Introduction
The incidence of neurodegenerative diseases, such as
Alzheimer's disease, has increased dramatically over the past few
decades (1,2). Although the molecular mechanism of
neurodegenerative diseases is not well understood, oxidative stress
is considered to be a common pathophysiological condition that
results in neurotoxicity (3,4). The redox system consists of a free
radical/reactive oxygen species (ROS)-generating system, oxidants
and antioxidant systems (5).
Following damage to the antioxidant system or an increase in ROS
generation, the balance of the redox status may be disrupted,
leading to the development of oxidative stress (5). The central nervous system is
particularly susceptible to ROS-induced damage and numerous studies
have demonstrated that oxidative stress is closely associated with
the development of neurodegenerative diseases. Greilberger et
al (6) compared the redox state
of the blood between healthy individuals and patients with
neurodegenerative diseases. Compared with healthy controls, there
were significant elevations in malondialdehyde, protein
carbonylation and oxidized albumin levels in patients with
neurodegenerative diseases, indicating that there is an association
between oxidative stress and the development of neurodegenerative
diseases (6). Furthermore,
4-hydroxynonenal-modified proteins have been detected in patients
with mild cognitive impairments and throughout the course of
Alzheimer's disease (7). In
addition, oxidative stress may serve an important role in
dopaminergic neurotoxicity (8) and
dopamine metabolism-induced alterations in redox status may
increase the risk of the onset or progression of Parkinson's
disease (9).
The fruits of Lycium barbarum L. (family
Solanaceae), more commonly known as Goji berry or wolfberry, have
been used in traditional Chinese herbal medicine for thousands of
years. Lycium barbarum polysaccharide (LBP) is the major active
component found in the fruits. It has been demonstrated that LBP
possesses various biological activities, including antioxidant,
anti-cancer, anti-inflammatory, anti-aging and immune-regulatory
activities (10–15). In particular, it has recently been
demonstrated that LBP exhibits potent neuroprotective effects in
vivo and in vitro (16–18). Bie
et al (16) reported that LBP
improved bipolar pulse current-induced microglia cell injury via
modulation of autophagy. Furthermore, LBP improved traumatic
cognition by reversing the imbalance of apoptosis/regeneration in
hippocampal neurons following stress (17). Wang et al (18) determined that LBP prevented focal
cerebral ischemic injury by inhibiting neuronal apoptosis in mice.
However, the mechanism of the neuroprotective effect of LBP remains
unclear.
The present study aimed to investigate the mechanism
of the LBP-exerted protective effect against oxidative
stress-induced neurotoxicity in vivo and in vitro.
The results demonstrated that LBP exhibited neuroprotective
activity via activation of nuclear factor erythroid 2-related
factor 2 (Nrf) 2/heme oxygenase (HO)-1 signaling.
Materials and methods
Reagents
β-actin (sc-130300) and Nrf2 (sc-722) antibodies
were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA) and the HO-1 (RT1270) antibody was purchased from Epitomics;
Abcam (Cambridge, MA, USA). MTT and CoCl2 were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Lipofectamine® 2000, the intracellular superoxide probe
dihydroethidium (DHE) and Rhodamine (Rho) 123 were all purchased
from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA).
Cell culture and treatment
PC12 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal calf serum (Gibco;
Thermo Fisher Scientific, Inc.), 100 µg/ml penicillin and 100 U/ml
streptomycin at 37°C with 5% CO2 in a humidified
incubator. Throughout the experimental treatments, cells were
exposed to H2O2 in the presence or absence of
LBP in serum-free DMEM.
Cell transfection
To test the role of Nrf2 in LBP-exerted protective
effect, PC12 cells were transfected with small interfering RNA
(siRNA) of Nrf2 and control siRNA before the treatment of
H2O2 and LBP. Nrf2 siRNA and control siRNA
were purchased from Santa Cruz Biotechnology (sc-156128).
Transfection was conducted using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol.
Determination of cell viability
Cells were seeded in a 96-well plate at a density of
1×104 cells/well. Cells in the exponential phase were
exposed to 0, 50, 100, 200 or 400 µM H2O2
and/or 125, 250, 500, 800 or 1,000 µg/ml LBP for 24 h. In some
experiments, cells were transfected with Nrf2 siRNA of Nrf2 and
control siRNA for 48 h prior to treatment. Cells were then exposed
to H2O2 and/or LBP in the presence of zinc
protoporphyrin IX (ZnPP) (10 µM; Sigma-Aldrich; Merck KGaA), an
inhibitor of HO-1. Following the experiments, cell viability was
determined by MTT assay. The purple formazan was dissolved in
dimethyl sulfoxide. Absorbance was measured at 550 nm and the
results are presented as folds of the control cells without
H2O2 treatment.
Determination of apoptosis
Cells (5×105) were seeded in culture
dishes and treated with 400 µM H2O2 in the
presence or absence of 125, 250 or 500 µg/ml LBP with or without
ZnPP for 24 h. In some experiments, cells were transfected with
siRNAs for 48 h prior to treatment. Following treatment, cells were
fixed with 4% formaldehyde for 15 min at room temperature.
Following washing with PBS, the cells were covered with proteinase
K solution for 15–20 min. After another PBS wash, the cells were
covered with the TUNEL reaction mixture (Roche Applied Science,
Penzberg, Germany) and incubated for 1 h in the dark. DAPI
counterstaining (10 min at room temperature) was followed by a
final PBS wash, and tissue sections were then examined and
photographed using confocal microscopy. The average number of
fluorescent dots in three images from each treatment group was
calculated. Results were expressed as folds of the control.
Determination of caspase activity
Activities of caspase 3 and 9 were determined using
a Caspase 3 Activity Assay kit (C1115) and Caspase 9 Activity Assay
kit (C1157; Beyotime Institute of Biotechnology, Haimen, China)
according to the manufacturer's protocol.
Determination of ROS levels
ROS levels were measured using oxidation-sensitive
probes. Cells were incubated with 10 µM DHE for 20 min at 37°C in
the dark and were subsequently observed under a confocal
fluorescence microscope. In addition, cells were also incubated
with 10 µM 2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA, an
intracellular hydrogen peroxide probe) (Sigma-Aldrich; Merck KGaA)
for 30 min at 37°C in the dark. Subsequently, cells were washed
twice with PBS and analyzed using a flow cytometer (BD Accuri™ C6,
BD Biosciences, Franklin Lakes, NJ, USA) and BD Accuri C6 software
(BD Biosciences). The results were expressed as folds of the
control.
Mitochondrial membrane potential
After the treatment of H2O2
and LBP, cells were stained with Rhodamine 123 (10 µM) for 20 min
at 37°C in the dark. Then the fluorescence was observed under a
confocal fluorescence microscope.
Determination of transcription
activity
A reporter gene assay was conducted to evaluate the
transcriptional activity of Nrf2. PC12 cells (5×104)
were seeded into 12-well plates. Subsequently, cells were
transfected with 0.2 µg antioxidant response element (ARE)-Luc
constructs (Beyotime Institute of Biotechnology, Haimen, China)
using Lipofectamine 2000, according to the manufacturer's protocol.
pRL-null plasmid (50 ng) encoding Renilla luciferase was included
in all samples to ensure transfection efficiency. A total of 48 h
after transfection, cells were treated with 400 µM
H2O2 in the presence or absence of 125, 250
or 500 µg/ml LBP for 24 h. Firefly and Renilla luciferase
activities were detected sequentially using the Dual-Glo™
Luciferase assay system (Promega Corporation, Madison, WI,
USA).
Chromatin immunoprecipitation (ChIP)
assay
Cells were treated with 400 µM
H2O2 in the presence or absence of 500 µg/ml
LBP for 24 h. Subsequently, a ChIP assay was conducted to examine
the binding of Nrf2 in HO-1 promoters. Following the experiment,
cells were washed, fixed in 1% formaldehyde for 10 min at room
temperature and sonicated (on 3 sec and off 10 sec for 10 times at
room temperature) to shear chromatin. Nrf2 antibody (dilution 1:50)
was added to the cleared lysate and binding was allowed to proceed
at 4°C overnight. The complex was eluted and the released DNA was
amplified by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) as stated below.
RNA isolation and RT-qPCR
Total RNA was isolated from cells and tissues using
Total RNA Isolation kit (Tiangen Biotech, Co., Ltd., Beijing,
China) according to the manufacturer's instructions. A total of 0.5
µg RNA was reverse transcribed using the First Strand cDNA
synthesis kit (Takara Bio, Inc., Otsu, Japan). qPCR was performed
to precisely quantify Nrf2 and HO-1 using SYBR Green reagent
(Takara Bio, Inc.). Amplification was performed with an initial
step at 94°C for 5 min, followed by 40 cycles of denaturation at
94°C for 30 sec, annealing at 63°C for 30 sec and extension at 72°C
for 10 sec. The 2−ΔΔCq method was used to determine gene
expression compared with the endogenous controls (GAPDH) (19). The primers for qPCR analysis were as
follows: Nrf2, forward, 5′-ATTGCCTGTAAGTCCTGGTCA-3′, reverse,
5′-ACTGCTCTTTGGACATCATTTCG-3′; HO-1, forward,
5′-TTCCTGGACTGATCCCAATTCTG-3′, reverse,
5′-CTTGGAAGCCACAGAAATGCAG-3′; GAP DH, forward,
5′-GCACCGTCAAGGCTGAGAAC-3′, reverse, 5′-TGGTGAAGACGCCAGTGGA-3′.
Protein extraction and western
blotting
Following treatment, cells were lysed and
cytoplasmic and nuclear proteins were extracted using NE-PER
Nuclear and Cytoplasmic Extraction reagents (Thermo Fisher
Scientific, Inc.). Protein concentration was determined using the
BCA assay. Lysates containing 20 µg protein were boiled at 100°C
for 5 min, separated on a 10% sodium dodecyl sulfate-polyacrylamide
gel and transferred to PVDF membranes. Membranes were blocked in 8%
skim milk in TBS buffer for 30 min at 37°C. Subsequently, membranes
were incubated with the appropriate primary antibodies (Nrf2, HO-1
and β-actin, dilution: 1:500) overnight at 4°C and washed four
times with TBST for 10 min each time. Following washing, membranes
were further incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies (goat anti-rabbit antibody, dilution: 1:5,000)
(32260; Invitrogen, Thermo Fisher Scientific, Inc.) for 30 min at
37°C and then washed another four times. Membranes were visualized
using Immobilon Western Chemiluminescent HRP Substrate (Merck
KGaA). Bands were captured using a VersaDoc image analysis system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) and quantified with
Quantity One software v.4.62 (Bio-Rad Laboratories, Inc.).
Animal treatment
All animal care was conducted in accordance with the
guidelines for the Care and Use of Laboratory Animals (15) and the principles presented by School
of Medicine, Xi'an Jiaotong University (Shaanxi, China). The
present study was approved by the Ethics Committee of Xi'an
High-Tech Hospital. All efforts were made to reduce suffering and
the number of animals used. A total of 64 adult male Wistar rats
weighing 250–300 g (8–10 weeks old) were obtained from the Animal
Centre of the School of Medicine, Xi'an Jiaotong University. Rats
were housed in a controlled environment, with a 12–12 h light/dark
cycle, controlled humidity (50%) and temperature (24°C), and had
free access to standard laboratory rat food and water.
Rats were randomly divided into four groups with 16
animals in each group: Control group, CoCl2 group,
CoCl2+LBP group and CoCl2+LBP+ZnPP group.
Surgery was conducted as previously reported (20–22).
Control group: Rats received saline solution. CoCl2
group: Rats received 50 mM CoCl2. CoCl2+LBP
group: Rats received 50 mM CoCl2 and LBP (100 mg/kg)
injection. CoCl2+LBP+ZnPP group: Rats received 50 mM
CoCl2, LBP (100 mg/kg) injection and ZnPP (10 mg/kg)
injection. Briefly, rats were placed in a stereotaxic apparatus and
received a unilateral lesion by drilling a small hole on the skull
in the frontoparietal cortex at Bregma-1.30 mm. Subsequently, a
sterile solution of CoCl2 (50 mM) or an equivalent
volume of saline solution was injected in the right hemisphere, 1
mm below the pia level in the cerebral cortex (layers 3–4) with a
Hamilton syringe. The CoCl2 solution was adjusted to
reach a physiological osmolarity of 310 mOsm/kg. Following the
injection, the lesion site was repaired using the temporal muscle
and the attached fascia. During the surgery period, the body
temperature of the animals was maintained using a heating pad.
Surgical procedures were performed under anesthesia induced with 8%
(v/v) sevofluorane inhalation (the dose was selected by preliminary
experiments). Concurrent preemptive analgesia was used and a
precision vaporiser was used to perform anaesthesia and each animal
was put in a separate cage for recovery. Following 2 days recovery,
rats in CoCl2+LBP group and CoCl2+LBP+ZnPP
group received intraperitoneal LBP (100 mg/kg) and rats in
CoCl2+LBP+ZnPP group received ZnPP (10 mg/kg) injection
once a day for 10 days. After the treatment, 8 rats in each group
were sacrificed using intraperitoneal injection of an overdose of
sodium pentobarbital (200 mg/kg; Merck KGaA). Frozen brain sections
(5 µm) were cut and then fixed in 4% formaldehyde overnight at room
temperature for the determination of apoptosis.
Morris water maze (MWM) test
Immediately after the treatment, an MWM test was
conducted to evaluate spatial learning and memory abilities, as
previously reported (22,23). The maze was a black circular pool
(120 cm in diameter and 45 cm high) divided into four quadrants,
with a depth of 30 cm and filled with clear tap water at a
temperature of 27±0.5°C. A black platform 10 cm in diameter was
located 2 cm below the surface of the water approximately in the
middle of one of the four quadrants. Each rat's path in the pool
was recorded with Videomex Water Maze Monitoring Software BL-420
(Chengdu Taimeng Technology Co., Ltd., Chengdu, China) and analyzed
off-line. Experiments were conducted in a soundproof room and the
light source and surrounding environment remained unaltered between
each experiment.
During the first 5 days, rats underwent four
swimming trials per day, with each trial period limited to 60 sec.
In the trials, the time taken to reach the platform, the swimming
distance and the speed were recorded. If the rat did not find the
platform within the set time, the computer would stop tracking and
record the time as 60 sec. If the platform was found within the 60
sec, the rat would be allowed to remain on the platform for 10 sec.
Otherwise, the rat would be guided to the platform to remain for 10
sec. On day 6, a probe trial test was conducted. The platform was
removed on day 6 for a 60 sec exploration test to record the
crossing index to the previous platform site.
Statistical analysis
Statistical analysis was performed using GraphPad
Software 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The
results are presented as the mean ± standard error of the mean. The
statistical significance of differences between groups was analyzed
via one-way analysis of variance followed by a Dunnett's t-test for
multiple comparisons. P<0.05 was considered to indicate a
significant difference.
Results
LBP inhibits
H2O2-induced mitochondrial apoptosis in PC12
cells
PC12 cells were incubated with 0, 50, 100, 200 or
400 µM H2O2 for 24 h and the results
indicated that 100–400 µM H2O2 significantly
decreased cell viability in a concentration-dependent manner
(P<0.05; Fig. 1A). Treatment with
400 µM H2O2 decreased cell viability to 20%
of the control; thus 400 µM H2O2 was used to
induce oxidative injury in PC12 cells in subsequent experiments.
PC12 cells were incubated with 0, 125, 250, 500, 800 or 1,000 µg/ml
LBP for 24 h and the results indicated that LBP significantly
decreased cell viability in a concentration-dependent manner at
concentrations >500 µg/ml (P<0.05; Fig. 1B). Thus, a concentration of 125–500
µg/ml LBP was selected to examine the effect of LBP on
neurotoxicity in PC12 cells in subsequent experiments.
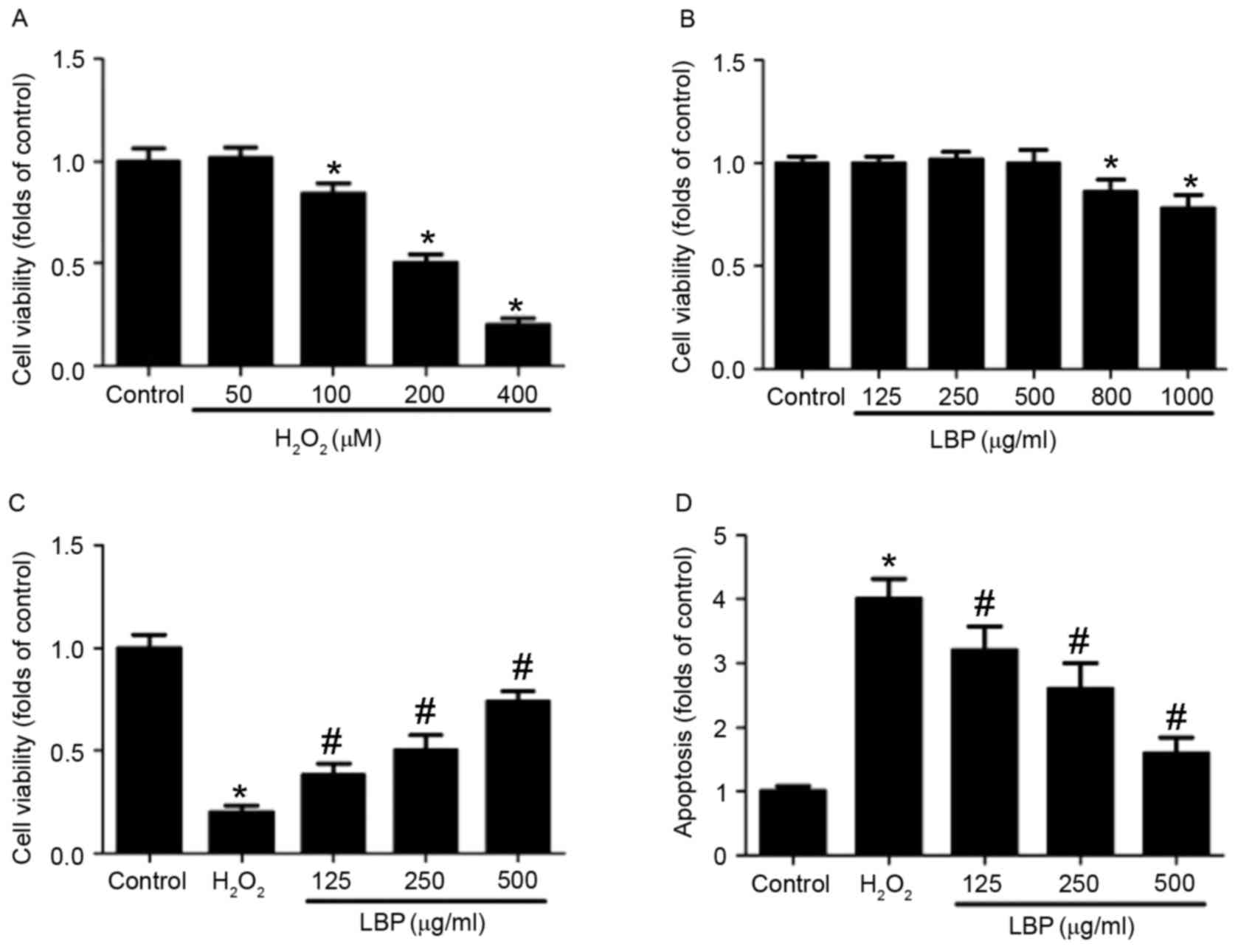 | Figure 1.Effect of LBP on
H2O2-induced apoptosis. PC12 cells were
incubated with (A) 0, 50, 100, 200 or 400 µM
H2O2 or (B) 0, 125, 250, 500, 800 or 1,000
µg/ml LBP for 24 h. Cell viability was determined by MTT assay and
the results are presented as folds of control. (C) PC12 cells were
incubated with 400 µM H2O2 in the presence or
absence of 125, 250 or 500 µg/ml LBP for 24 h. Cell viability was
determined by MTT assay and the results are presented as folds of
control. (D) Apoptosis was determined by TUNEL staining and the
results are presented as folds of control. *P<0.05 vs. control;
#P<0.05 vs. H2O2 treatment.
LBP, Lycium barbarum polysaccharide. |
LBP dose-dependently reversed the decrease in cell
viability induced by H2O2 (P<0.05;
Fig. 1C). Furthermore, LBP treatment
decreased TUNEL-positive cell numbers in
H2O2-treated cells in a
concentration-dependent manner (P<0.05; Fig. 1D). H2O2-induced
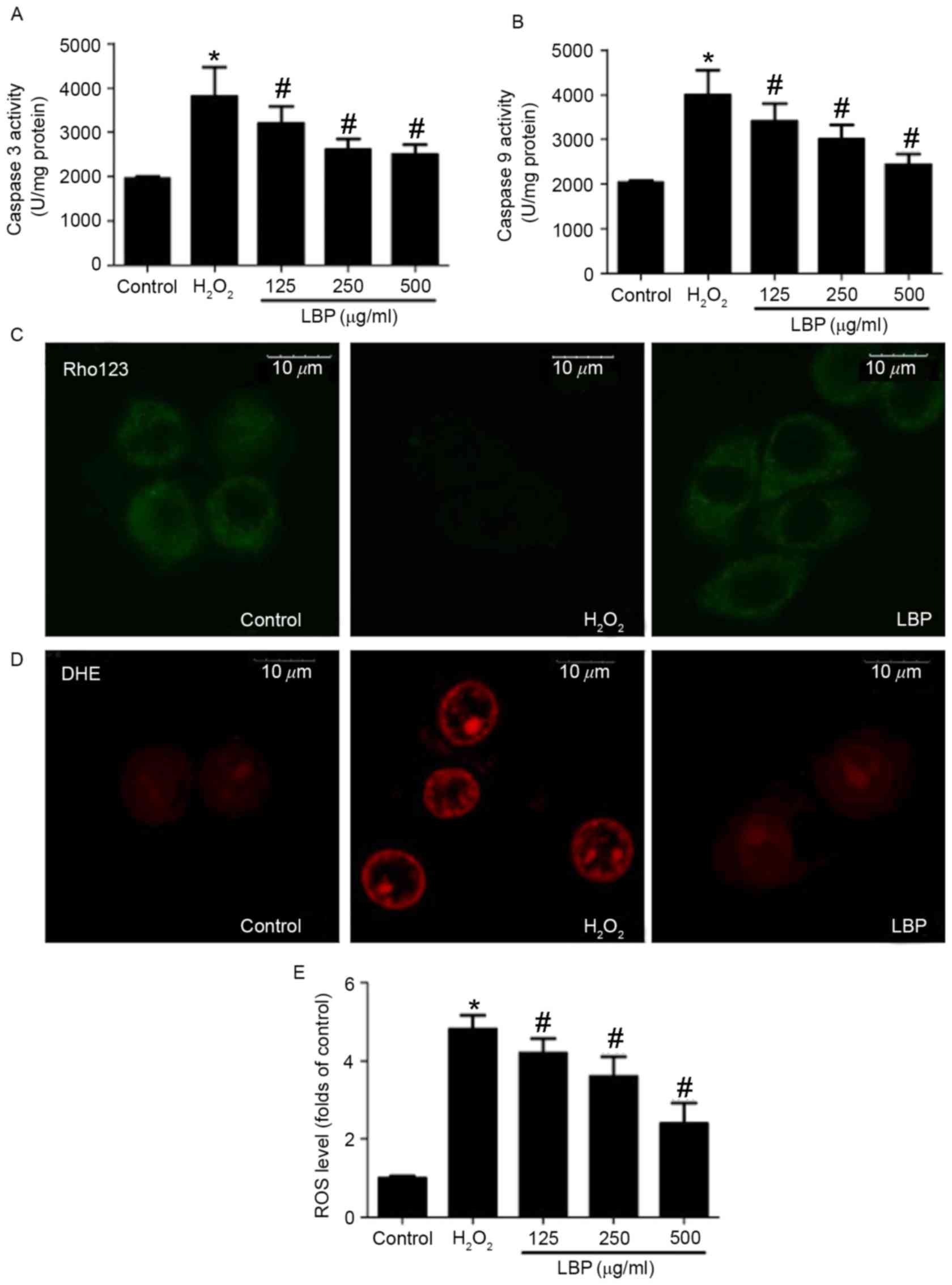
increases in caspase-3 and −9 activity were significantly reversed
by LBP in a concentration-dependent manner (P<0.05; Fig. 2A and B). Mitochondrial membrane
potential was determined using Rho 123 staining and fluorescence
was observed using a confocal microscope. The results demonstrated
that LBP markedly inhibited the H2O2-induced
decrease in Rho 123 fluorescence, indicating a reversal in the
decrease of the mitochondrial membrane potential (Fig. 2C). ROS levels were determined by DHE
and DCFH-DA staining. The H2O2-induced
increase in DHE staining was markedly inhibited by LBP (Fig. 2D). Furthermore, LBP significantly
suppressed the H2O2-induced increase of
DCFH-DA-positive cell numbers in a concentration dependent manner
(P<0.05; Fig. 2E). These results
indicate that LBP concentration-dependently inhibits
H2O2-induced mitochondrial apoptosis.
LBP inhibits the
H2O2-induced decrease of Nrf2/HO-1 signaling
in PC12 cells
The potential mechanism responsible for the
LBP-induced protective effects against
H2O2-induced neurotoxicity in PC12 cells was
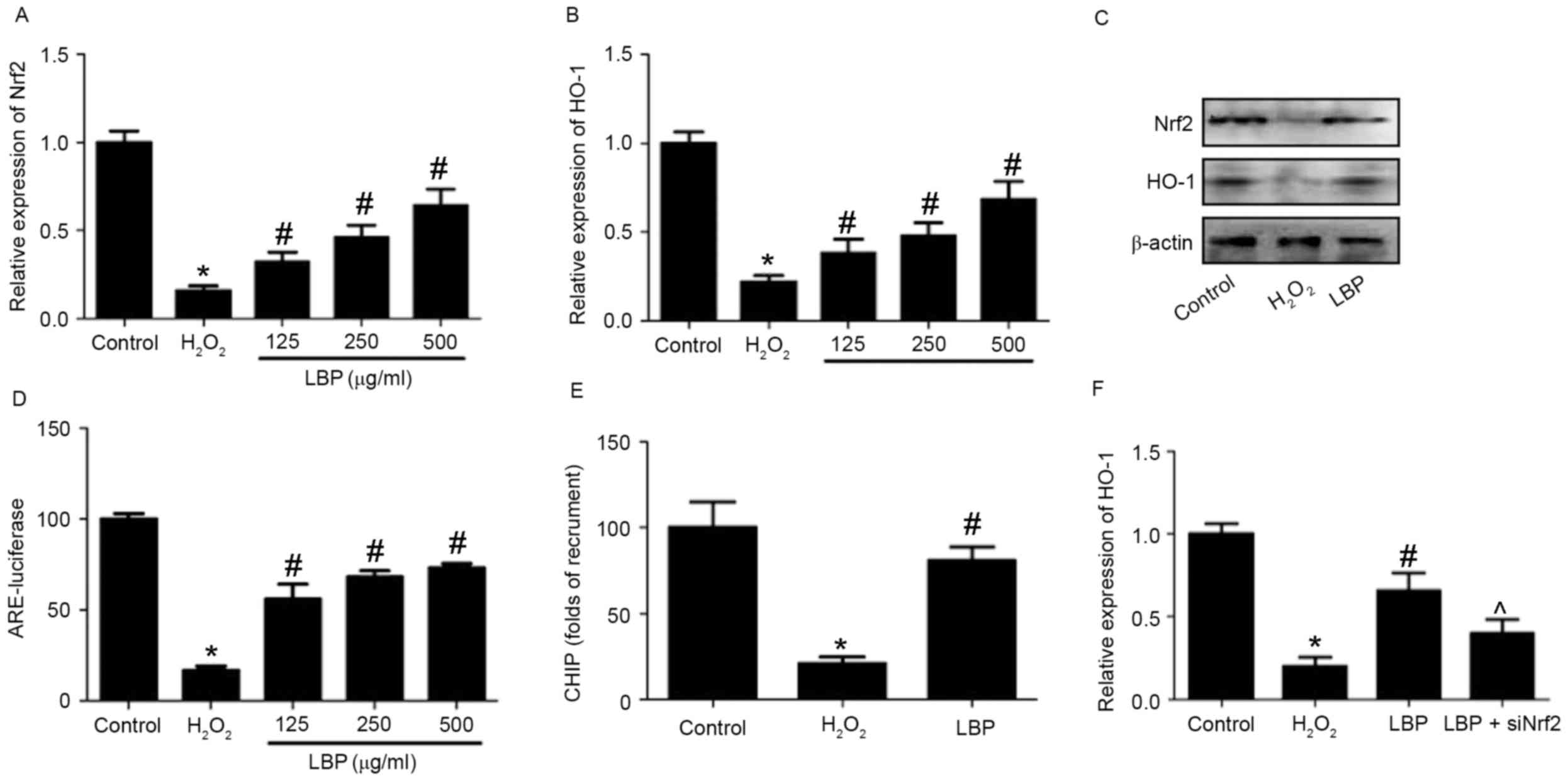
subsequently evaluated. Levels of Nrf2 and HO-1 mRNA and protein
were measured. The results demonstrated that the
H2O2-induced decreases in Nrf2 and HO-1 mRNA
expression were significantly reversed by LBP in a
concentration-dependent manner (P<0.05; Fig. 3A and B). LBP also markedly reversed
the decrease in Nrf2 and HO-1 protein expression induced by
H2O2 (Fig.
3C). ARE-luciferase activity was significantly reduced by
H2O2 (P<0.05), however this reduction was
reversed by LBP (P<0.05; Fig.
3D). The results of the ChIP assay identified that LBP
significantly reversed the H2O2-induced
decrease of Nrf2 binding to the promoters of HO-1 (P<0.05;
Fig. 3E). Cells were transfected
with siNrf2 and then exposed to H2O2 in the
presence or absence of LBP. The results determined that the
LBP-induced increase of HO-1 expression in
H2O2-treated cells was significantly
inhibited by Nrf2 silencing induced by siNrf2 (P<0.05; Fig. 3F). These results suggest that
Nrf2/HO-1 signaling is involved in the LBP-induced protective
effects against neurotoxicity induced by H2O2
and indicate that LBP inhibits the
H2O2-induced decrease of Nrf2/HO-1 signaling
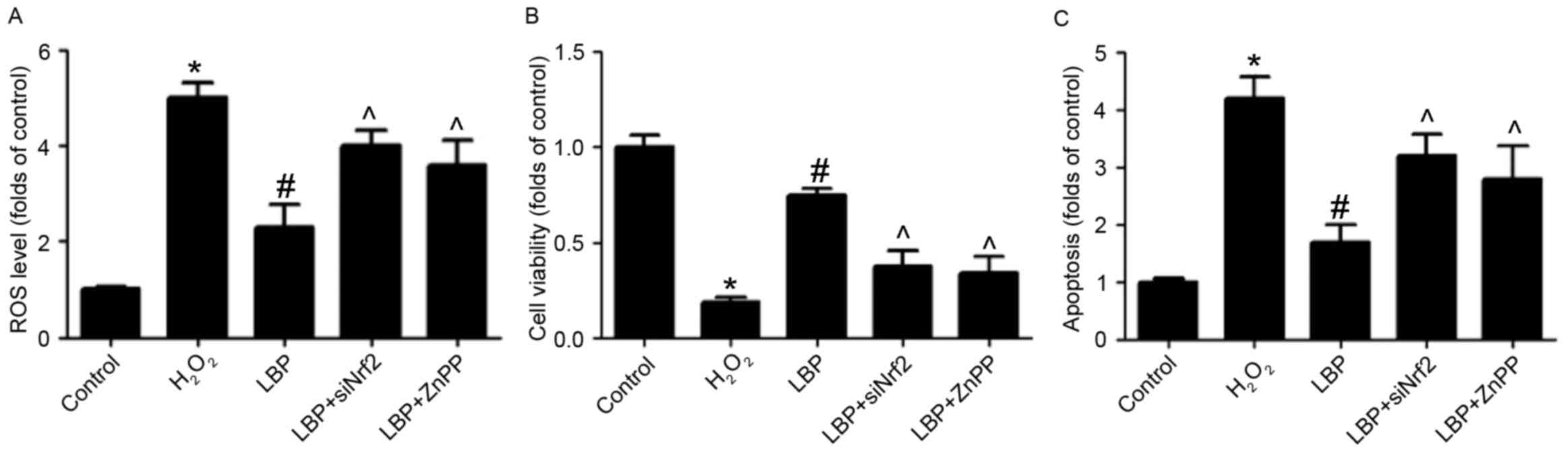
in PC12 cells. To determine whether the upregulation of Nrf2/HO-1
signaling is involved in the protective effect of LBP, cells were
transfected with siNrf2, then exposed to H2O2
in the presence or absence of LBP with or without ZnPP, an
inhibitor of HO-1. It was demonstrated that in
H2O2-treated cells, the LBP-induced decrease
in ROS levels (Fig. 4A), increase in
cell viability (Fig. 4B) and
decrease in cell apoptosis (Fig. 4C)
was significantly reversed by siNrf2 and ZnPP (all P<0.05).
These results indicate that Nrf2/HO-1 signaling is involved in the
LBP-induced protective effects against neurotoxicity induced by
H2O2 in PC12 cells.
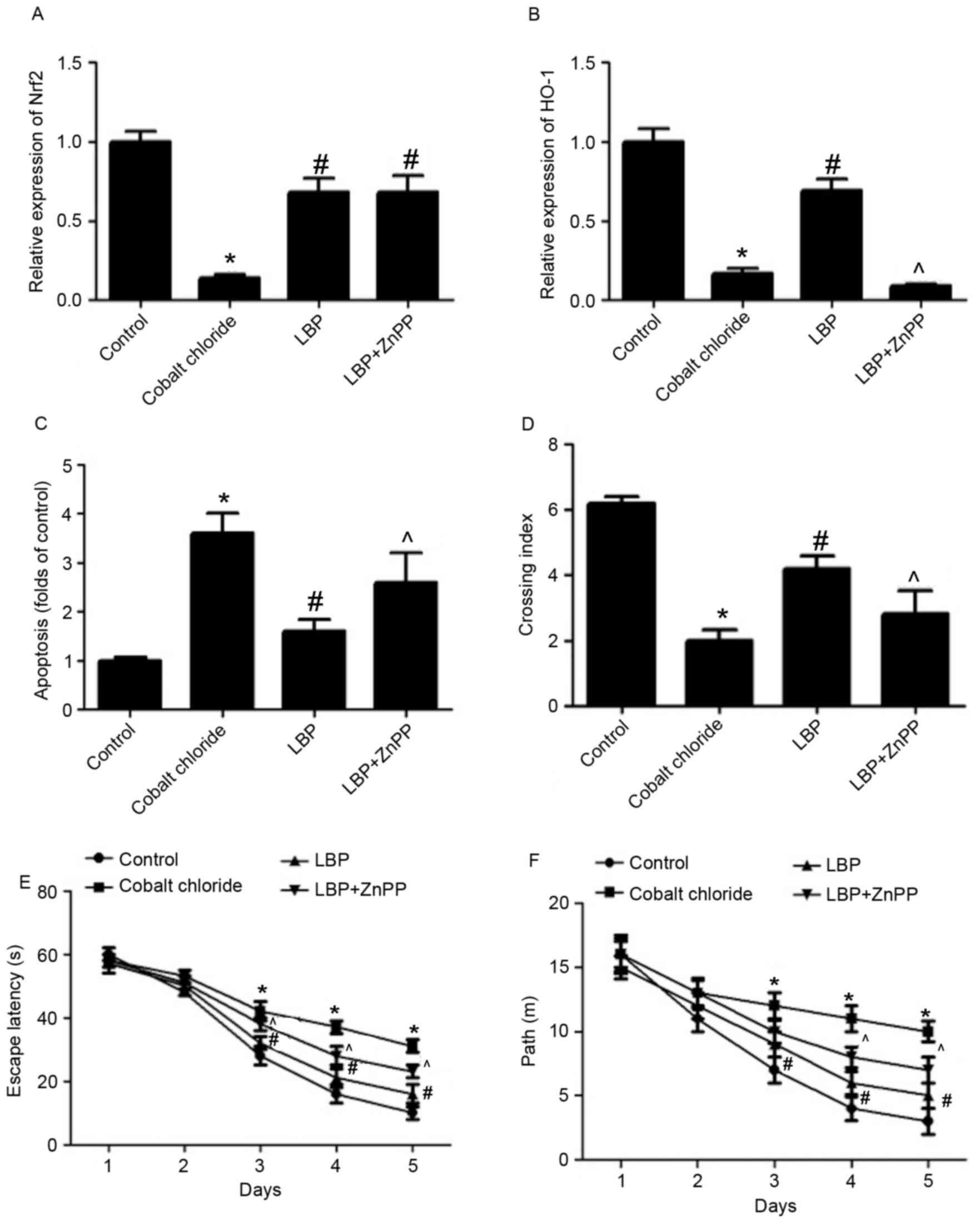
Neuroprotective effect of LBP on
CoCl2 in vivo
Subsequently, the neuroprotective effect of LBP
against CoCl2 in vivo was evaluated. Rats were
injected with CoCl2 to construct an animal model of
neurotoxicity and subsequently received LBP with or without ZnPP.
Following treatment, a MWM test was conducted to evaluate the
spatial learning and memory abilities of rats. The
CoCl2-induced decrease of Nrf2 expression was
significantly inhibited by LBP (P<0.05) and ZnPP did not
significantly affect Nrf2 expression in LBP-treated rats (Fig. 5A). LBP reversed the
CoCl2-induced decrease of HO-1 expression in rat brains
(P<0.05; Fig. 5B), however this
effect was significantly attenuated by ZnPP (P<0.05; Fig. 5B). A CoCl2-induced
increase in the number of TUNEL-positive cells was inhibited by
LBP, indicating a reduction in apoptosis (P<0.05; Fig. 5C). However, ZnPP significantly
reversed the anti-apoptotic effect of LBP in
CoCl2-treated rats (P<0.05; Fig. 5C). During the MWM test, rats in
CoCl2 group spent more time finding the platform site,
indicated by a significantly lower crossing index compared with
control rats (P<0.05; Fig. 5D).
Compared with the CoCl2 group, rats in LBP group spent
significantly less time finding the platform site (P<0.05;
Fig. 5D), however this decrease in
time spent to find the platform site induced by LBP was reversed by
ZnPP (P<0.05; Fig. 5D).
Rats in the CoCl2 group exhibited
significantly longer escape latencies on test days 3, 4, and 5 than
those in the control group (P<0.05; Fig. 5E). Compared with the CoCl2
group, rats in the LBP group had shorter escape latencies on test
days 3, 4, and 5 (P<0.05; Fig.
5E); however this decrease in escape latencies induced by LBP
was reversed by ZnPP (P<0.05; Fig.
5E). The distance traveled by the rats in each group to find
the platform was also gradually reduced by training and the
changing patterns of traveling distance induced by LBP and ZnPP
were largely consistent with those for the latency (Fig. 5F). The results demonstrated that LBP
may protect against CoCl2-induced neurotoxicity in
vivo and that this may involve the regulation of Nrf2/HO-1
signaling (Fig. 6).
Discussion
There has been a marked increase in the incidence of
neurodegenerative diseases including Alzheimer's and Parkinson's
disease over the last few years (1,2).
Previous studies have suggested that oxidative stress serves a
crucial role in the development of different types of neurotoxicity
(24–26). It has been demonstrated that LBP, the
major active component of the Lycium barbarum L. fruit, possesses
potent antioxidant activity (27–29),
thus the current study examined the potential neuroprotective
effect of LBP.
In the current study, in vitro experiments
were performed on PC12 cells that had undergone
H2O2-induced neurotoxicity to evaluate the
neuroprotective effect of LBP. In addition,
CoCl2-mediated hypoxic neurotoxicity was established in
rats to examine the neuroprotective effect of LBP in vivo.
It was demonstrated that LBP exhibits potent neuroprotective
activity, as indicated by the increase in cell viability and
decrease in mitochondrial apoptosis in
H2O2-treated PC12 cells, as well as the
decreased apoptosis in rat brain tissue, decreased time to find the
platform site, shorter escape latencies and a shorter distance
traveled to find the platform in CoCl2-treated rats.
There have been few studies assessing the
neuroprotective effects of LBP. It has been demonstrated that LBP
improves bipolar pulse current-induced microglia cell injury via
modulation of autophagy (16).
Furthermore, LBP improved traumatic cognition by reversing the
imbalance of apoptosis/regeneration that occurs in hippocampal
neurons following the induction of stress (17). Wang et al (18) indicated that LBP prevents focal
cerebral ischemic injury by inhibiting neuronal apoptosis in mice.
Additionally, Rui et al (30)
determined that LBP protects rat primary cultured hippocampal
neurons against injury induced by oxygen-glucose deprivation and
reperfusion. Li et al (31)
demonstrated that LBP reduces neuronal damage, disruption of the
blood-retinal barrier and oxidative stress in retinal
ischemia/reperfusion injury. Based on the aforementioned results
and the results of the current study, LBP may exhibit potent
neuroprotective activity.
The antioxidant activities of LBP may serve an
important role in the protective effects against neurotoxicity, as
reflected by the significant reduction in ROS levels that occurs in
H2O2-treated PC12 cells. Nrf2 is a key
transcription factor that determines redox status by regulating
numerous antioxidant enzymes (32,33).
HO-1 is an important target gene of Nrf2; HO-1 exhibits
antioxidant, anti-apoptotic and anti-inflammatory properties via
bilirubin/biliverdin and carbon monoxide, the products of
HO-1-catalyzed heme degradation (34). It has been demonstrated that
disruption to the Nrf2-ARE pathway contributes to the development
of neurotoxicity and neurodegenerative diseases (35–37).
Activation of Nrf2/HO-1 signaling may be an important method of
protecting against neurotoxicity (38–40). It
has been indicated that dietary LBP stimulates the Nrf2/ARE pathway
and ameliorates insulin resistance induced by a high-fat diet
(41). Furthermore, activation of
the Nrf2/HO-1 antioxidant pathway may contribute to the protective
effects of LBP in the rodent retina following
ischemia-reperfusion-induced damage (42). In the present study, the potential
role of Nrf2/HO-1 signaling in the neuroprotective effects of LBP
was examined. LBP inhibited the reduction of Nrf2/HO-1 signaling in
H2O2-treated PC12 cells and
CoCl2-treated rats. Nrf2 silencing significantly
inhibited the protective effects of LBP in
H2O2-treated PC12 cells. Furthermore,
administration of ZnPP suppressed the protective effects of LBP in
H2O2-treated PC12 cells and
CoCl2-treated rats. These results demonstrate that the
Nrf2/HO-1 pathway may, at least partly, be responsible for the
neuroprotective effects of LBP.
In conclusion, the current study demonstrated that
LBP exhibits protective effects against neurotoxicity via
upregulation of Nrf2/HO-1 signaling. Enhancement of Nrf2/HO-1
signaling contributed to an improvement of oxidative stress and the
amelioration of apoptosis. These data may improve understanding of
the neuroprotective activities of LBP.
References
|
1
|
Paulsen JS, Nance M, Kim JI, Carlozzi NE,
Panegyres PK, Erwin C, Goh A, McCusker E and Williams JK: A review
of quality of life after predictive testing for and earlier
identification of neurodegenerative diseases. Prog Neurobiol.
110:2–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gratwicke J, Jahanshahi M and Foltynie T:
Parkinson's disease dementia: A neural networks perspective. Brain.
138:1454–1476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhat AH, Dar KB, Anees S, Zargar MA,
Masood A, Sofi MA and Ganie SA: Oxidative stress, mitochondrial
dysfunction and neurodegenerative diseases; A mechanistic insight.
Biomed Pharmacother. 74:101–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao Y and Zhao B: Oxidative stress and
the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev.
2013:3165232013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X and Hai C: Redox modulation of
adipocyte differentiation: Hypothesis of ‘Redox Chain’ and novel
insights into intervention of adipogenesis and obesity. Free
Radical Bio Med. 89:99–125. 2015. View Article : Google Scholar
|
|
6
|
Greilberger J, Koidl C, Greilberger M,
Lamprecht M, Schroecksnadel K, Leblhuber F, Fuchs D and Oettl K:
Malondialdehyde, carbonyl proteins and albumin-disulphide as useful
oxidative markers in mild cognitive impairment and Alzheimer's
disease. Free Radic Res. 42:633–638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sultana R, Perluigi M and Butterfield
Allan D: Lipid peroxidation triggers neurodegeneration: A redox
proteomics view into the Alzheimer disease brain. Free Radic Biol
Med. 62:157–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blesa J, Trigo-Damas I, Quiroga-Varela A
and Jackson-Lewis VR: Oxidative stress and Parkinson's disease.
Front Neuroanat. 9:912015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong ES, Tan JM, Wang C, Zhang Z, Tay SP,
Zaiden N, Ko HS, Dawson VL, Dawson TM and Lim KL: Relative
sensitivity of parkin and other cysteine-containing enzymes to
stress-induced solubility alterations. J Biol Chem.
282:12310–12318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HX and Ng TB: Natural products with
hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic
and antithrombotic activities. Life Sci. 65:2663–2677. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Liang L, Wang Y, Diao J, Zhao C,
Chen G, He Y, Luo C, Wu X and Zhang Y: Synergistic
immunotherapeutic effects of Lycium barbarum polysaccharide and
interferon-α2b on the murine Renca renal cell carcinoma cell line
in vitro and in vivo. Mol Med Rep. 12:6727–6737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao R, Cai Y, Shao X and Ma B: Improving
the activity of Lycium barbarum polysaccharide on sub-health mice.
Food Funct. 6:2033–2040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Lv J, Yang B, Liu F, Tian Z, Cai Y,
Yang D, Ouyang J, Sun F, Shi Y and Xia P: Lycium barbarum
polysaccharide attenuates type II collagen-induced arthritis in
mice. Int J Biol Macromol. 78:318–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao J, Zhu Y, Liu Y, Tipoe GL, Xing F and
So KF: Lycium barbarum polysaccharide attenuates alcoholic cellular
injury through TXNIP-NLRP3 inflammasome pathway. Int J Biol
Macromol. 69:73–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Q, Dong B, Chen J, Zhao B, Wang X,
Wang L, Zha S and Wang Y, Zhang J and Wang Y: Effect of drying
methods on physicochemical properties and antioxidant activities of
wolfberry (Lycium barbarum) polysaccharide. Carbohydr Polym.
127:176–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bie M, Lv Y, Ren C, Xing F, Cui Q, Xiao J
and So KF: Lycium barbarum polysaccharide improves bipolar pulse
current-induced microglia cell injury through modulating autophagy.
Cell Transplant. 24:419–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao J, Chen C, Liu Y, Li Y, Long Z, Wang
H, Zhang Y, Sui J, Wu Y, Liu L and Yang C: Lycium barbarum
polysaccharide improves traumatic cognition via reversing imbalance
of apoptosis/regeneration in hippocampal neurons after stress. Life
Sci. 121:124–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang T, Li Y, Wang Y, Zhou R, Ma L, Hao Y,
Jin S, Du J, Zhao C, Sun T and Yu J: Lycium barbarum polysaccharide
prevents focal cerebral ischemic injury by inhibiting neuronal
apoptosis in mice. PLoS One. 9:e907802014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caltana L, Merelli A, Lazarowski A and
Brusco A: Neuronal and glial alterations due to focal cortical
hypoxia induced by direct cobalt chloride (CoCl2) brain injection.
Neurotox Res. 15:348–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Caltana L, Rutolo D, Nieto ML and Brusco
A: Further evidence for the neuroprotective role of oleanolic acid
in a model of focal brain hypoxia in rats. Neurochem Int. 79:79–87.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guan D, Su Y, Li Y, Wu C, Meng Y, Peng X
and Cui Y: Tetramethylpyrazine inhibits CoCl2 -induced
neurotoxicity through enhancement of Nrf2/GCLc/GSH and suppression
of HIF1α/NOX2/ROS pathways. J Neurochem. 134:551–565. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai Y, Li W, Zhong M, Chen J, Liu Y, Cheng
Q and Li T: Preconditioning and post-treatment with cobalt chloride
in rat model of perinatal hypoxic-ischemic encephalopathy. Brain
Dev. 36:228–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pearson JN and Patel M: The role of
oxidative stress in organophosphate and nerve agent toxicity. Ann N
Y Acad Sci. 1378:17–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lan AP, Chen J, Chai ZF and Hu Y: The
neurotoxicity of iron, copper and cobalt in Parkinson's disease
through ROS-mediated mechanisms. Biometals. 29:665–678. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Venkatesan R, Subedi L, Yeo EJ and Kim SY:
Lactucopicrin ameliorates oxidative stress mediated by
scopolamine-induced neurotoxicity through activation of the NRF2
pathway. Neurochem Int. 99:133–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao K, Liu M, Cao J, Yao M, Lu Y, Li J,
Zhu X, Yang Z and Wen A: Protective effects of Lycium barbarum
polysaccharide on 6-OHDA-induced apoptosis in PC12 cells through
the ROS-NO pathway. Molecules. 20:293–308. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu X, Hu S, Zhu L, Ding J, Zhou Y and Li
G: Effects of Lycium barbarum polysaccharides on oxidative stress
in hyperlipidemic mice following chronic composite psychological
stress intervention. Mol Med Rep. 11:3445–3450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi B, Ji Q, Wen Y, Liu L, Guo X, Hou G,
Wang G and Zhong J: Lycium barbarum polysaccharides protect human
lens epithelial cells against oxidative stress-induced apoptosis
and senescence. PLoS One. 9:e1102752014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rui C, Yuxiang L, Yinju H, Qingluan Z,
Yang W, Qipeng Z, Hao W, Lin M, Juan L, Chengjun Z, et al:
Protective effects of Lycium barbarum polysaccharide on neonatal
rat primary cultured hippocampal neurons injured by oxygen-glucose
deprivation and reperfusion. J Mol Histol. 43:535–542. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li SY, Yang D, Yeung CM, Yu WY, Chang RC,
So KF, Wong D and Lo AC: Lycium barbarum polysaccharides reduce
neuronal damage, blood-retinal barrier disruption and oxidative
stress in retinal ischemia/reperfusion injury. PLoS One.
6:e163802011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vriend J and Reiter RJ: The
Keap1-Nrf2-antioxidant response element pathway: A review of its
regulation by melatonin and the proteasome. Mol Cell Endocrinol.
401:213–220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Na HK and Surh YJ: Oncogenic potential of
Nrf2 and its principal target protein heme oxygenase-1. Free Radic
Biol Med. 67:353–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ollinger R, Yamashita K, Bilban M, Erat A,
Kogler P, Thomas M, Csizmadia E, Usheva A, Margreiter R and Bach
FH: Bilirubin and biliverdin treatment of atherosclerotic diseases.
Cell Cycle. 6:39–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gan L and Johnson JA: Oxidative damage and
the Nrf2-ARE pathway in neurodegenerative diseases. Biochim Biophys
Acta. 1842:1208–1218. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Narasimhan M, Riar AK, Rathinam ML,
Vedpathak D, Henderson G and Mahimainathan L: Hydrogen peroxide
responsive miR153 targets Nrf2/ARE cytoprotection in paraquat
induced dopaminergic neurotoxicity. Toxicol Lett. 228:179–191.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Park SY, Kim DY, Kang JK, Park G and Choi
YW: Involvement of activation of the Nrf2/ARE pathway in protection
against 6-OHDA-induced SH-SY5Y cell death by α-iso-cubebenol.
Neurotoxicology. 44:160–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ye F, Li X, Li L, Yuan J and Chen J: t-BHQ
provides protection against lead neurotoxicity via Nrf2/HO-1
pathway. Oxid Med Cell Longev. 2016:20759152016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dwivedi S, Rajasekar N, Hanif K, Nath C
and Shukla R: Sulforaphane ameliorates okadaic Acid-Induced memory
impairment in rats by activating the Nrf2/HO-1 antioxidant pathway.
Mol Neurobiol. 53:5310–5323. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kwon SH, Ma SX, Hwang JY, Lee SY and Jang
CG: Involvement of the Nrf2/HO-1 signaling pathway in
sulfuretin-induced protection against amyloid beta25-35
neurotoxicity. Neuroscience. 304:14–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Li W, Li Y, Wang Q, Gao L and Zhao
J: Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway
and ameliorates insulin resistance induced by high-fat via
activation of PI3K/AKT signaling. Oxid Med Cell Longev.
2014:1456412014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He M, Pan H, Chang RC, So KF, Brecha NC
and Pu M: Activation of the Nrf2/HO-1 antioxidant pathway
contributes to the protective effects of Lycium barbarum
polysaccharides in the rodent retina after
ischemia-reperfusion-induced damage. Plos One. 9:e848002014.
View Article : Google Scholar : PubMed/NCBI
|