The majority of patients with NSCLC have an advanced
stage tumor at the time of diagnosis (15). In order to increase the efficacy of
anticancer drugs and decrease mortality rates, the focus of lung
cancer management has shifted to early diagnosis and personalized
anticancer therapy (16,17). Hence, different diagnostics tools for
patients with NSCLC have been explored (11,18).
Clinically, computerized tomography (CT) scanning is frequently
used to diagnose NSCLC (19).
Subsequently, histopathology is applied to confirm the final
diagnosis (20). Aberle et al
(21) reported that CT screening had
altered the landscape of lung-cancer screening and decreased lung
cancer-associated mortalities by 20%, indicating the potential
efficacy of screening for early stage lung cancer using CT imaging.
However, CT used alone is not sensitive enough for the detection of
early stage NSCLC (22,23). Seigneurin et al indicated that
the presence of positron emission tomography (PET) in the work-up
protocol is associated with higher recall rates, detection rates
and positive predictive values of CT screening for lung cancer
(24).
The aforementioned diagnostic methods typically
diagnose patients with NSCLC at an advanced clinical stage, losing
the optimal time frame for treatment and thus shortening survival
times. Therefore, modified and optimized CT screening techniques
should be developed for the diagnosis of early stage NSCLC. Novel
imaging techniques could enhance the sensitivity of the detection
of early stage tumor morphology. A previous study has evaluated the
application of dynamic contrast-enhanced CT imaging in the study of
the differentiation of benign and malignant tumors, observed by
tumor vessel and permeability nodule perfusion (25). However, conventional contrast agents
present lower efficacy for tumor analysis due to their rapid
diffusion away from the lungs (26).
Furthermore, previous reports have demonstrated that iodinated
contrast agents are less sensitive to changes in cell morphology
(27,28).
Nanoparticle-based imaging contrast agents exhibit
potential in diagnosing early-stage cancer through providing a more
accurate and sensitive detection of tumor nodules (29,30). Cho
et al (31) investigated
inorganic nanoparticles containing semiconductor quantum dots, iron
oxide and gold, which revealed their potential for use as contrast
agents combined with CT for diagnostics.
The present study investigated the use of a
nanoscale microbubble contrast agent for contrast-enhanced (CE) CT
to detect early stage NSCLC. This highlighted the potential
application of CECT-targeted nanoparticle contrast agent (TNCA)
imaging in the diagnosis of NSCLC. The results revealed the
advantages of CECT-TNCA compared with CT alone in the early
diagnosis and final confirmation of NSCLC. CECT-TNCA, with the
contrast agent inhaled by nebulization, led to lesions being
augmented and amplified in the lung during imaging, resulting in a
reliable and sensitive assessment for the clinical diagnosis of
NSCLC.
The present study was performed in accordance with
the recommendations for the Guide for the Care and Use of
Laboratory Animals of The First Hospital of Shijiazhuang
(Shijiazhuang, China), and all animal work was approved by the
Ethics Committee of The First Hospital of Shijiazhuang. All surgery
and euthanasia were performed under sodium pentobarbital
anesthesia, and all efforts were made to minimize suffering.
A CECT diagnosis system (MX4000, Philips Medical
Systems, Inc., Bothell, WA, USA) was used to analyze the lungs
using a preprogrammed setting, which was optimized to obtain the
best image. Details of the settings used are described in previous
study (32).
A novel liposome-encapsulated contrast agent
comprising lenvatinib-bound nanoparticles was used in the present
study. The lenvatinib was bound to superparamagnetic iron oxide
nanoparticles via a covalent bond, as described in a previous study
(33). The route of administration
for the TNCA or Optison™ (GE Healthcare Life Sciences, Little
Chalfont, UK; used as a control) was inhalation using an atomizer.
The microbubbles containing lenvatinib could reach the lesion
through the pulmonary circulation due to their small diameter.
After 30 min, the TNCA could be visualized using the CECT system.
No side effects were observed from the nanoparticle-lenvatinib
contrast agent.
Data from the CECT-TNCA images was analyzed using
the CECT system, including the volume of the tumors.
All data are presented as the mean ± standard
deviation of triplicate experiments. Data was analyzed by SPSS 19.0
software (IBM Corp., Armonk, NY, USA) using one-way analysis of
variance with Tukey's multiple comparison test. The Kaplan-Meier
estimator was used to produce survival curves for the mice over the
120-day long treatment period. P<0.05 was considered to indicate
a statistically significant difference.
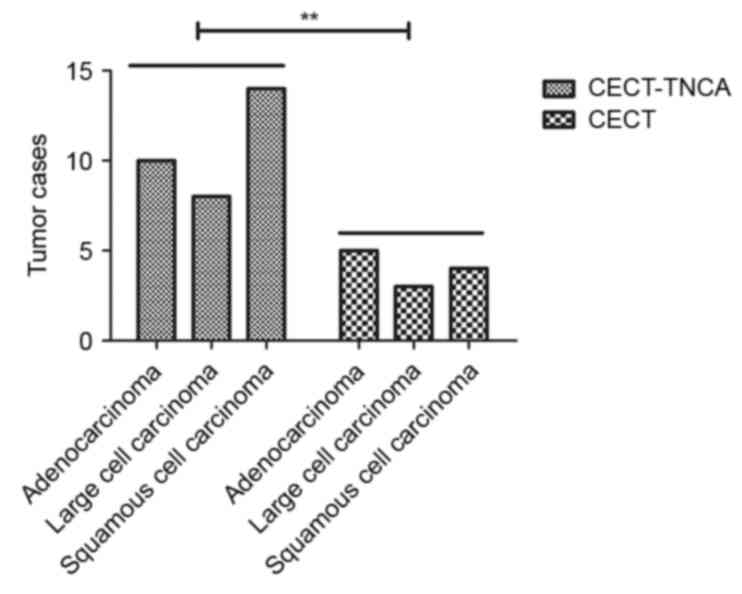
A total of 120 mice with early stage NSCLC
(adenocarcinoma, large cell carcinoma and squamous cell carcinoma;
n=40/group) were used to analyze the efficacy of CECT-TNCA on mice
with early stage NSCLC. Tumor metastasis was not observed in any of
the mice. CECT-TNCA identified significantly more of the mice with
NSCLC compared with CECT (32/120 vs. 7/120, respectively;
P<0.001; Fig. 1). The
characteristics of mice with NSCLC are detailed in Table I. These data suggest that, compared
with CECT, CECT-TNCA is more sensitive for the diagnosis of early
NSCLC.
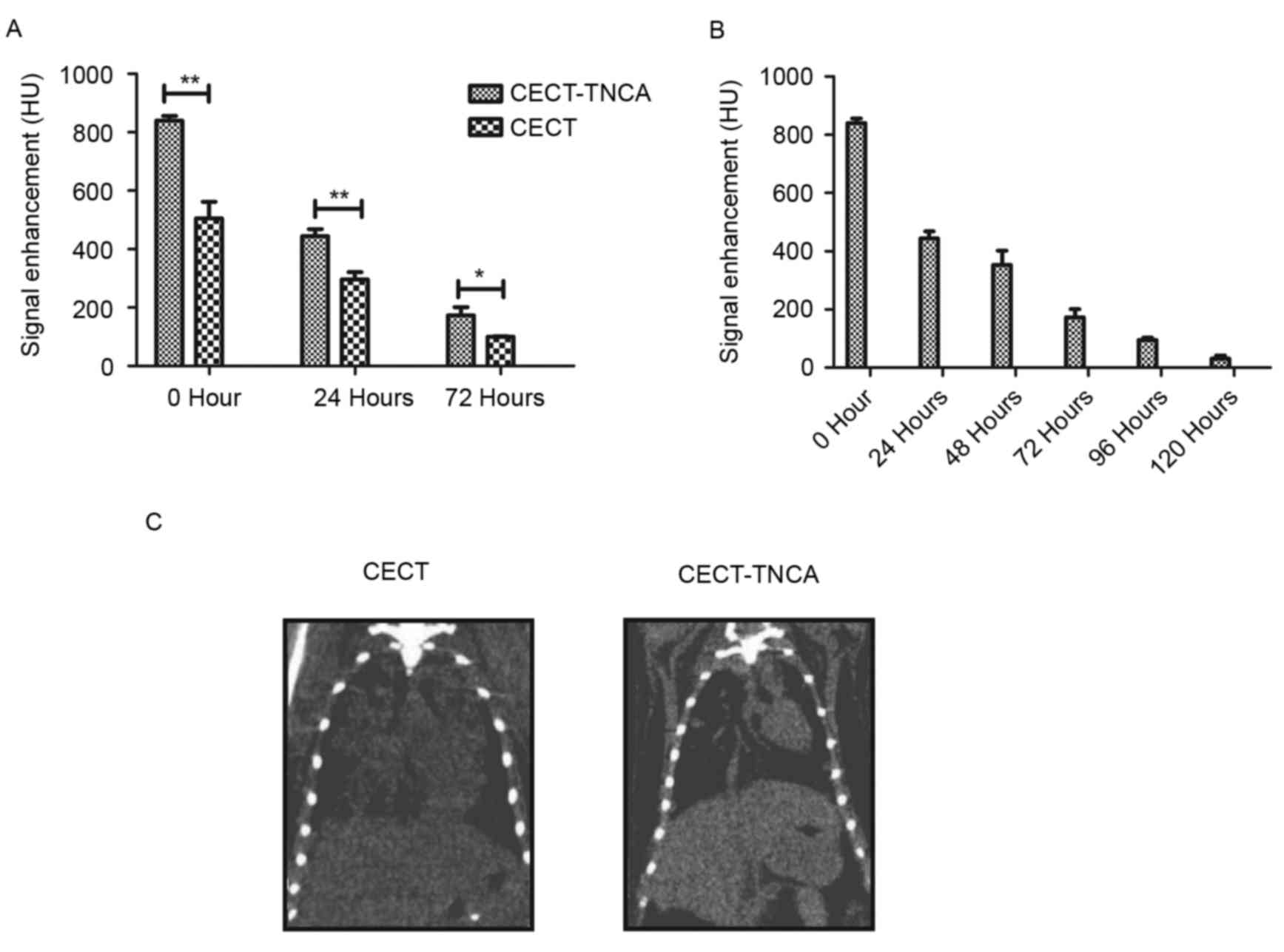
In order to analyze the efficacy of CECT-TNCA,
signal intensity was detected after administration of the liposomal
contrast agent. This revealed that the nanoscale microbubble
contrast agent significantly enhanced the signal intensity compared
with CECT (P<0.05; Fig. 2A). The
nanoscale microbubble contrast agent was cleared from systemic
circulation in ~120 h (Fig. 2B).
Dynamic analysis of lung tumor nodules revealed an enhanced signal
after inhalation of the TNCA (Fig.
2C). These results suggest that the TNCA significantly improved
signal intensity in lung tumor nodules.
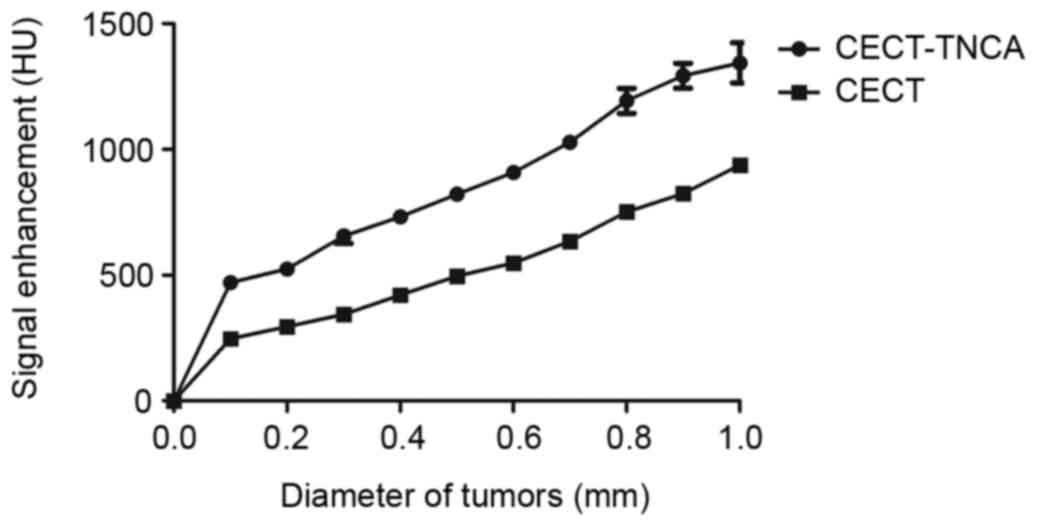
The pulmonary tumor nodules were further analyzed by
CECT-TNCA and CECT, with results revealing a positive association
between diameter and signal intensity (Fig. 5). Imaging analysis included NSCLC
volume and fractional blood volume as a function of nodule
diameter. In addition, signal intensity fed back by lesions in the
NSCLC nodules was improved after administration of the targeted
nanoscale microbubble contrast agent. These factors enabled the
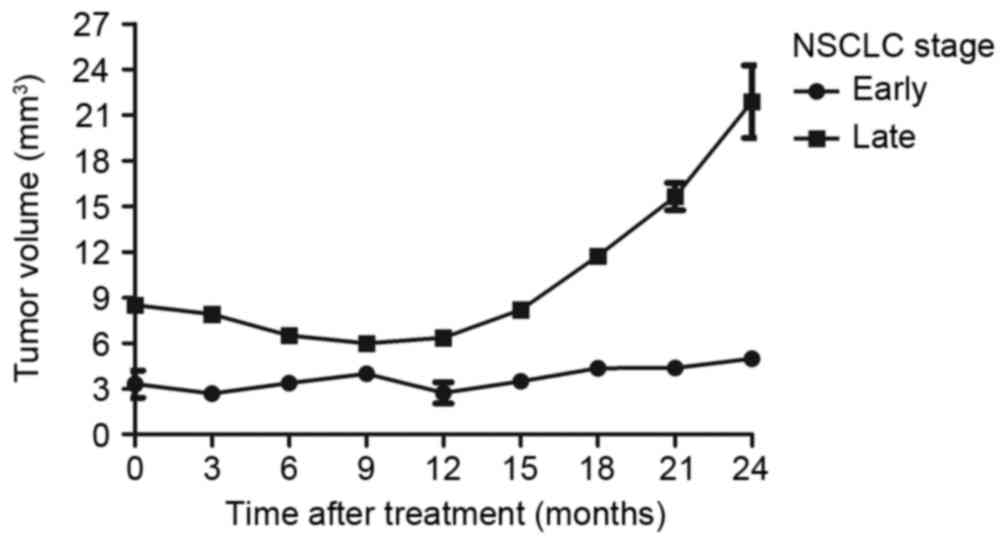
visualization of tiny tumor lesions in the lung (Fig. 6). Furthermore, the longitudinal
aspect of CECT-TNCA allowed for the imaging of early-stage NSCLC
tumors in mice (Fig. 7). In
addition, the data demonstrated that survival after treatment was
improved in mice with early-stage NSCLC compared with late-stage
NSCLC (Fig. 8). These results
indicate that CECT-TNCA-diagnosed early-stage tumors exhibited
relatively higher signal enhancement compared with CT tumors in
xenograft mice.
NSCLC is one of the most common of types of
respiratory cancer and a leading cause of cancer-associated
mortality worldwide (35). The
incidence of NSCLC and the number of NSCLC-associated mortalities
is growing (36). Notably, the
majority of newly diagnosed patients with NSCLC are already in a
late phase, which decreases the probability of recovery and
shortens survival time (37).
Therapeutic regimens for advanced NSCLC include targeted
intervention, chemotherapy, radiotherapy and immunotherapy
(38).
The present study investigated a comprehensive
approach of CECT combined with a TNCA in mice, in order to improve
the accuracy of early stage NSCLC diagnosis. Lenvatinib
encapsulated by liposomes was used as the TNCA. Lenvatinib is a
multi-target tyrosine kinase inhibitor of vascular endothelial
growth factor receptor (VEGFR) 1–3, fibroblast growth factor
receptor (FGFR) 1–4, platelet-derived growth factor receptor
(PDGFR) β, proto-oncogene tyrosine-protein kinase receptor Ret
(Ret) and mast/stem cell growth factor receptor Kit (Kit). VEGF1-3,
FGFR1-4, PDGFR-β, Ret and Kit-mediated angiogenesis have been
identified as key factors in the development of human cancer
(48,49). Lenvatinib has potent antitumor
activity against a number of human tumors (50). A previous report has suggested
targeting the receptor of lenvatinib in NSCLC (51). The results of the present study
demonstrated that liposome-encapsulated lenvatinib has a potential
application as a TNCA to improve the accuracy of early-stage NSCLC
diagnosis. This technique enhanced the signal intensity in lesions
in the lung, improving the spatial resolution of CECT.
Previously, liposomal and iodinated contrast agents
have provided methods to detect solid tumors (52,53). The
feasibility of spectral CT imaging for the detection of tumors with
target-based contrast agents was evidenced in a previous study.
Contrast agents have improved CT diagnosis in terms of image
quality, as observed in a prospective randomized trial (54). However, the development of a
non-invasive assay for the accurate diagnosis of NSCLC remains a
challenge. The present study investigated the efficacy of a
liposome-encapsulated lenvatinib TNCA for the diagnosis of early
stage NSCLC in a xenograft mice model. The results revealed that
CECT-TNCA-diagnosed early stage NSCLC tumors exhibited increased
signal enhancement compared with CT-diagnosed tumors. This suggests
that the liposome-encapsulated targeted contrast agent enables
high-resolution imaging of early phase NSCLC.
In conclusion, the current study provided insights
into improving CT imaging resolution using a liposome-encapsulated
targeted contrast agent. The preclinical application of thus
imaging procedure would provide novel opportunities to assess the
efficacy of early-stage NSCLC diagnosis and determine whether
nanoparticles exhibit absorbability without affecting the
respiratory system. The results of the present study indicate that
CECT-TNCA may be of significant value in the diagnosis of NSCLC.
Further studies are required to validate these results.
|
1
|
van der Wekken AJ, Saber A, Hiltermann TJ,
Kok K, van den Berg A and Groen HJ: Resistance mechanisms after
tyrosine kinase inhibitors afatinib and crizotinib in non-small
cell lung cancer, a review of the literature. Crit Rev Oncol
Hematol. 100:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joseph SS, Yentz SE, Mikkilineni S, Nelson
C and Kalemkerian GP: Eyelid metastasis in non-small cell lung
cancer: Diagnosis and management. Am J Med. 129:e169–e172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeCotiis C, Hu Y, Greenberg AK, Huie M,
Tsay JC, Pass H, Goldberg JD and Rom WN: Inflammatory cytokines and
non-small cell lung cancer in a CT-scan screening cohort:
Background review of the literature. Cancer Biomark. 16:219–233.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kepka L and Socha J: PET-CT use and the
occurrence of elective nodal failure in involved field radiotherapy
for non-small cell lung cancer: A systematic review. Radiother
Oncol. 115:151–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morinaga R, Okamoto I, Furuta K, Kawano Y,
Sekijima M, Dote K, Satou T, Nishio K, Fukuoka M and Nakagawa K:
Sequential occurrence of non-small cell and small cell lung cancer
with the same EGFR mutation. Lung Cancer. 58:411–413. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khreish F, Hellwig D, Mathews J, Bücker A,
Kirsch CM and Grgic A: Simultaneous occurrence of typical carcinoid
and non-small-cell lung cancer in the same lung lobe: Value of
nuclear medicine. Clin Nucl Med. 36:481–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Targowski T, Janda P, Owczarek W, Raczka
A, Jahnz-Rózyk K and Plusa T: Evaluation of occurrence frequency of
circulating p53 protein in serum of patients with chronic
obstructive pulmonary diseases and non-small cell lung cancer. Pol
Merkur Lekarski. 28:265–267. 2010.(In Polish). PubMed/NCBI
|
|
8
|
Xie FJ, Lu HY, Zheng QQ, Qin J, Gao Y,
Zhang YP, Hu X and Mao WM: The clinical pathological
characteristics and prognosis of FGFR1 gene amplification in
non-small-cell lung cancer: A meta-analysis. Onco Targets and Ther.
9:171–181. 2016. View Article : Google Scholar
|
|
9
|
Moro-Sibilot D, Smit E, de Castro Carpeno
J, Lesniewski-Kmak K, Aerts JG, Villatoro R, Kraaij K, Nacerddine
K, Dyachkova Y, Smith KT, et al: Non-small cell lung cancer
patients with brain metastases treated with first-line
platinum-doublet chemotherapy: Analysis from the European FRAME
study. Lung Cancer. 90:427–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim SH, Sun JM, Lee SH, Ahn JS, Park K and
Ahn MJ: Pembrolizumab for the treatment of non-small cell lung
cancer. Expert Opin Biol Ther. 16:397–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ulivi P, Mercatali L, Casoni GL, Scarpi E,
Bucchi L, Silvestrini R, Sanna S, Monteverde M, Amadori D, Poletti
V and Zoli W: Multiple marker detection in peripheral blood for
NSCLC diagnosis. PloS One. 8:e574012013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mroczko B, Szmitkowski M and Czygier M:
Granulocyte colony stimulating factor (G-CSF) in diagnosis and
monitoring of non-small-cell lung cancer (NSCLC). Pol Arch Med
Wewn. 103:163–168. 2000.(In Polish). PubMed/NCBI
|
|
13
|
Muller B, Bovet M, Yin Y, Stichel D, Malz
M, González-Vallinas M, Middleton A, Ehemann V, Schmitt J, Muley T,
et al: Concomitant expression of far upstream element (FUSE)
binding protein (FBP) interacting repressor (FIR) and its splice
variants induce migration and invasion of non-small cell lung
cancer (NSCLC) cells. J Pathol. 237:390–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Q, Yue J, Zhang C, Gu X, Chen H and
Xu L: Inactivation of M2 AChR/NF-κB signaling axis reverses
epithelial-mesenchymal transition (EMT) and suppresses migration
and invasion in non-small cell lung cancer (NSCLC). Oncotarget.
6:29335–29346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim DS, Park KM, Won YS, Kim JY, Lee JK,
Kim JG, Oh ST, Jung SS and Kang WK: Occurrence and prognosis of
symptomatic venous thromboembolism in colorectal cancer surgery
patients. Vasc Specialist Int. 30:49–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thunnissen E, Kerr KM, Herth FJ,
Lantuejoul S, Papotti M, Rintoul RC, Rossi G, Skov BG, Weynand B,
Bubendorf L, et al: The challenge of NSCLC diagnosis and predictive
analysis on small samples. Practical approach of a working group.
Lung Cancer. 76:1–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geng J, Sun J, Lin Q, Gu J, Zhao Y, Zhang
H, Feng X, He Y, Wang W, Zhou X and Yu J: Methylation status of
NEUROG2 and NID2 improves the diagnosis of stage I NSCLC. Oncol
Lett. 3:901–906. 2012.PubMed/NCBI
|
|
18
|
Peters S, Adjei AA, Gridelli C, Reck M,
Kerr K and Felip E: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 23
Suppl 7:vii56–vii64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jafri SH, Shi R and Mills G: Advance lung
cancer inflammation index (ALI) at diagnosis is a prognostic marker
in patients with metastatic non-small cell lung cancer (NSCLC): A
retrospective review. BMC Cancer. 13:1582013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vilmar AC, Santoni-Rugiu E and Sørensen
JB: ERCC1 and histopathology in advanced NSCLC patients randomized
in a large multicenter phase III trial. Ann Oncol. 21:1817–1824.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Lung Screening Trial Research
Team, . Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD,
Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM and Sicks JD:
Reduced lung-cancer mortality with low-dose computed tomographic
screening. N Engl J Med. 365:395–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saldias PF, Díaz PJ, Rain MC, Illanes CP,
Díaz TR and Díaz PO: Early detection of lung cancer using computed
tomography among patients with chronic obstructive pulmonary
disease. Rev Med Chil. 144:202–210. 2016.(In Spanish). PubMed/NCBI
|
|
23
|
Rubin GD: Lung nodule and cancer detection
in computed tomography screening. J Thorac Imaging. 30:130–138.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seigneurin A, Field JK, Gachet A and Duffy
SW: A systematic review of the characteristics associated with
recall rates, detection rates and positive predictive values of
computed tomography screening for lung cancer. Ann Oncol.
25:781–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sudarski S, Henzler T and Schoenberg SO:
Post-therapeutic positron emission tomography/computed tomography
for early detection of non-small cell lung cancer recurrence.
Transl Lung Cancer Res. 2:295–303. 2013.PubMed/NCBI
|
|
26
|
Hagberg GE, Mamedov I, Power A, Beyerlein
M, Merkle H, Kiselev VG, Dhingra K, Kubìček V, Angelovski G and
Logothetis NK: Diffusion properties of conventional and
calcium-sensitive MRI contrast agents in the rat cerebral cortex.
Contrast Media Mol Imaging. 9:71–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turetschek K, Preda A, Novikov V, Brasch
RC, Weinmann HJ, Wunderbaldinger P and Roberts TP: Tumor
microvascular changes in antiangiogenic treatment: Assessment by
magnetic resonance contrast media of different molecular weights. J
Magn Reson Imaging. 20:138–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samei E, Saunders RS, Badea CT, Ghaghada
KB, Hedlund LW, Qi Y, Yuan H, Bentley RC and Mukundan S Jr:
Micro-CT imaging of breast tumors in rodents using a liposomal,
nanoparticle contrast agent. Int J Nanomedicine. 4:277–282. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palekar RU, Jallouk AP, Lanza GM, Pan H
and Wickline SA: Molecular imaging of atherosclerosis with
nanoparticle-based fluorinated MRI contrast agents. Nanomedicine
(Lond). 10:1817–1832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oghabian MA and Farahbakhsh NM: Potential
use of nanoparticle based contrast agents in MRI: A molecular
imaging perspective. J Biomed Nanotechnol. 6:203–213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cho EC, Glaus C, Chen J, Welch MJ and Xia
Y: Inorganic nanoparticle-based contrast agents for molecular
imaging. Trends Mol Med. 16:561–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nakamoto Y, Ishimori T, Sano K, Temma T,
Ueda M, Saji H and Togashi K: Clinical efficacy of dual-phase
scanning using (68)Ga-DOTATOC-PET/CT in the detection of
neuroendocrine tumours. Clin Radiol. 71:1069.e1–e5. 2016.
View Article : Google Scholar
|
|
33
|
Chen CL, Hu GY, Mei Q, Qiu H, Long GX and
Hu GQ: Epidermal growth factor receptor-targeted ultra-small
superparamagnetic iron oxide particles for magnetic resonance
molecular imaging of lung cancer cells in vitro. Chin Med J (Engl).
125:2322–2328. 2012.PubMed/NCBI
|
|
34
|
Yasugi M, Takigawa N, Ochi N, Ohashi K,
Harada D, Ninomiya T, Murakami T, Honda Y, Ichihara E, Tanimoto M
and Kiura K: Everolimus prolonged survival in transgenic mice with
EGFR-driven lung tumors. Exp Cell Res. 326:201–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fenton-Ambrose L and Kazerooni EA:
Preventative care: Lung-cancer screens now worth the cost. Nature.
514:352014. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kulkarni S, Vella ET, Coakley N, Cheng S,
Gregg R, Ung YC and Ellis PM: The use of systemic treatment in the
maintenance of patients with non-small cell lung cancer: A
systematic review. J Thorac Oncol. 11:989–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshiba S, Jansen M, Matsushima N, Chen S
and Mendell J: Population pharmacokinetic analysis of patritumab, a
HER3 inhibitor, in subjects with advanced non-small cell lung
cancer (NSCLC) or solid tumors. Cancer Chemother Pharmacol.
77:987–996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Weller A, O'Brien ME, Ahmed M, Popat S,
Bhosle J, McDonald F, Yap TA, Du Y, Vlahos I and deSouza NM:
Mechanism and non-mechanism based imaging biomarkers for assessing
biological response to treatment in non-small cell lung cancer. Eur
J Cancer. 59:65–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim SH, Cho BC, Choi HJ, Chung KY, Kim DJ,
Park MS, Kim SK, Chang J, Shin SJ, Sohn JH and Kim JH: The number
of residual metastatic lymph nodes following neoadjuvant
chemotherapy predicts survival in patients with stage III NSCLC.
Lung Cancer. 60:393–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Satouchi M, Negoro S, Funada Y, Urata Y,
Shimada T, Yoshimura S, Kotani Y, Sakuma T, Watanabe H, Adachi S,
et al: Predictive factors associated with prolonged survival in
patients with advanced non-small-cell lung cancer (NSCLC) treated
with gefitinib. Br J Cancer. 96:1191–1196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Soldan K, Pooley FD, Hansen J, Andersen A,
Chang-Claude J, Ferro G, Ohgaki H, Skov BG, Cherrie JW, Saracci R
and Boffetta P: Lung fibre burden in lung cancer cases employed in
the rock and slag wool industry. Ann Occup Hyg. 50:241–248.
2006.PubMed/NCBI
|
|
42
|
Garcia Duenas OF, Villanueva Kerckoff H,
Olvera Rico H and Plascencia Lira J: Benign peritoneal cystic
mesothelioma as differential diagnose of an ovarian dependant
tumor. Case report and review of the literature. Ginecol Obstet
Mex. 75:111–114. 2007.(In Spanish). PubMed/NCBI
|
|
43
|
Garg PK, Deo SV, Kumar R, Shukla NK,
Thulkar S, Gogia A, Sharma DN and Mathur SR: Staging PET-CT
scanning provides superior detection of lymph nodes and distant
metastases than traditional imaging in locally advanced breast
cancer. World J Surg. 40:2036–2042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nogami Y, Banno K, Irie H, Iida M, Masugi
Y, Murakami K and Aoki D: Efficacy of 18-FDG PET-CT dual-phase
scanning for detection of lymph node metastasis in gynecological
cancer. Anticancer Res. 35:2247–2253. 2015.PubMed/NCBI
|
|
45
|
Davison CA, Chapman SE, Sasser TA, Wathen
C, Diener J, Schafer ZT and Leevy WM: Multimodal optical, X-ray CT
and SPECT imaging of a mouse model of breast cancer lung
metastasis. Curr Mol Med. 13:368–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Genestreti G, Burgio MA, Matteucci F,
Piciucchi S, Scarpi E, Monti M, Bucchi L, Parisi E, Crociani L,
Gurioli C, et al: Endobronchial/Endoesophageal Ultrasound
(EBUS/EUS) guided fine needle aspiration (FNA) and 18F-FDG PET/CT
scanning in restaging of locally advanced non-small cell lung
cancer (NSCLC) Treated with Chemo-radiotherapy: A
Mono-institutional pilot experience. Technol Cancer Res Treat.
14:721–727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Manowitz A, Sedlar M, Griffon M, Miller A,
Miller J and Markowitz S: Use of BMI guidelines and individual dose
tracking to minimize radiation exposure from low-dose helical chest
CT scanning in a lung cancer screening program. Acad Radiol.
19:84–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rini BI and Atkins MB: Resistance to
targeted therapy in renal-cell carcinoma. Lancet Oncol.
10:992–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Eichelberg C, Junker K, Ljungberg B and
Moch H: Diagnostic and prognostic molecular markers for renal cell
carcinoma: A critical appraisal of the current state of research
and clinical applicability. Eur Urol. 55:851–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hutson TE: Targeted therapies for the
treatment of metastatic renal cell carcinoma: Clinical evidence.
Oncologist. 16 Suppl 2:S14–S22. 2011. View Article : Google Scholar
|
|
51
|
Nishio M, Horai T, Horiike A, Nokihara H,
Yamamoto N, Takahashi T, Murakami H, Yamamoto N, Koizumi F, Nishio
K, et al: Phase 1 study of lenvatinib combined with carboplatin and
paclitaxel in patients with non-small-cell lung cancer. Br J
Cancer. 109:538–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Achenbach S, Paul JF, Laurent F, Becker
HC, Rengo M, Caudron J, Leschka S, Vignaux O, Knobloch G, Benea G,
et al: Erratum to: Comparative assessment of image quality for
coronary CT angiography with iobitridol and two contrast agents
with higher iodine concentrations: Iopromide and iomeprol. A
multicentre randomized double-blind trial. Eur Radiol. 27:8312017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mannheim JG, Schlichthaerle T, Kuebler L,
Quintanilla-Martinez L, Kohlhofer U, Kneilling M and Pichler BJ:
Comparison of small animal CT contrast agents. Contrast Media Mol
Imaging. 11:272–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Honoris L, Zhong Y, Chu E, Rosenthal D, Li
D, Lam F and Budoff MJ: Comparison of contrast enhancement, image
quality and tolerability in Coronary CT angiography using 4
contrast agents: A prospective randomized trial. Int J Cardiol.
186:126–128. 2015. View Article : Google Scholar : PubMed/NCBI
|