Introduction
Heterotopic ossification is the pathological
formation of lamellar bone in the soft tissue outside the skeleton
(1). Heterotopic ossification occurs
frequently following severe head injury, spinal injury,
non-traumatic intracranial lesions and long-term coma, and the
etiology of heterotopic ossification has been classified as
neurogenic, traumatic and hereditary (2). It is a debilitating condition as soft
tissues, such as muscle, tendon and ligaments convert into
cartilage and bone, leading to immobility (3). Heterotopic ossification typically
occurs following severe head injury, spinal injury, non-traumatic
intracranial lesion and long-term coma. However, the occurrence of
excessive, symptomatic heterotopic ossification bilaterally around
the hips and knees is rarely described in the literature (2).
In the recovery from articular cartilage injury, the
cartilage exhibits little capacity for self-repair and regeneration
(4). Bone marrow-derived mesenchymal
stem cells (BMSCs) are able to facilitate the regeneration of
cartilage (5). Studies using animal
models have demonstrated that transplanted BMSCs are able to
improve the repair of damaged bone, tendons and ligaments in
vivo (6,7).
The most potent growth factors for enhancing bone
formation are the bone morphogenetic proteins (BMPs), including
BMP-7 and BMP-2 (8). BMP-7 induces
osteoblast-like genes and matrix mineralization in primary
mesenchymal stem cell (MSC) cultures. The appropriate biological
and mechanical properties of scaffold materials are important in
the attachment and differentiation of MSCs (9).
Wnt/β-catenin signaling is activated following the
binding of Wnt ligands to the frizzled family of receptors
(10). β-catenin signaling helps to
regulate the differentiation of pluripotent stem cells into the
cartilage lineage during fracture healing (11). Wnt signaling regulates the
development of cartilage from MSCs and improves the efficiency of
bone tissue engineering. Fromigue (12) previously demonstrated that the Wnt
signaling pathway was involved in strontium-induced proliferation
and differentiation of murine cartilage.
As Wnt/β-catenin signaling is important in cartilage
development, the present study supplemented the cartilage
differentiation culture media of rabbit MSCs (rMSCs) with BMP-7 to
activate Wnt/β-catenin signaling. The results of the current study
provide novel insights into the mechanism by which BMP-7 regulates
the differentiation of MSCs into cartilage through the
Wnt/β-catenin signaling pathway.
Materials and methods
Reagents
BMP-7 was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Antibodies targeting β-actin (cat no. 12262),
GAPDH (cat no. 5174) alkaline phosphatase (ALP; cat no. 8681),
runt-related transcription factor 2 (Runx2; cat no. 8486), cluster
of differentiation (CD)34 (cat no. 3569), CD44 (cat no. 3516),
β-catenin (cat no. 11887), glycogen synthase kinase 3β (GSK3β; cat
no. 94331) and phosphorylated-GSK3β (cat no. 9322) and
peroxidase-conjugated secondary antibody against immunoglobulin
(Ig)G (cat no. 9087) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). β-catenin signaling inhibitor XAV-939 was
purchased from Sigma-Aldrich (Merck KGaA).
Isolation and culture of rMSCs
The rMSCs were obtained from a male neonatal New
Zealand white rabbit (0.75 kg, 1 month old). Rabbits were allowed
free access to food and water at 25°C and 50–60% humidity with a 12
h light/dark cycle. The rabbit was purchased from Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China). The
present study was carried out in strict accordance with the
Guidelines on the Care and Use of Laboratory Animals issued by the
Chinese Council on Animal Research and the Guidelines of Animal
Care. Bone marrow (32 ml) was aspirated from the iliac crest of the
rabbit following sacrifice. The bone marrow was flushed with low
glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) using a 1-ml syringe.
The bone marrow-PBS mixture (8 ml) was centrifuged for 5 min at
1,200 × g and 20°C (Labofuge 400R; Thermo Fisher Scientific, Inc.)
and the supernatant was removed. Pellets were washed with PBS (8
ml) and centrifuged at 1,200 × g again. The resultant cells were
plated in a culture dish, and incubated at 37°C with 5% CO2 for 4
days. The animal protocol was approved by the Inner Mongolia
Medical University Experimental Animal Management Committee (Inner
Mongolia, China).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide (MTT)
assay
To determine the cell growth percentage, an MTT
assay was performed. Cells were subsequently divided into the
following groups: Control cells and BMP-7-treated cells (final
concentration 400, 500, 600, 700 or 800 ng/ml). Cells in the BMP-7
groups were grown in 96-well plates (1×103 cells/well,
37°C for 1, 2, 3, 4, 5 and 6 days) supplemented with different
concentrations of BMP-7 (400, 500, 600, 700 or 800 ng/ml). Control
cells were stored in DMEM containing 0.1% dimethyl sulfoxide
(DMSO). At 1, 2, 3, 4, 5 and 6 days following BMP-7 treatment, a
total of 20 µl of MTT was added to each well to give final
concentration of 0.5%. Cells were incubated for 4 h at 37°C in the
dark and 150 µl DMSO was added to each well for 10 min to dissolve
the formazan crystals. The absorbance was measured using a
microplate reader (EXL800; Cole-Parmer, Vernon Hills, IL, USA) at
490 nm. All experiments were repeated three times. The viability of
the BMP-7 treated cells was expressed as the percentage of
population growth plus the standard error of the mean relative to
that of untransfected control cells. Cell growth was calculated as
follows: growth percentage = (mean experimental absorbance-mean
control absorbance)/mean control absorbance ×100.
Immunofluorescence
The rMSCs, were fixed in 3.7% paraformaldehyde at
room temperature for 30 min. rMSCs were then blocked with 1% bovine
serum albumin (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) in PBS with 10% goat serum (Sigma-Aldrich; Merck KGaA)
overnight at 4°C. The samples were subsequently incubated with
primary antibodies (CD44, 1:500, and CD34, 1:800) diluted in PBS at
37°C for 2 h. Primary antibody binding was detected using an IgG
(H+L) secondary antibody (cat no. 9087; 1:2,000; Cell Signaling
Technology, Inc.) at 37°C for 1 h. The fluorescence images were
captured using a fluorescence microscope.
Western blot analysis
Total proteins were extracted from rMSCs using a
Beyotime Cell Protein Extraction kit (Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer's
protocol. Proteins (20 µg) were separated by 12% SDS-PAGE and
transferred to nitrocellulose membranes for immunoblotting.
Membranes were blocked with 5% fat-free milk for 30 min at room
temperature in PBS buffer containing 0.1% Tween-20, and
subsequently incubated with primary antibodies against β-actin
(1:2,000), ALP (1:800), Runx2 (1:1,000), β-catenin (1:1,500), GSK3β
(1:1,000) and phosphorylated-GSK3β (1:8,00) overnight at 4°C. The
membranes were subsequently washed three times with PBS, and then
incubated with peroxidase-conjugated secondary antibodies IgG (cat
no. 9087; 1:4,000; Cell Signaling Technology, Inc.) at 37°C for 1
h. ECL reagents were used to visualize the results (Pierce; Thermo
Fisher Scientific, Inc.; cat no. 32132).
Cartilage differentiation
The rMSCs were plated at a density of 5,000
cells/cm2 and exposed to standard
differentiation-inducing media (cat no. D4902; Sigma-Aldrich; Merck
KGaA) for 21 days. The medium (10 mg/ml transforming growth factor
b1 and BMP-7) was refreshed twice per week. Cartilage
differentiation was achieved following standard in vitro
protocols (13). Endothelial
differentiation was stimulated by culturing the cells in
endothelial growth medium-2 (Sigma-Aldrich; Merck KGaA) at 37°C for
2 h.
Statistical analysis
One-way analysis of variance was used to complete
the statistical analysis with GraphPad Prism version 5 software
(GraphPad Software, La Jolla, CA, USA). Unpaired Student's t-tests
were used as appropriate to evaluate the statistical significance
of differences between two group means. Data are presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Identification of rMSCs
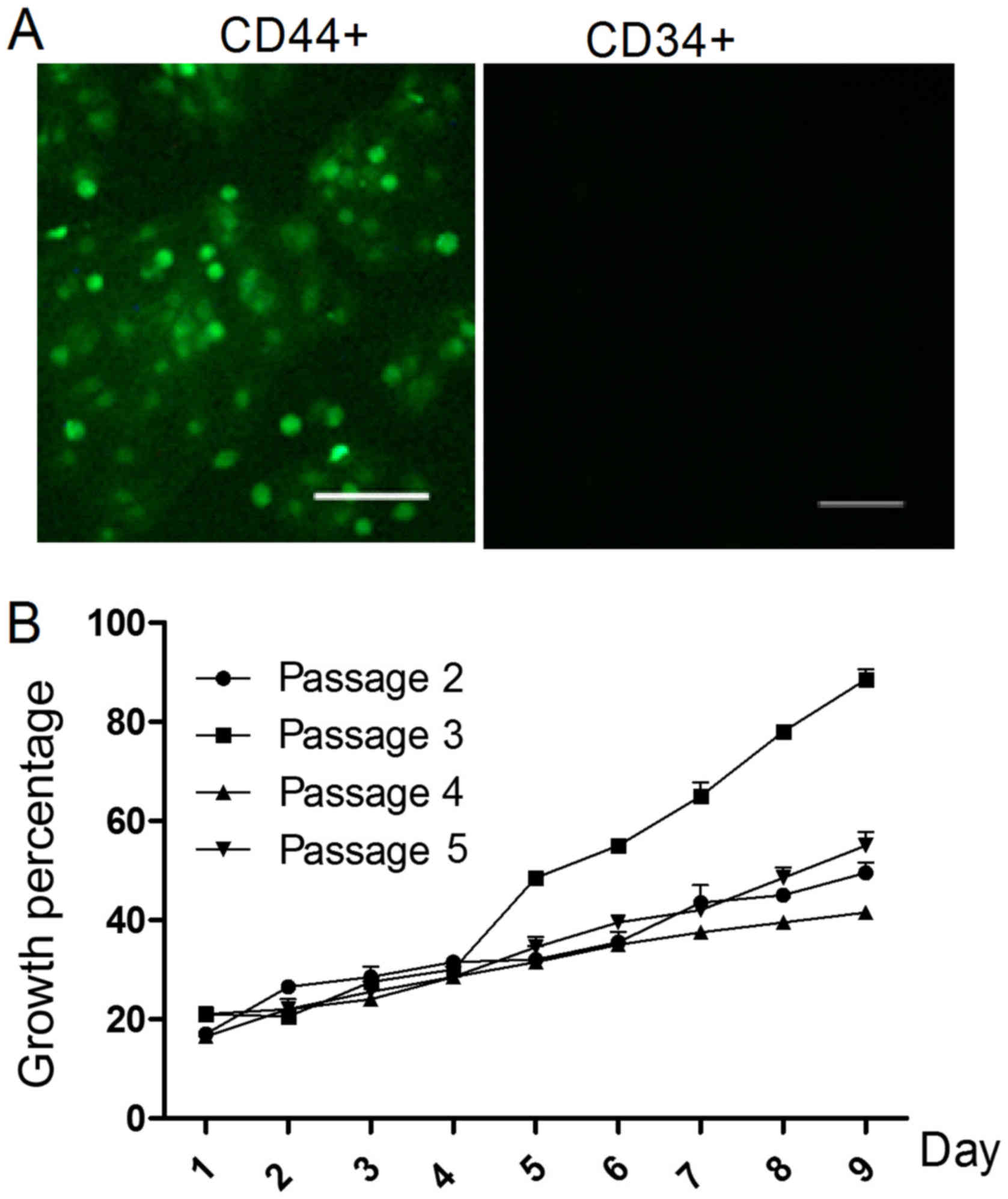
The morphology of the rMSCs was observed using a
microscope. On day 10, cells reached 80% confluence. To identify
the rMSCs, the expression of CD34 and CD44 was evaluated.
Observation under a fluorescence microscope detected no expression
of CD34, but CD44 was observed as green fluorescence (Fig. 1A).
From the second to the fifth cell passages, the
growth status was analyzed. As presented in Fig. 1B, the cells demonstrated the greatest
ability to proliferate in the third passage (P<0.05).
Optimal concentration and time for
BMP-7-induced effects on the differentiation of rMSCs into
cartilage
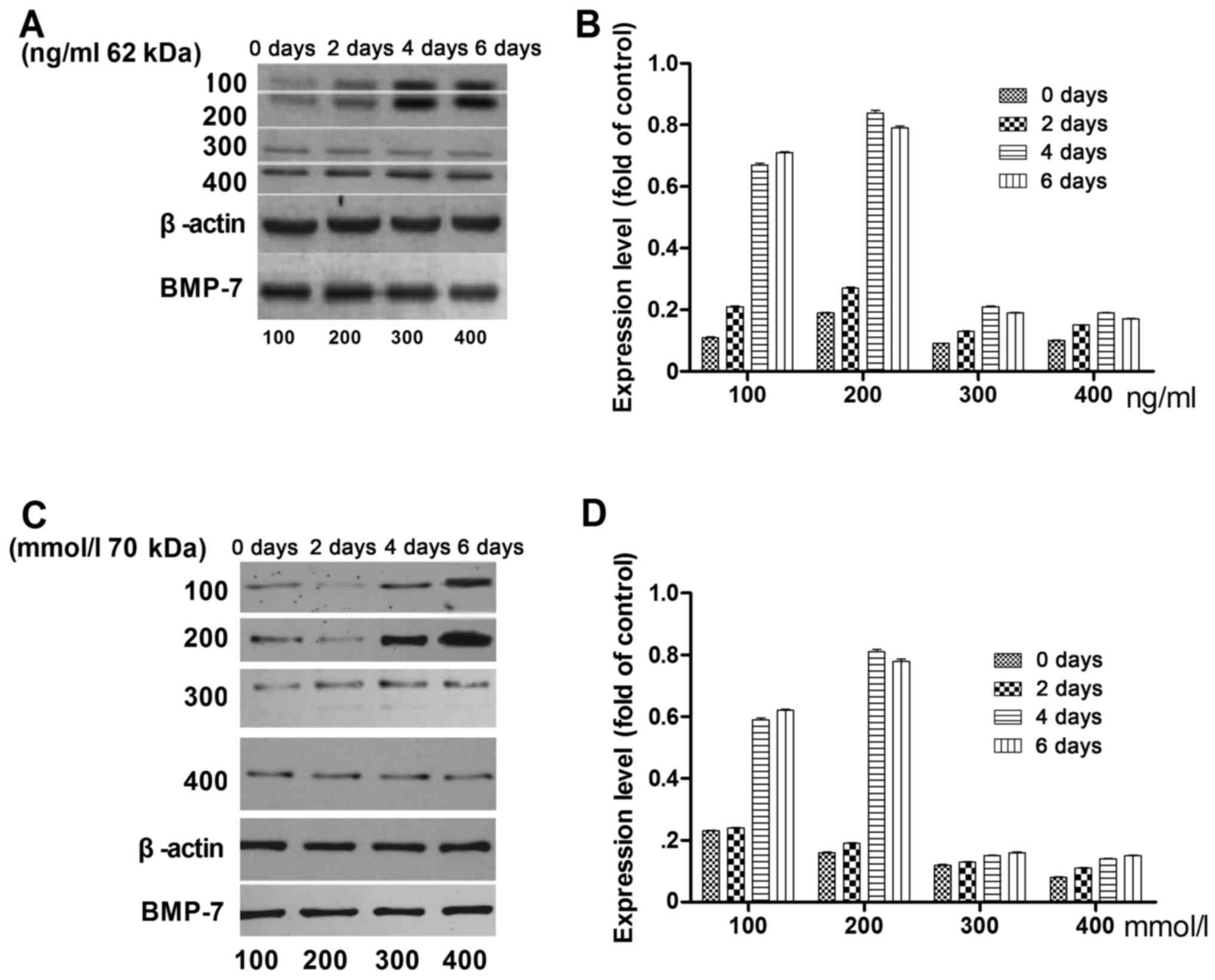
The optimal concentration and application time of
BMP-7 in the differentiation of rMSCs into cartilage were
investigated. Fig. 2 presents the
expression levels of Runx2 (Fig. 2A)
and ALP (Fig. 2B) on days 0, 2, 4
and 6 with BMP-7 concentrations of 100, 200, 300, and 400 ng/ml.
The highest expression level for both Runx2 and ALP was observed on
day 4 with 200 ng/ml BMP-7.
BMP-7 promotes the differentiation of
rMSCs into cartilage via the Wnt/β-catenin pathway
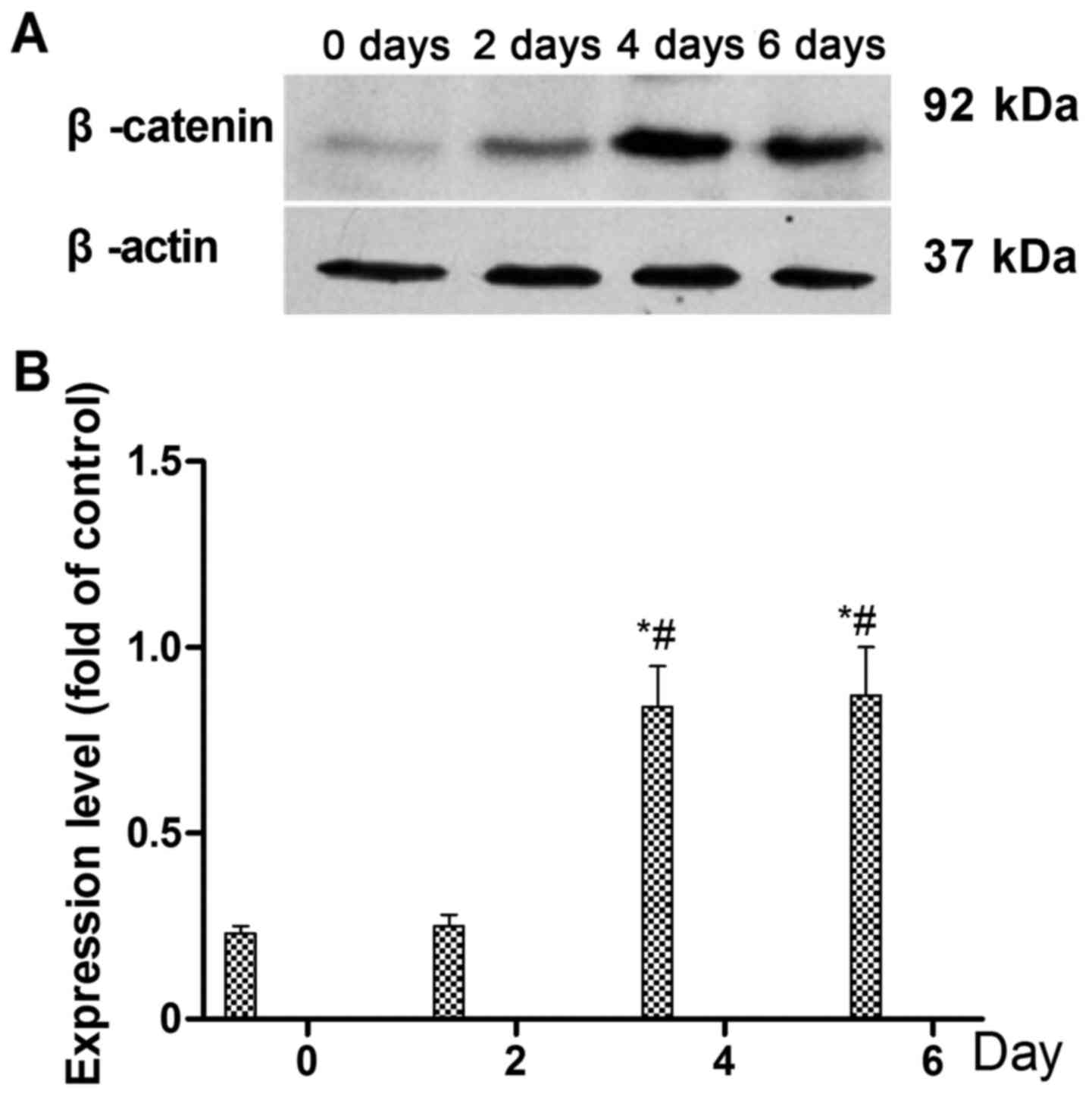
To identify the signaling pathway through which
BMP-7 acts to promote the differentiation of rMSCs into cartilage,
cells were treated with 200 ng/ml of BMP-7 for 0, 2, 4 and 6 days,
and the expression level of β-catenin was examined by western
blotting on days 0, 2, 4 and 6 (Fig.
3A). The results shown in Fig.
3B indicate that the expression of β-catenin was significantly
increased on day 4 and 6, compared with that on days 0 and 2
(P<0.05; Fig. 3B). The
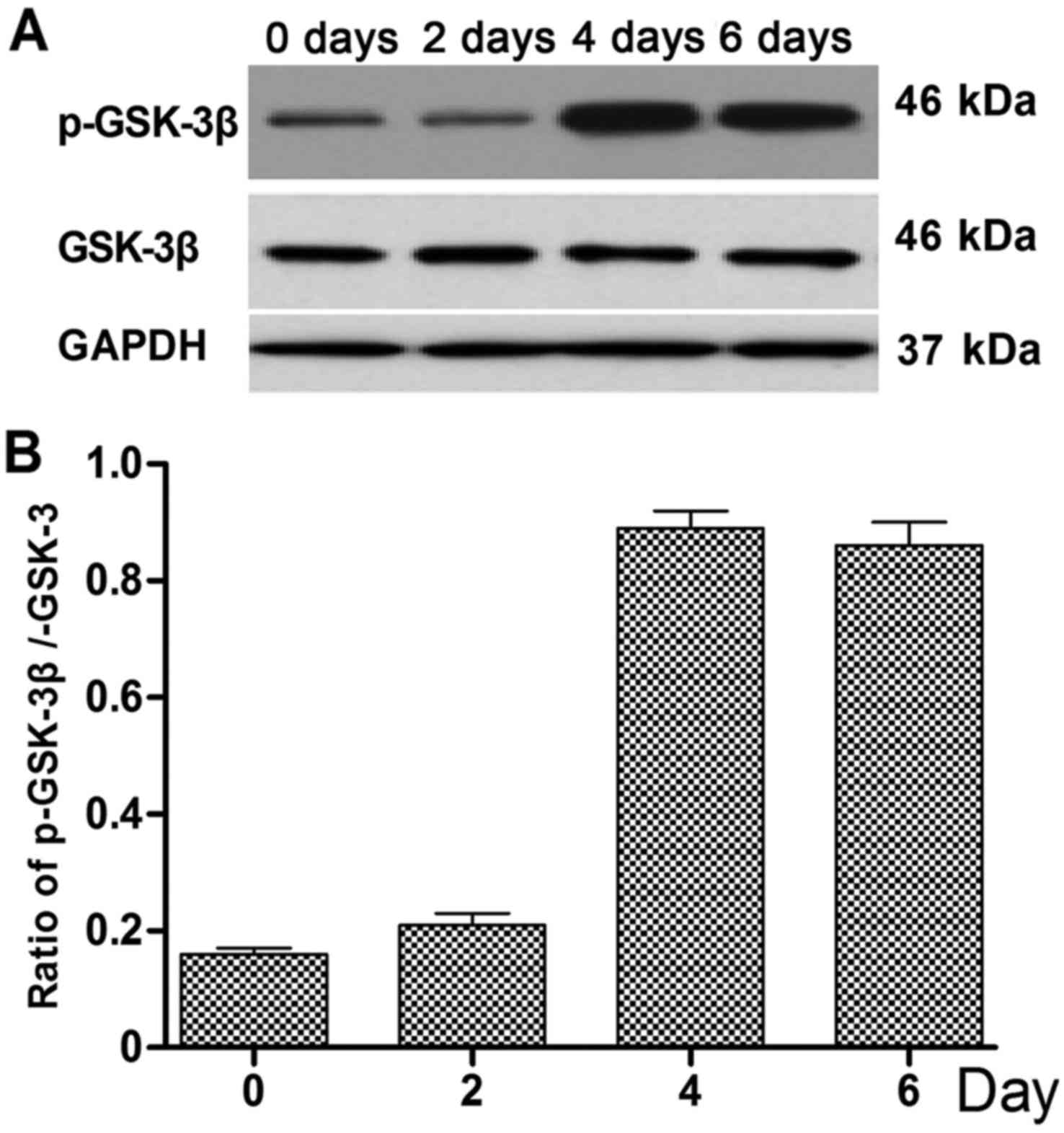
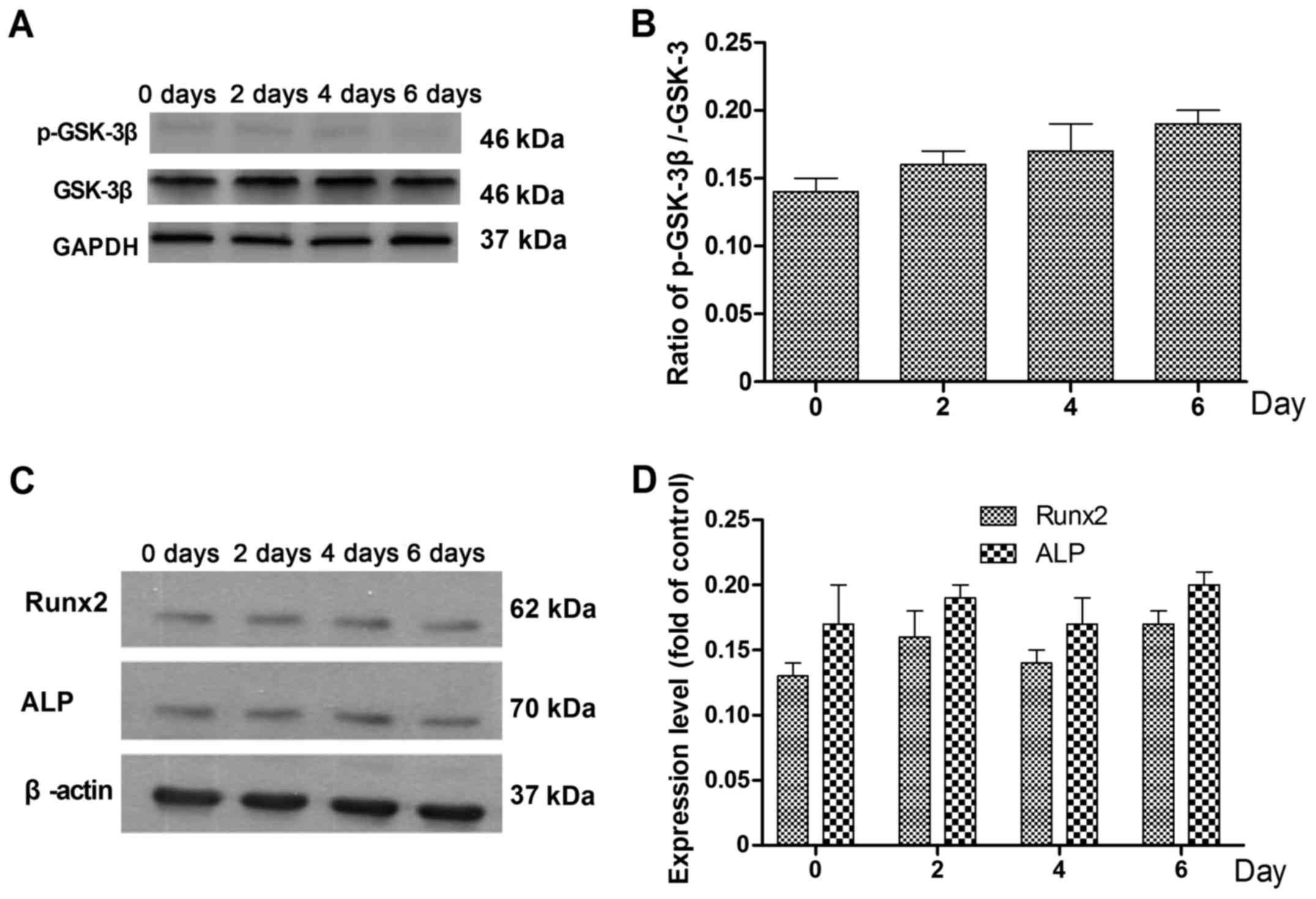
phosphorylation of GSK-3β was also detected by western blotting
(Fig. 4A), and was found to be
increased on days 4 and 6 compared with days 0 and 2 (Fig. 4B).
To verify that BMP-7 promotes the differentiation of
rMSCs into cartilage via the Wnt/β-catenin pathway, the β-catenin
signaling inhibitor XAV-939 (1.0 µM) was used to inhibit
Wnt/β-catenin activity. Following the addition of XAV-939, the
cells were treated with BMP-7 for 0, 2, 4 and 6 days. The
phosphorylation of GSK-3β in the presence of XAV-939 (Fig. 5A and B) was decreased compared with
that measured in the absence of XAV-939. In addition, the
expression levels of ALP and Runx2 were also decreased in the
presence of XAV-939 (Fig. 5C and D)
compared with those in its absence.
Discussion
Identification of a trigger for the differentiation
of rMSCs into cartilage may assist the development of novel
therapeutic approaches for the treatment of heterotopic
ossification (14). The present
study demonstrates that BMP-7 promoted the differentiation of rMSCs
into cartilage via the Wnt/β-catenin pathway.
The CD44 and CD34 markers to identify the rMSCs were
verified via immunofluorescence (15). Observation under a fluorescence
microscope indicated that there was no expression of CD34, whereas
CD44 was clearly expressed. Cell surface markers are key to
identifying BMSCs (16). The BMSC
preparations in the present study were positive for CD44 and
negative for hematopoietic markers and endothelial markers such as
CD34. Immunofluorescence confirmed that all cells were positive for
expressions of CD44 and negative for CD34, thus confirming BMSCs.
The optimal BMP-7 concentration and application time for the
differentiation rMSCs into cartilage in vitro was
investigated, and was found to be 200 ng/ml BMP-7 with 4 days
incubation.
Furthermore, the mechanism and signaling pathway by
which BMP-7 promotes the differentiation of rMSCs was evaluated.
The Wnt/β-catenin pathway serves an important role in cell activity
(15). The results suggested that
BMP-7 promoted rMSC differentiation via the Wnt/β-catenin pathway.
Following 4 days of treatment with BMP-7 (200 ng/ml),
phosphorylation of GSK-3β was stimulated and the expression of
β-catenin, ALP and Runx2 was increased. Previous studies have
reported that PI3K-activated Akt is able to phosphorylate GSK-3β at
Ser9, thereby inactivating GSK-3β and triggering related signaling
pathways (17,18). Furthermore, inhibiting β-catenin
signaling with XAV-939 suppressed the BMP-7-mediated changes
observed in the rMSCs. In recent years, GSK-3β has been reported to
serve important roles in regulating osteoblast differentiation
(17). Several researchers have
shown that inhibition of GSK-3β promotes osteogenic differentiation
of mesenchymal progenitors but not adipogenic differentiation
(17) and found that GSK-3β
inactivation upon receptor activator of NF-kB ligand (RANKL)
stimulation is crucial for osteoclast differentiation (18). The present study has limitations. For
instance, only the Wnt/β-catenin pathway was considered; future
studies should investigate additional cell pathways. The results of
the present study demonstrate that BMP-7 accelerates the
differentiation of rMSCs into cartilage via the Wnt/β-catenin
pathway. These findings may provide a basis for the development of
BMP-7 treatments for patients with cartilage degeneration.
Acknowledgements
This study was supported by the Natural Science
Foundation of Inner Mongolia of China (grant no. 2014MS08103).
References
|
1
|
Alport B, Horne D and Burbridge B:
Heterotopic ossification of the quadratus lumborum muscle. J Radiol
Case Rep. 8:41–46. 2014.PubMed/NCBI
|
|
2
|
Convente MR, Wang H, Pignolo RJ, Kaplan FS
and Shore EM: The immunological contribution to heterotopic
ossification disorders. Curr Osteoporos Rep. 13:116–124. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Edwards DS and Clasper JC: Heterotopic
ossification: A systematic review. J R Army Med Corps. 161:315–321.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng H, Martin JA, Duwayri Y, Falcon G
and Buckwalter JA: Impact of aging on rat bone marrow-derived stem
cell chondrogenesis. J Gerontol A Biol Sci Med Sci. 62:136–148.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang C, Jin C, Du X, Yan C, Min BH, Xu Y
and Wang L: An autologous bone marrow mesenchymal stem cell-derived
extracellular matrix scaffold applied with bone marrow stimulation
for cartilage repair. Tissue Eng Part A. 20:2455–2462. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi J, Zhang X, Zeng X, Zhu J, Pi Y, Zhou
C and Ao Y: One-step articular cartilage repair: Combination of in
situ bone marrow stem cells with cell-free
poly(L-lactic-co-glycolic acid) scaffold in a rabbit model.
Orthopedics. 35:e665–e671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maeda S, Fujitomo T, Okabe T, Wakitani S
and Takagi M: Shrinkage-free preparation of scaffold-free
cartilage-like disk-shaped cell sheet using human bone marrow
mesenchymal stem cells. J Biosci Bioeng. 111:489–492. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lavery K, Hawley S, Swain P, Rooney R,
Falb D and Alaoui-Ismaili MH: New insights into BMP-7 mediated
osteoblastic differentiation of primary human mesenchymal stem
cells. Bone. 45:27–41. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burastero G, Scarfì S, Ferraris C, Fresia
C, Sessarego N, Fruscione F, Monetti F, Scarfò F, Schupbach P,
Podestà M, et al: The association of human mesenchymal stem cells
with BMP-7 improves bone regeneration of critical-size segmental
bone defects in athymic rats. Bone. 47:117–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aguilar JS, Begum AN, Alvarez J, Zhang XB,
Hong Y and Hao J: Directed cardiomyogenesis of human pluripotent
stem cells by modulating Wnt/β-catenin and BMP signalling with
small molecules. Biochem J. 469:235–241. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
de Boer J, Siddappa R, Gaspar C, van
Apeldoorn A, Fodde R and van Blitterswijk C: Wnt signaling inhibits
osteogenic differentiation of human mesenchymal stem cells. Bone.
34:818–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fromigué O, Marie PJ and Lomri A: Bone
morphogenetic protein-2 and transforming growth factor-beta2
interact to modulate human bone marrow stromal cell proliferation
and differentiation. J Cell Biochem. 68:411–426. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Culbert AL, Chakkalakal SA, Theosmy EG,
Brennan TA, Kaplan FS and Shore EM: Alk2 regulates early
chondrogenic fate in fibrodysplasia ossificans progressiva
heterotopic endochondral ossification. Stem Cells. 32:1289–1300.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kara M, Ekiz T, Öztürk GT, Onat ŞŞ and
Özçakar L: Heterotopic Ossification and Peripheral Nerve
Entrapment: Ultrasound is a Must-use Imaging Modality. Pain Med.
16:1643–1644. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang LL, Liu JJ, Liu F, Liu WH, Wang YS,
Zhu B and Yu B: MiR-499 induces cardiac differentiation of rat
mesenchymal stem cells through wnt/β-catenin signaling pathway.
Biochem Biophys Res Commun. 420:875–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W, Zhang F, Shi H, Tan R, Han S, Ye
G, Pan S, Sun F and Liu X: Comparisons of rabbit bone marrow
mesenchymal stem cell isolation and culture methods in vitro. PLoS
One. 9:e887942014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Cai J, Cai XH and Chen L: miR-346
regulates osteogenic differentiation of human bone marrow-derived
mesenchymal stem cells by targeting the Wnt/β-catenin pathway. PLoS
One. 8:e722662013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kramer I, Halleux C, Keller H, Pegurri M,
Gooi JH, Weber PB, Feng JQ, Bonewald LF and Kneissel M: Osteocyte
Wnt/beta-catenin signaling is required for normal bone homeostasis.
Mol Cell Biol. 30:3071–3085. 2010. View Article : Google Scholar : PubMed/NCBI
|