bismas SK and Kode A: Oxidant
and antioxidant balance in the airways and airway disease. Eur J
Pharmacol. 533:222–239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Driscoll KE: TNFα and MIP-2: Role in
particle-induced inflammation and regulation by oxidative stress.
Toxicol Lett. 112–113:177–183. 2000. View Article : Google Scholar
|
|
27
|
Cui S, He ZZ, Zhu ZW, Sun Z, Xu YT, Wang
JL, Bao YY, Ji DY, Liu S, Liu JT, et al: Microfluidic analysis of
PM2.5-induced epithelial-mesenchymal transition in human bronchial
epithelial 16HBE cells. Microfluid Nanofluidics. 19:263–272. 2015.
View Article : Google Scholar
|
|
28
|
Jing Y, Zhang H, Cai Z, Zhao Y, Wu Y,
Zheng X, Liu Y, Qin Y, Gu M and Jin J: Bufei huoxue capsule
attenuates PM2.5-induced pulmonary inflammation in mice. Evid Based
Complement Alternat Med. 2017:15757932017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Song L, Ju W, Wang X, Dong L,
Zhang Y, Ya P, Yang C and Li F: The acute airway inflammation
induced by PM2.5 exposure and the treatment of essential oils in
Balb/c mice. Sci Rep. 7:442562017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung KH, Beak H, Park S, Shin D, Jung J,
Park S, Kim J and Bae H: The therapeutic effects of tuberostemonine
against cigarette smoke-induced acute lung inflammation in mice.
Eur J Pharmacol. 774:80–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin YY, Jing Y, Liu Y, Gu MJ, Jin J, Pan
L, Cai Z and Zhang HC: Influence of Guben Zhike Granules on lung
function and morphology of lung injury mouse model induced by
PM2.5. China J Trad Chin Med Pharm. 31:1028–L1031.
2016.(In Chinese).
|
|
32
|
Park SH, Chen WC, Durmus N, Bleck B,
Reibman J, Riemekasten G and Grunig G: The effects of
antigen-specific IgG1 antibody for the
pulmonary-hypertension-phenotype and B cells for inflammation in
mice exposed to antigen and fine particles from air pollution. PLoS
One. 10:e01299102015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Su WY, Jaskot RH, Richards J, Abramson SR,
Woessner JF Jr, Yu WH and Dreher KL: Induction of pulmonary
matrilysin expression by combustion and ambient air particles. Am J
Physiol Lung Cell Mol Physiol. 279:L152–L160. 2000.PubMed/NCBI
|
|
34
|
Veranth JM, Kaser EG, Veranth MM, Koch M
and Yost GS: Cytokine responses of human lung cells (BEAS-2B)
treated with micron-sized and nanoparticles of metal oxides
compared to soil dusts. Part Fibre Toxicol. 4:22007. View Article : Google Scholar : PubMed/NCBI
|