Introduction
Endometriosis (EMs) is characterized by the growth
of endometrial glands and stroma outside the uterine cavity and is
a common disease among women of childbearing age (1). EMs affects 6–10% fertile women and may
cause abdominal pain and decreased fertility and its recurrence
rate is slightly higher than its incidence rate (2). EMs can migrate to distant tissues,
including the peritoneum, ovarian and inteestinal walls (3). Although EMs is a benign disease, it has
malignant capabilities (4).
MicroRNAs (miRNAs) are a class of endogenous and
highly evolutionarily conserved non-coding RNAs of 18–24
nucleotides in length (5,6). miRNA can induce mRNA degradation or the
translational repression of target genes through complementary
pairing with the 3′ untranslated region (UTR) of target mRNA
(7). It has been demonstrated that
miRNA serves a variety of roles in physiological processes,
including growth, differentiation, proliferation and apoptosis
(8–10). In addition, miRNA can change the
physiological and biological characteristics of the endometrium by
targeting multiple genes (11,12).
Therefore, differences in the miRNA expression profile between
patients with and those without EMs may indicate associations with
the development of EMs. Previous studies have demonstrated that
miR-30c may be involved in the progression of numerous diseases,
including the regulation of plasminogen activator inhibitor (PAI)
in sickle cell anemia (13), the
reorganization of myocardial connective tissue in myocardial
infarction (14), adipocyte
differentiation in diabetes (15),
inhibition of the invasion of non-small cell lung cancer (16) and promotion of the toxicity of
natural killer cells to inhibit the development of liver cancer
(17). It has been reported that
miR-30c negatively regulates endometrial cancer cells by repressing
the metastasis-associated gene-1 (MAG-1) (18). However, the role of miR-30c in EMs
has not yet been assessed.
PAI type 1 (PAI-1) belongs to the serine protease
inhibitor superfamily and is single-chain glycoprotein composed of
379 or 381 amino acids, with a molecular mass of 50×103
Da (19,20). PAI-1 protein is unstable as it does
not contain a disulfide bond; however, it can become stable by
combining with vitronectin (19,21).
PAI-1 has three types of structures; an active, inactive and
unstable type, and its conformation can be mutually transformed
(19,22). PAI-1 is widely expressed in various
cell types, including platelets, monocytes, megakaryocytes, liver
cells, mesangial cells, fibroblasts and basal cells in adipose
tissue (23). In addition, vascular
smooth muscle cells and endothelial cells are the primary producers
of PAI-l (19). Secreted PAI-1 can
enter the stromal cells or blood circulation and certain PAI-1
proteins in circulating blood can be stored in the α particles of
platelets, while others present in plasma serve important
physiological functions (24). PAI-1
has an important regulatory role in the fibrinolytic system and the
coagulation system, which is the primary inhibitor of the
fibrinolytic system, to suppress urinary plasminogen
activator/tissue plasminogen activator activity and to block the
conversion of plasminogen to plasmin (25). Furthermore, PAI-1 serves an important
role in cell signaling, adhesion and metastasis (26). PAI-1 can inhibit fibrinolysis,
stabilize the extracellular matrix and promote invasion (27,28). It
has been demonstrated that PAI-1 may stabilize the basement
membrane by inhibiting protein degradation in a number of
physiological processes, including the evolution of connective
tissue, blood coagulation, fibrinolysis, complement activation,
inflammatory overreaction and tumor angiogenesis and stability
(29–31).
The development and progression of EMs includes
numerous steps, including cell adhesion, invasion and angiogenesis
(4). In the present study, the
expression of miR-30c and PAI-1 in EMs was measured and the role of
miR-30c and PAI-1 in EMs was analyzed.
Materials and methods
Patients and samples
The present study included 20 female patients with
EMs who were admitted to the Department of Gynaecology and
Obstetrics of the Affiliated Hospital of Jining Medical University
(Jining, China) between May 2013 and December 2014. Their eutopic
and ectopic endometrium were collected. As a control, normal
endometrial tissues were collected from 18 female patients with
primary cervical cancers who had undergone complete hysterectomy.
The average age of patients with EMs was (43±2.4 years) and that of
the control group was (41±3.3 years); there were no significant
differences between two groups with regard to age. All patients
experienced regular menses and the endometrium was in the secretory
phase (confirmed by menstrual cycle and histological examination).
Only patients who had not received any hormone treatment and who
had not experienced any serious complications in the three months
preceding surgery were included in the present study. Prior written
and informed consent was obtained from every patient and the study
was approved by the ethics review board of the Affiliated Hospital
of Jining Medical University (Jining, China).
Isolation and culture of primary
endometrial stromal cells (ESCs)
ESCs were isolated from endometrial tissues. In
brief, following rinsing 2–3 times with phosphate-buffered saline
(PBS), endometrial tissue was cut into 0.5–1 mm3
sections, which were incubated with collagenase (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) at 37°C for 50–80 min. An equal
volume of Dulbecco's modified Eagle's medium (DMEM)/F12 (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) with 10% fetal bovine
serum (FBS) (Hyclone; GE Healthcare Life Sciences) was added when
no obvious tissue mass was observed. Tissue residues were removed
using a 100-mesh filter and the filtrate was centrifuged at 800 ×
g, for 5 min at 4°C. The pellet was re-suspended in serum-free
DMEM/F12 (HyClone; GE Healthcare Life Sciences) and then filtered
through a 200-mesh filter. The filtrate was centrifuged at 1,000 ×
g for 10 min at 4°C and the supernatant was discarded. The cells
were suspended in the DMEM/F12 with 10% FBS (containing 100 IU/ml
penicillin and 100 IU/ml streptomycin), seeded in culture flasks
and incubated at 37°C in 5% CO2 for 24 h. The DMEM/F12
medium with 10% FBS was replaced and cells were cultured at 37°C in
an incubator with 5% CO2. The medium was changed every
48 h and cell growth was measured every day. Once the cells had
reached 80–90% confluence, cells were passaged following a standard
procedure.
miRNA transfection
At 24 h prior to transfection, cells were seeded in
6-well plates at 70–90% confluency. ESCs were divided into four
groups based on transfection: An miR-30c mimics group, a miR-30c
inhibitor group, a negative control group (NC) and a blank group.
These miRs were purchased from Genepharma Co., Ltd. (Shanghai,
China). Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used for transfection
following the manufacturer's instructions. Cells were harvested 48
h after transfection and reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) was performed to detect the
expression of miR-30c and PAI-1 in the cells.
RNA extraction and RT-qPCR
Total RNA was isolated from tissues using 1 ml
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) per 100 mg tissue according to the manufacturer's protocol.
For transfected cells, 2×105 cells were treated with 1
ml TRIzol reagent. RNA integrity was checked by gel electrophoresis
and the purity of RNA was assessed using the ratio of absorbance at
260 and 280 nm (Thermo Scientific™ Evolution 300; Thermo Fisher
Scientific, Inc.). Complementary (c)DNA was synthesized from total
RNA by reverse transcription with the PrimeScript™ RT regent kit
(Takara Biotechnology, Inc., Dalian, China) and stored at −20°C.
The 20-µl reverse transcription system included 6 µl miRNA
template, 2×10 µl miRNA Reaction Buffer mix, 2 µl 0.1% bovine serum
albumin (BSA) and 2 µl miRNA PrimeScript RT Enzyme Mixture (all
Takara Biotechnology, Inc.). The reaction was performed using ABI
Veriti 96-Well Thermo Cycler (Thermo Fisher Scientific, Inc.) at
37°C for 60 min with Poly A primer. The SYBR-Green RT-PCR Master
mix (Takara Biotechnology, Inc.) was used to detect the expression
of miR-30c and PAI-1 in tissues and ESCs. U6 was used as internal
reference for detecting miR-30c, while GAPDH was used for PAI-1.
The primer sequences were as follows: U6 forward, GCT TCG GCA GCA
CAT ATA CTA AA AT and reverse, CGC TTC ACG AAT TTG CGT GTC AT;
GAPDH forward, TTA GCA CCC CTG GCC AAG G and reverse, CTT ACT CCT
TGG AGG CCA TG. The primers for miR-30c were
5′-TGTGTAAACATCCTACACTCTCAG-3′ and Uni-miR qRT-PCR Primer (Sangon
Biotech Co., Ltd., Shanghai China). The primers for PAI-1 were:
Forward, 5′-ACCTGGGAATGACCGACATGT-3′ and reverse,
5′-CTCTCGTTCACCTCGATCTTCACT-3′. The miRNA reaction system included
12.5 µl SYBR RT-PCR Master mix, 1 µl forward primer and 1 µl
reverse primer, 2 µl cDNA and 8.5 µl double distilled
(dd)H2O, and the cycling conditions were as follows:
95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 60°C
for 20 sec. The PAI-1 system included 10 µl SYBR RT-PCR Master mix,
0.5 µl forward primer and 0.5 µl reverse primer, 1 µl cDNA and 8 µl
ddH2O. The PCR cycling conditions were as follows: 95°C
for 10 min, followed by 40 cycles at 95°C for 1 min, 60°C for 40
sec, 72°C for 30 sec and 72°C for 1 min. Relative expression was
calculated using the 2−ΔΔCq method (32).
Western blot analysis
For protein isolation, each 50-mg tissue sample was
ground into powder with liquid nitrogen and lysed with 600 µl
radioimmunoprecipitation assay (50 mM Tris-base, 1 mM EDTA, 150 mM
NaCl, 0.1% SDS, 1% Triton X-100 and 1% sodium deoxycholate) lysis
buffer. Following centrifugation at the speed of 12,000 × g at 4°C
for 5 min, the supernatant was obtained and the protein
concentration was assessed using Pierce BCA Protein Assay kit.
(Thermo Fisher Scientific, Inc.) A similar method was applied to
extract proteins in ESCs. Isolated proteins (10 µl) were separated
by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride
membrane. The proteins were incubated with primary antibodies for 1
h at room temperature. The primary antibodies were rabbit
anti-human PAI-1 (no. 11907; 1:800) and rabbit anti-human GAPDH
antibody (no. 2118; 1:2,000). The membranes were then incubated
with secondary antibody overnight at 4°C. The secondary antibody
was horseradish peroxidase-conjugated goat anti-rabbit
immunoglobulin G (no. 7074; 1:1,000). All antibodies were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Finally,
the membrane was developed by enhanced chemiluminescence plus
reagent (EMD Millipore, Billerica, MA, USA). Image Lab™ software
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was applied to
analyze the blot images and the intensity of the bands.
Transwell migration assay
The migration assays were performed using Transwell
chambers (Corning Inc., New York, NY, USA). The re-suspended ESCs
were seeded (1×105 cell/well) in 200 µl serum-free RPMI
1640 medium (Hyclone; GE Healthcare Life Sciences) and placed into
the upper chambers, while the bottom chambers contained 750 µl RPMI
medium with 10% FBS. After 48 h of culture, cells on the lower side
of the membrane were fixed with 4% formaldehyde at room
temperature, washed with PBS and stained using crystal violet.
Finally, images of the cells were captured under a light microscope
at ×200 magnification with five random views and the number of
invaded cells was counted.
MTT assay
Four groups of cells transfected with miR-30c
mimics, miR-30c inhibitor or negative control as well as blank
cells were seeded in 96-well plates (2×103/well) and 3
replicates were performed for each group. The MTT solution
(Beyotime Institute of Biotechnology, Haimen, China) was added to
each well at 24, 48, and 72 h. Following incubation at 37°C for 4
h, the absorbance of each well was measured using a BioTek
spectrophotometer (Biotek Instruments, Inc., Winooski, VT, USA) at
492 nm and a cell growth curve was constructed.
Cell adhesion assay
In each well of a 96-well plate, 50 µl Matrigel
(serum-free medium at 1:8 dilution) was added and dried under
sterile conditions. Following transfection for 48 h,
2×104 cells/well were cultured in each incubated well.
After 60 min of incubation, non-adherent cells were rinsed off and
a Cell Counting Kit-8 (Beyotime Company, Jiangsu, China) was used
to quantify the attached cells by detection of absorbance at 450 nm
using a BioTek spectrophotometer.
Cell invasion assay
The invasion of ESCs was analyzed using Matrigel
invasion chambers (growth-factor depleted Matrigel invasion
chambers; BD Biosciences, Franklin Lakes, NJ, USA). In the Matrigel
chambers, 500 µl serum-free DMEM was added and incubated at room
temperature for 1 h to hydrate the Matrigel. Subsequently, 750 µl
DMEM containing 20% fetal bovine serum (Hyclone; GE Healthcare Life
Sciences) was added to the lower chamber. Successfully transfected
cells were collected and re-suspended to 4×105 cells/ml
with DMEM containing 0.1% BSA. Following 18 h of incubation at 37°C
with 5% CO2, the cells on the upper side of the membrane
were wiped with a cotton swab. The invaded cells on the other side
of the chamber were fixed with 4% methanol at room temperature for
10 min. Following staining with 0.1% crystal violet, cells were
counted under a microscope.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 16
statistical software (SPSS, Inc., Chicago, IL, USA). All data were
analyzed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
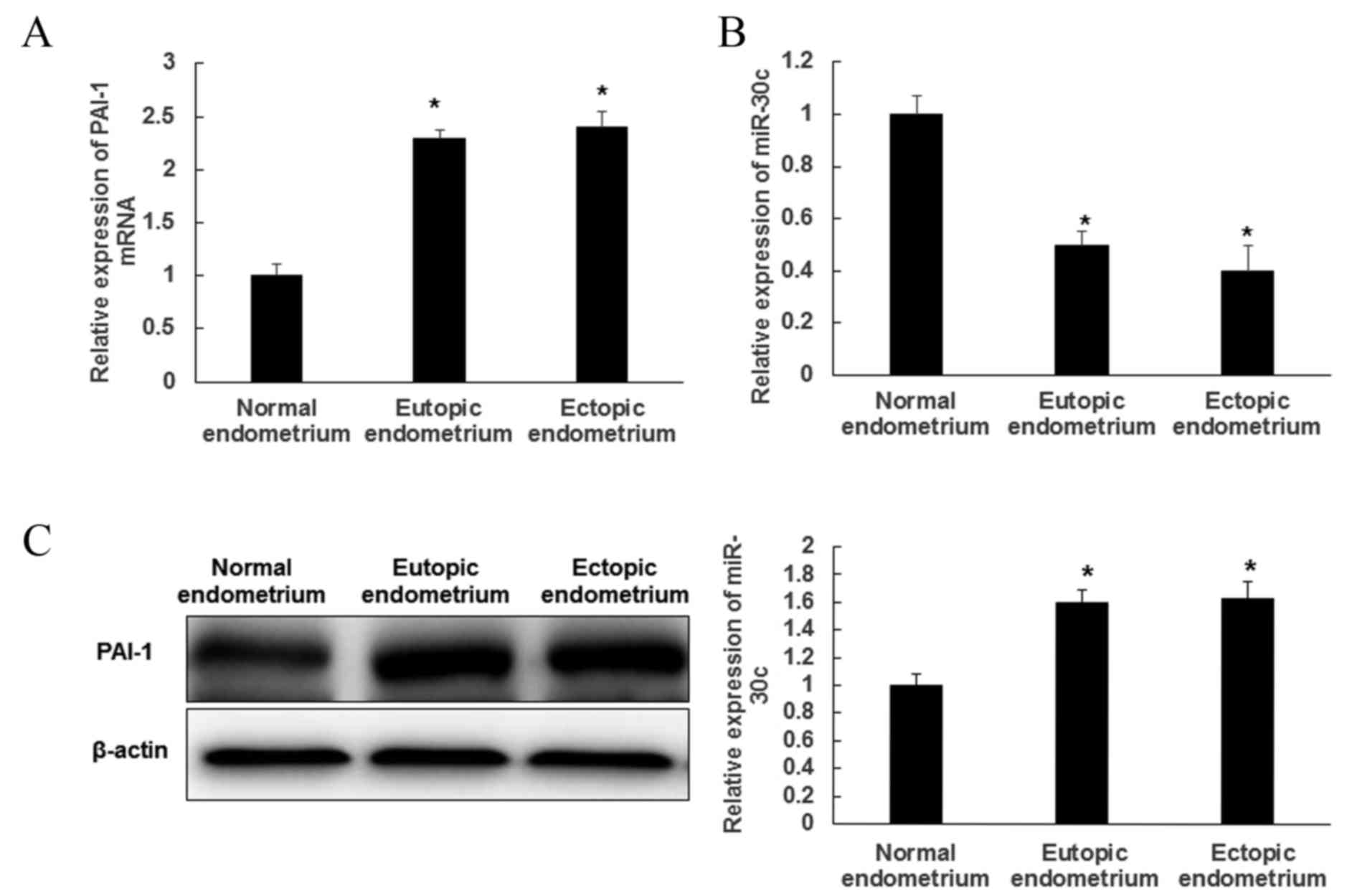
Expression of PAI-1 and miR-30c in EMs
tissues
RT-qPCR was performed to evaluate the expression of
PAI-1 and miR-30c in EMs tissues. PAI-1 expression was
significantly increased in ectopic and eutopic endometrium compared
with normal tissues (P<0.05; Fig.
1A). PAI-I expression did not significantly differ between
ectopic and eutopic endometrium. By contrast, the expression of
miR-30c was significantly decreased in ectopic and eutopic
endometrium compared with normal tissues (P<0.05); however, no
difference in miR-30c expression was observed between ectopic and
eutopic endometrium (Fig. 1B). In
addition, the levels of PAI-1 protein in tissues were detected by
western blot analysis and were similar to its mRNA expression.
Levels of PAI-1 protein were significantly higher in eutopic and
ectopic endometrium than in normal tissues (P<0.05), while there
was no significant difference between PAI-1 expression in ectopic
and eutopic endometriosis tissue (Fig.
1C). These results demonstrated that miR-30c expression was
decreased, whereas PAI-1 expression was increased in EMs
tissue.
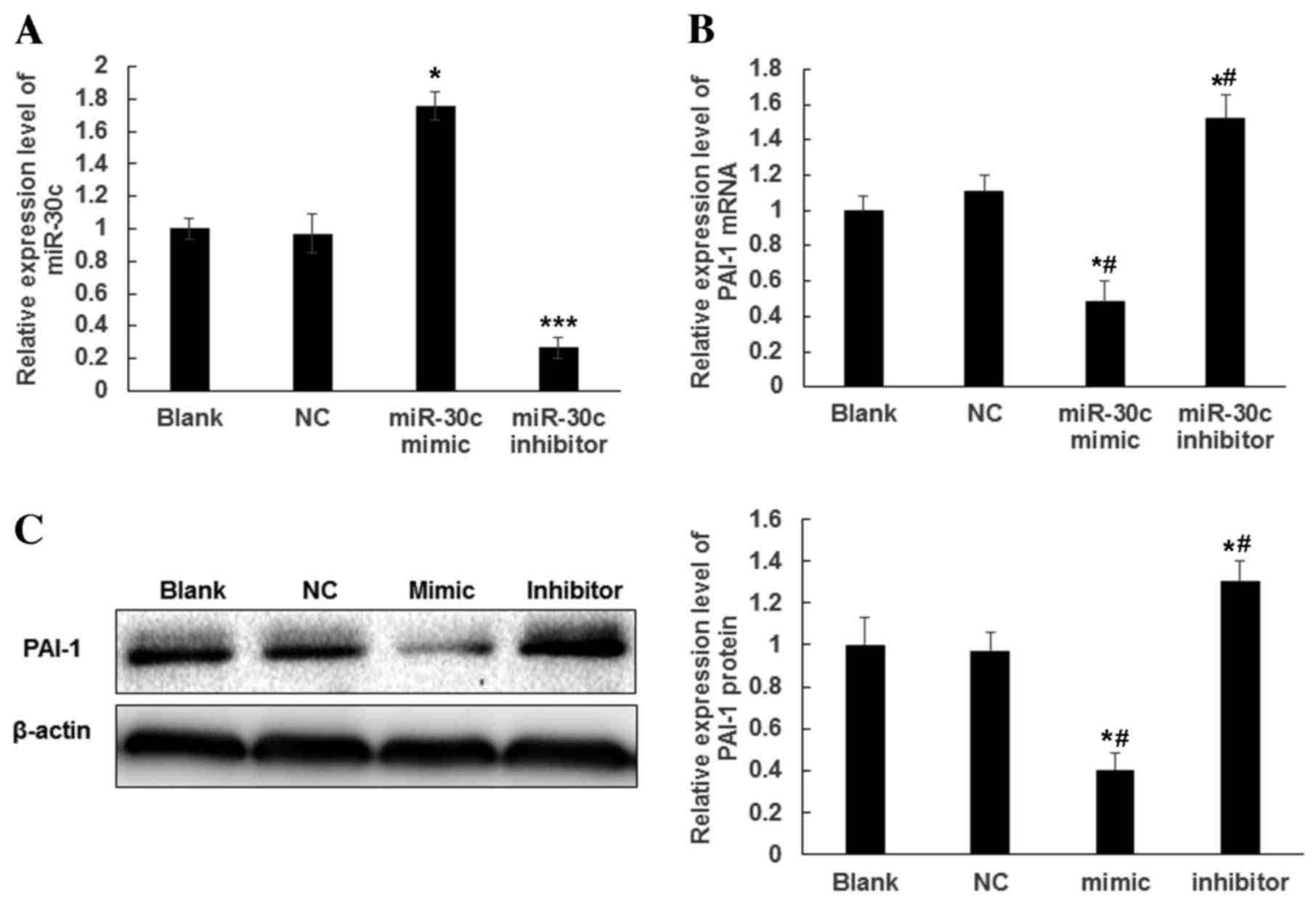
miR-30c inhibits the expression of
PAI-1 in transfected ESC cells
To determine whether PAI-1 expression is regulated
by miR-30c, ESCs were transfected with miR-30c mimics and miR-30c
inhibitor. Following transfection for 48 h, miR-30c expression was
significantly increased in ESCs transfected with miR-30c mimics and
was >70% higher than that in the NC and Blank groups (P<0.05
vs. Blank; Fig. 2A). Furthermore,
miR-30c expression was significantly decreased in ESCs transfected
with miR-30c inhibitor and was ~30% of that in the NC and Blank
groups (P<0.001 vs. Blank; Fig.
2A). Total RNA and protein was extracted from transfected ESCs
to detect the expression of PAI-1 mRNA and protein by RT-qPCR and
western blotting, respectively. Compared with the NC and Blank
groups, cells transfected with miR-30c mimics exhibited
significantly decreased expression of PAI-1 at the mRNA (P<0.05;
Fig. 2B) and protein level
(P<0.05; Fig. 2C). By contrast,
inhibition of miR-30c expression increased the expression of PAI-1
at the mRNA and protein level (P<0.05; Fig. 2B and C). These results indicated that
overexpression of miR-30c may repress the expression of PAI-1 mRNA
and protein.
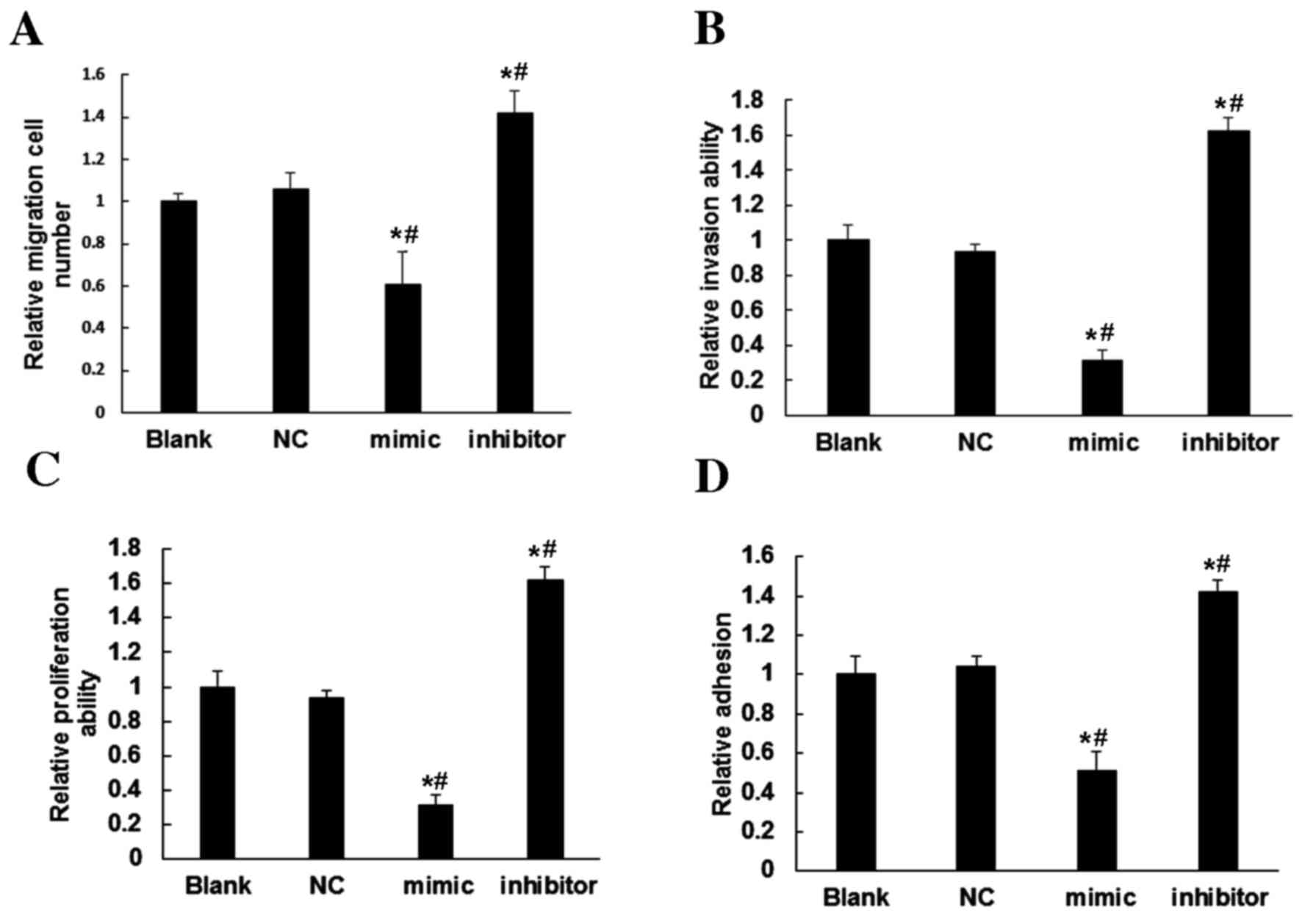
ESC proliferation, adhesion and
migration are regulated by miR-30c
To further assess the regulatory roles of miR-30c in
ESCs, its effect on migration and invasion were assessed. Compared
with the Blank and NC groups, ESCs overexpressing miR-30c exhibited
significantly reduced cell migration (P<0.05; Fig. 3A) and invasion (P<0.05; Fig. 3B). By contrast, inhibition of miR-30c
expression in ESCs increased the ability of cells to migrate and
invade (P<0.05; Fig. 3A and B).
These results indicated that miR-30c expression inhibits the
motility of ESCs.
To elucidate the effect of miR-30c on cell
proliferation, an MTT assay was performed. The results demonstrated
that, compared with the Blank and NC groups, overexpression of
miR-30c in ESCs significantly reduced the proliferation of ESCs
(P<0.05), whereas inhibition of miR-30c in ESCs significantly
increased ESC proliferation (P<0.05; Fig. 3C). This suggested that miR-30c may
repress the proliferation of ESCs.
Compared with the Blank and NC groups, the results
of an adhesion test using Matrigel showed that overexpression of
miR-30c in the miR-30 mimics group decreased the number of adhesive
cells, while the inhibition of miR-30c in the group transfected
with the miR-30 inhibitor exhibited an increased number of adhesive
cells (Fig. 3D). This indicated that
miR-30c may reduce the adhesion ability of ESCs.
Discussion
EMs is a type of benign disease with malignant
behaviors (33). The pathology of
EMs includes adhesion of ectopic endometrial cells, invasive growth
and angiogenesis (34,35). The etiology and pathogenesis of EMs
have remained to be fully elucidated. miRNA, as a
post-transcriptional regulator, may influence the biological
behavior of cells by targeting a wide range of mRNAs (36). Accounting for ~2% of the total number
of human genes, miRNA can regulate >30% genes in the human
genome (37). It is important to
identify miRNA and associated target genes that impact EMs
progression. As the primary inhibitor of the fibrinolysis system,
PAI-1 is widely expressed in various tissues. Casslen et al
(38) found that PAI-1 was expressed
in the human endometrium and Bruse et al (39) demonstrated that PAI-1 was
overexpressed in ectopic endometrial tissues. The results of the
present study showed that PAI-1 expression in ectopic and eutopic
endometrial tissues was higher than in normal tissues, which is
consistent with the results of the previous study by Bruse et
al (39).
A study by Lagos-Quintana et al (40) detected the expression of miR-30c in
the heart and brain tissues of mice. Furthermore, abnormal
expression of miR-30c was found in the reticulocytes of patients
with Polycythemia vera (41).
Downregulated miR-30c has been identified in a number of
malignancies, including breast (42), colorectal (43) and bladder cancer (44). The present study found that miR-30c
was downregulated in ectopic and eutopic endometrial tissues,
indicating that miR-30c may suppress the progression and
development of EMs. Primary ESCs were cultured and subsequently
transfected with miR-30c mimics or inhibitor. It was demonstrated
that miR-30c was able to regulate the expression of PAI-1 in ESCs.
It has been demonstrated that miR-30c can directly bind to the
bases 1,704–1,760 in the 3′untranslated region (UTR) of PAI-1 in
human endothelial cells (13).
Therefore, miR-30c may regulate PAI-1 expression by directly
targeting the 3′UTR of PAI-1. In the present study, the biological
functions of miR-30c in ESCs were investigated by targeting PAI-1.
It was demonstrated that repressed expression of PAI-1 induced by
increased miR-30c expression may decrease the migration and
invasion of ESCs. Furthermore, inhibition of miR-30c expression
induced the upregulation of PAI-1 expression and promoted ESC
migration and invasion. In addition, the MTT assay showed that
miR-30c inhibited ESC proliferation. The effects of miR-30c on the
adhesion ability of ESCs were also examined. It was found that
overexpression of miR-30c downregulated PAI-1 expression, thus
reducing the number of cells attached to the Matrigel, while
inhibition of miR-30c increased the number of adhesive cells by
upregulating the expression of PAI-1. The results suggested that
miR-30c may be involved in endometriosis by targeting PAI-1, thus
affecting the migration, invasion, proliferation and adhesion of
ESCs.
In conclusion, the present study demonstrated that
downregulation of miR-30c may be involved in the occurrence and
progression of EMs. The negative regulation of PAI-1 by miR-30c
appears to be important in Ems-associated processes. Through
designing targeted inhibition strategies and monitoring the
expression of miR-30c and PAI-1, the diagnosis and prognosis of Ems
may improve.
Acknowledgements
The authors would like to thank Professor Li Xu
(Vice President of The Affiliated Hospital of Jining Medical
University) for providing the experimental materials and her advice
and Professor Weihua Wu (The Office of President) for his
experimental suggestions and thesis modification. The authors also
wish to thank Professor Hongchun Hou (Department of Reproductive
Medicine, The Affiliated Hospital of Jining Medical University) for
his kind help during the present study.
References
|
1
|
Parazzini F, Vercellini P and Pelucchi C:
Endometriosis: Epidemiology and etiological factorsEndometriosis:
Science and Practice. Wiley-Blackwell; New York, NY: pp. 19–26.
2012, View Article : Google Scholar
|
|
2
|
Tandoi I, Somigliana E, Riparini J,
Ronzoni S, Vigano' P and Candiani M: High rate of endometriosis
recurrence in young women. J Pediatr Adolesc Gynecol. 24:376–379.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vercellini P, Frontino G, Pietropaolo G,
Gattei U, Daguati R and Crosignani PG: Deep endometriosis:
Definition, pathogenesis, and clinical management. J Am Assoc
Gynecol Laparosc. 11:153–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vercellini P, Viganò P, Somigliana E and
Fedele L: Endometriosis: Pathogenesis and treatment. Nat Rev
Endocrinol. 10:261–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai EC, Tomancak P, Williams RW and Rubin
GM: Computational identification of Drosophila microRNA genes.
Genome Biol. 4:R422003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim VN: Small RNAs: Classification,
biogenesis, and function. Mol Cells. 19:1–15. 2005.PubMed/NCBI
|
|
7
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.PubMed/NCBI
|
|
8
|
Rácz Z, Kaucsár T and Hamar P: The huge
world of small RNAs: Regulating networks of microRNAs (review).
Acta Physiol Hung. 98:243–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bueno MJ, de Castro IP and Malumbres M:
Control of cell proliferation pathways by microRNAs. Cell Cycle.
7:3143–3148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chegini N: Uterine microRNA signature and
consequence of their dysregulation in uterine disorders. Anim
Reprod. 7:117–128. 2010.PubMed/NCBI
|
|
12
|
Pan Q and Chegini N: MicroRNA signature
and regulatory functions in the endometrium during normal and
disease states. Semin Reprod Med. 26:479–493. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patel N, Tahara S, Malik P and Kalra VK:
Involvement of miR-30c and miR-301a in immediate induction of
plasminogen activator inhibitor-1 by placental growth factor in
human pulmonary endothelial cells. Biochem J. 434:473–482. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Divakaran V and Mann DL: The emerging role
of microRNAs in cardiac remodeling and heart failure. Circ Res.
103:1072–1083. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Karbiener M, Neuhold C, Opriessnig P,
Prokesch A, Bogner-Strauss JG and Scheideler M: MicroRNA-30c
promotes human adipocyte differentiation and co-represses PAI-1 and
ALK2. RNA Biol. 8:850–860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia Y, Chen Q, Zhong Z, Xu C, Wu C, Liu B
and Chen Y: Down-regulation of miR-30c promotes the invasion of
non-small cell lung cancer by targeting MTA1. Cell Physiol Biochem.
32:476–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong J, Liu R, Zhuang R, Zhang Y, Fang L,
Xu Z, Jin L, Wang T, Song C, Yang K, et al: miR-30c-1* promotes
natural killer cell cytotoxicity against human hepatoma cells by
targetingthe transcription factor HMBOX1. Cancer Sci. 103:645–652.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou H, Xu X, Xun Q, Yu D, Ling J, Guo F,
Yan Y, Shi J and Hu Y: microRNA-30c negatively regulates
endometrial cancer cells by targeting metastasis-associated gene-1.
Oncol Rep. 27:807–812. 2012.PubMed/NCBI
|
|
19
|
Gils A and Declerck PJ: The structural
basis for the pathophysiological relevance of PAI-I in
cardiovascular diseases and the development of potential PAI-1
inhibitors. Thromb Haemost. 91:425–437. 2004.PubMed/NCBI
|
|
20
|
Yildiz Yasar S, Kuru P, Oner Toksoy E and
Agirbasli M: Functional stability of plasminogen activator
inhibitor-1. ScientificWorldJournal. 2014:8582932014.PubMed/NCBI
|
|
21
|
Zhou A, Huntington JA, Pannu NS, Carrell
RW and Read RJ: How vitronectin binds PAI-1 to modulate
fibrinolysis and cell migration. Nat Struct Biol. 10:541–544. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wind T, Hansen M, Jensen JK and Andreasen
PA: The molecular basis for anti-proteolytic and non-proteolytic
functions of plasminogen activator inhibitor type-1: Roles of the
reactive centre loop, the shutter region, the flexible joint region
and the small serpin fragment. Biol Chem. 383:21–36. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cesari M, Pahor M and Incalzi RA:
Plasminogen activator inhibitor-1 (PAI-1): A key factor linking
fibrinolysis and age-related subclinical and clinical conditions.
Cardiovasc Ther. 28:e72–e91. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fay WP, Eitzman DT, Shapiro AD, Madison EL
and Ginsburg D: Platelets inhibit fibrinolysis in vitro by both
plasminogen activator inhibitor-1-dependent and-independent
mechanisms. Blood. 83:351–356. 1994.PubMed/NCBI
|
|
25
|
Kozlova N, Jensen JK, Chi TF, Samoylenko A
and Kietzmann T: PAI-1 modulates cell migration in a LRP1-dependent
manner via β-catenin and ERK1/2. Thromb Haemost. 113:988–998. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Czekay RP, Wilkins-Port CE, Higgins SP,
Freytag J, Overstreet JM, Klein RM, Higgins CE, Samarakoon R and
Higgins PJ: PAI-1: An integrator of cell signaling and migration.
Int J Cell Biol. 2011:5624812011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Placencio VR, Miyata T and DeClerck YA:
Pharmacologic inhibition of PAI-1 increases apoptosis and inhibits
macrophage migration in cancer. Cancer Research. 73:Abstract 1548.
2013. View Article : Google Scholar
|
|
28
|
Loskutoff DJ, Curriden SA, Hu G and Deng
G: Regulation of cell adhesion by PAI-1. Apmis. 107:54–61. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee CC and Huang TS: Plasminogen activator
inhibitor-1: The expression, biological functions, and effects on
tumorigenesis and tumor cell adhesion and migration. J Cancer Mole.
1:25–36. 2005.
|
|
30
|
Erem C, Ersoz HO, Karti SS, Ukinç K,
Hacihasanoglu A, Değer O and Telatar M: Blood coagulation and
fibrinolysis in patients with hyperthyroidism. J Endocrinol Invest.
25:345–350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Binder BR, Christ G, Gruber F, Grubic N,
Hufnagl P, Krebs M, Mihaly J and Prager GW: Plasminogen activator
inhibitor 1: Physiological and pathophysiological roles. News
Physiol Sci. 17:56–61. 2002.PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Somigliana E, Vigano' P, Parazzini F,
Stoppelli S, Giambattista E and Vercellini P: Association between
endometriosis and cancer: A comprehensive review and a critical
analysis of clinical and epidemiological evidence. Gynecol Oncol.
101:331–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Witz CA: Current concepts in the
pathogenesis of endometriosis. Clin Obstet Gynecol. 42:566–585.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laschke MW and Menger MD: In vitro and in
vivo approaches to study angiogenesis in the pathophysiology and
therapy of endometriosis. Hum Reprod Update. 13:331–342. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Casslen B, Urano S and Ny T: Progesterone
regulation of plasminogen activator inhibitor 1 (PAI-1) antigen and
mRNA levels in human endometrial stromal cells. Thromb Res.
66:75–87. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bruse C, Radu D and Bergqvist A: In situ
localization of mRNA for the fibrinolytic factors uPA, PAI-1 and
uPAR in endometriotic and endometrial tissue. Mol Hum Reprod.
10:159–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bruchova H, Merkerova M and Prchal JT:
Aberrant expression of microRNA in polycythemia vera.
Haematologica. 93:1009–1016. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tanic M, Yanowsky K, Rodriguez-Antona C,
Andrés R, Márquez-Rodas I, Osorio A, Benitez J and Martinez-Delgado
B: Deregulated miRNAs in hereditary breast cancer revealed a role
for miR-30c in regulating KRAS oncogene. PloS One. 7:e388472012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bandrés E, Cubedo E, Agirre X, Malumbres
R, Zárate R, Ramirez N, Abajo A, Navarro A, Moreno I, Monzó M and
García-Foncillas J: Identification by real-time PCR of 13 mature
microRNAs differentially expressed in colorectal cancer and
non-tumoral tissues. Mol Cancer. 5:292006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang G, Zhang H, He H, Tong W, Wang B,
Liao G, Chen Z and Du C: Up-regulation of microRNA in bladder tumor
tissue is not common. Int Urol Nephrol. 42:95–102. 2010. View Article : Google Scholar : PubMed/NCBI
|