Introduction
The treatment of bone defects caused by trauma and
tumors, in particular radial bone defect, is challenging in
clinical practice. Approximately 800,000 bone repair surgeries with
bone grafting are performed each year in the US alone. In China,
>30 million bone defect cases are caused by trauma each year
(1). However, the bone substitutes
currently in use do not adequately combine the advantages of
biocompatibility, high mechanical strength, easy degradation and
low cost (2). With the developments
in tissue engineering, tissue-engineered bones are being widely
used as an alternative to allografts and autografts due to their
low immunogenicity, absence of secondary trauma and easy
availability (3,4). Different scaffolds made of a single
biomaterial have respective advantages and defects in vitro
and in vivo. To address this problem, a compounding
technique that adjusts the proportion of each material and the way
of forming the composite is currently applied (5,6).
Budiraharjo et al (7)
co-cultured a chitosan (CS) scaffold coated with
nano-hydroxyapatite (nHA), and the stem cells and scaffold
demonstrated excellent biocompatibility and osteoinductive
capacity. Furthermore, Alves da Silva et al prepared a
composite scaffold by polymerization of CS and silk fibroin (SF),
which was then used for co-culture of bone marrow stem cells. This
composite scaffold was capable of inducing chondrocyte
differentiation (8).
An SF/CS composite scaffold was applied in the
repair of knee joint defects in rabbits in a preliminary study
(9). On the basis of good
biocompatibility and appropriate degradation rates, the SF/CS/nHA
composite scaffold with improved performance was prepared in the
present study and used for the repair of defect in rabbit radial
bone. The osteogenic capacity and mechanism were discussed in the
present study using X-ray scanning and pathological
observation.
Materials and methods
Materials
A total of 45 New Zealand white rabbits (age, 2
months; 24 females and 21 males; weight, 2.5±3.0 kg) were obtained
from the Zunyi Medical College Animal Experiment Center (Zunyi,
China). Animals were housed at 15°C and 45% humidity, with ad
libitum access to standard rabbit food and water. The
experiments were conducted at the Central Laboratory of Zunyi
Medical College. The study design involved randomized grouping of
animals and in vivo experiments. The study was approved by
the Ethics Committee of Zunyi Medical College.
Preparation of SF/CS/nHA composite
scaffold
The SF/CS/nHA scafford was prepared as previously
described (10). SF, CS and nHA were
prepared into 2% solutions and then combined in three proportions,
namely 1:1:0.5, 1:1:1 and 1:1:1.5. The mixture was stirred at 55°C
using a magnetic stirrer until the bubbles disappeared and the
mixing was uniform. Next, the mixture was transferred to a 9-well
plate using a 20 ml syringe (~50 ml/well) and frozen at −80°C for
24 h. The frozen scaffolds transformed from fluid into solid state
but still contained ice, so were sealed with parafilm. A pattern of
holes was made on the parafilm so that the water could be absorbed.
The scaffolds were placed in a vacuum drier for 36 h. The scaffolds
were then removed and soaked in a mixture of 75% methanol and 1
mol/l sodium hydroxide for 15 h. Subsequently, the scaffolds were
repeatedly washed with ultra-pure water and dried again in the
vacuum drier. The scaffolds were then placed for 10 h in a solution
of crosslinking agents, containing
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and
N-hydroxysuccinimide. After rinsing, the wet composite
scaffolds were frozen at −80°C for 24 h, followed by vacuum drying
for 36 h. Finally, the prepared composite scaffolds were sealed for
later use (11,12).
Modeling
A total of 45 New Zealand white rabbits were fasted
from food and water for 6 h before surgery. Anesthesia was induced
by intravenous injection of 2.5% sodium pentobarbital via the ear
margin. The rabbits were immobilized in the four limbs to prevent
damage to the vessels or nerves caused by body twitching during
surgery. Next, the right forelimb was exposed, the skin was
disinfected conventionally with iodophor and alcohol, and incised
from the midpoint of the radial bone. Dissection was performed
layer by layer with caution in order not to damage the vessels or
nerves. In the exposed radial bone, a defect of approximately 2 cm
in length was performed using a sterilized saw blade, as previously
described (13–15). After the radial bone defect was
performed, rabbits were divided into three groups (n=15 each) and
treated as follows: Group A, in which SF/CS/nHA composite scaffold
was implanted; group B, in which SF/CS scaffold was implanted,
serving as the negative control group; and group C, in which no
scaffold was implanted, serving as the blank control group
(16). The incision was sutured
layer by layer and disinfected with iodophor following surgery, and
wrapped with gauze without applying external fixation. To prevent
postoperative infection, penicillin (500,000 U) and streptomycin
(0.1 g) were injected intramuscularly. The rabbits were kept in
separate cages subsequent to surgery.
Rough observation and X-ray
scanning
The diet and mobility of rabbits were observed
regularly subsequent to surgery, and the incisions were checked for
reddening and infection. At 4, 6, 8 and 12 weeks after surgery, the
rabbits were immobilized to obtain anteroposterior X-ray scans of
the radial bone. Bony calluses and replacement of the scaffold by
bone tissues were observed in X-ray scans. Rabbits were sacrificed
and bone tissues were harvested at different times postoperatively
(4, 6, 8 and 12 weeks after surgery, n=3 at each time point) and
made into pathological specimens to evaluate the repair of bone
defects, the scaffold association with the surrounding tissues and
the absorption of the scaffold.
Histopathological observation
Vertical and transverse sections were prepared for a
comprehensive observation. Briefly, the specimens were fixed with
4% formaldehyde, decalcified and sliced into 4-µm sections. Next,
specimens were dewaxed in xylene, dehydrated through an ethanol
gradient, washed with water and air dried for 24 h. Pathological
observation was based on hematoxylin-eosin (HE) and toluidine blue
staining. The sections were dehydrated, sealed and observed under
an inverted microscope to evaluate the growth of normal bone
tissues, osteogenic capacity and degradation of the scaffold. The
lesions in the surrounding tissues were also observed. Lane-Sandhu
histologic criteria were used (17)
i) Healing: None, 0; fiber, 1; fibrocartilage, 2; bone, 3;
trabecular and cortical bone, 4. ii) Callus: None, 0; small amount
of callus, 1; medium callus, 2; large amount of callus, 3; fully
callus, 4. iii) Bone marrow: No bone marrow, 0, bone marrow
started, 1; >50% of the defect area has bone marrow, 2; complete
marrow, 3; mature marrow, 4.
Statistical analysis
The results of rough observation, X-ray scan and
pathological observation were analyzed using SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA). F-test was conducted to
compare the mean values of multiple samples. Differences with
P<0.05 were considered to be statistically significant.
Results
Postoperative conditions and
observations
Rabbits were provided with food 12 h after surgery
in each group. The incisions healed within 1 week without severe
infection, reddening or festering. At 1 week after surgery, the
rabbits in groups A and B appeared to be more active compared with
those in group C, while the mobility was moderate in all groups 3
weeks later. As indicated by pathological observation, the
scaffolds in groups A and B at 4 weeks after surgery were enclosed
in fibrous connective tissues. The scaffolds under the periosteum
presented signs of calcification and a grayish white color. Little
difference was observed between groups A and B with the naked eye.
At 8 weeks, the scaffold was gradually replaced by bony calluses in
groups A and B. The scaffolds of group B had a light yellow color.
Between weeks 12 and 16, osseointegration with a similar color and
texture as that of the normal bone tissues was observed in group A.
In group B, the scaffolds had a light yellow color, similar to that
of the cartilage. Although the broken ends of the bone presented
slight elongation at the early stage in group C, no
osseointegration was observed and the defect was filled with soft
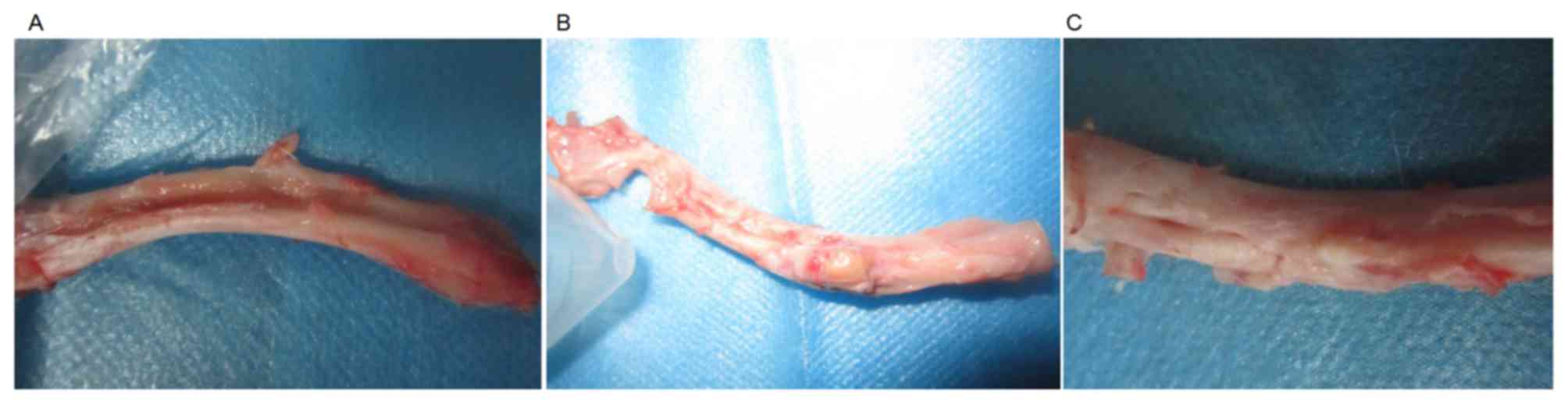
tissues during the entire observational period. Fig. 1 shows the radial bone defect of each
group at 16 weeks after surgery.
Osteogenic capacity evaluated by X-ray
scanning in each group
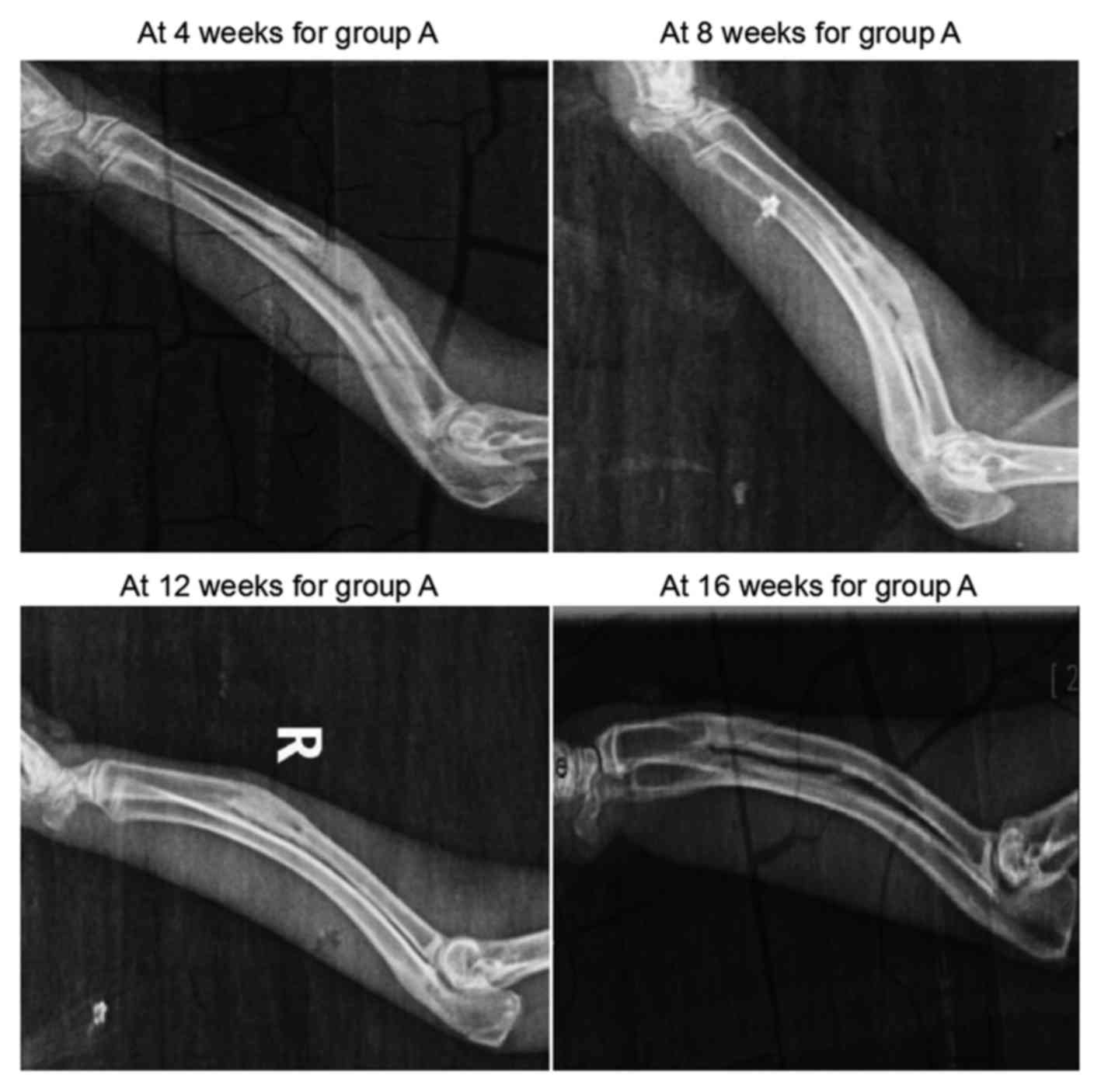
The results of X-ray examination conducted in group
A are shown in Fig. 2. At 4 weeks
after surgery, the scaffolds in the defect region presented with a
grayish white high-density shadow. At 8 weeks after surgery, the
calcified shadow was darkened with distinct boundary from the
surrounding soft tissues. At 12 weeks, the marrow cavity was
unobstructed partially between the two broken ends. Finally, at 16
weeks, no evident disparity was observed on the X-ray scans between
the defect region and the normal bone tissue, while the marrow
cavity was completely unobstructed.
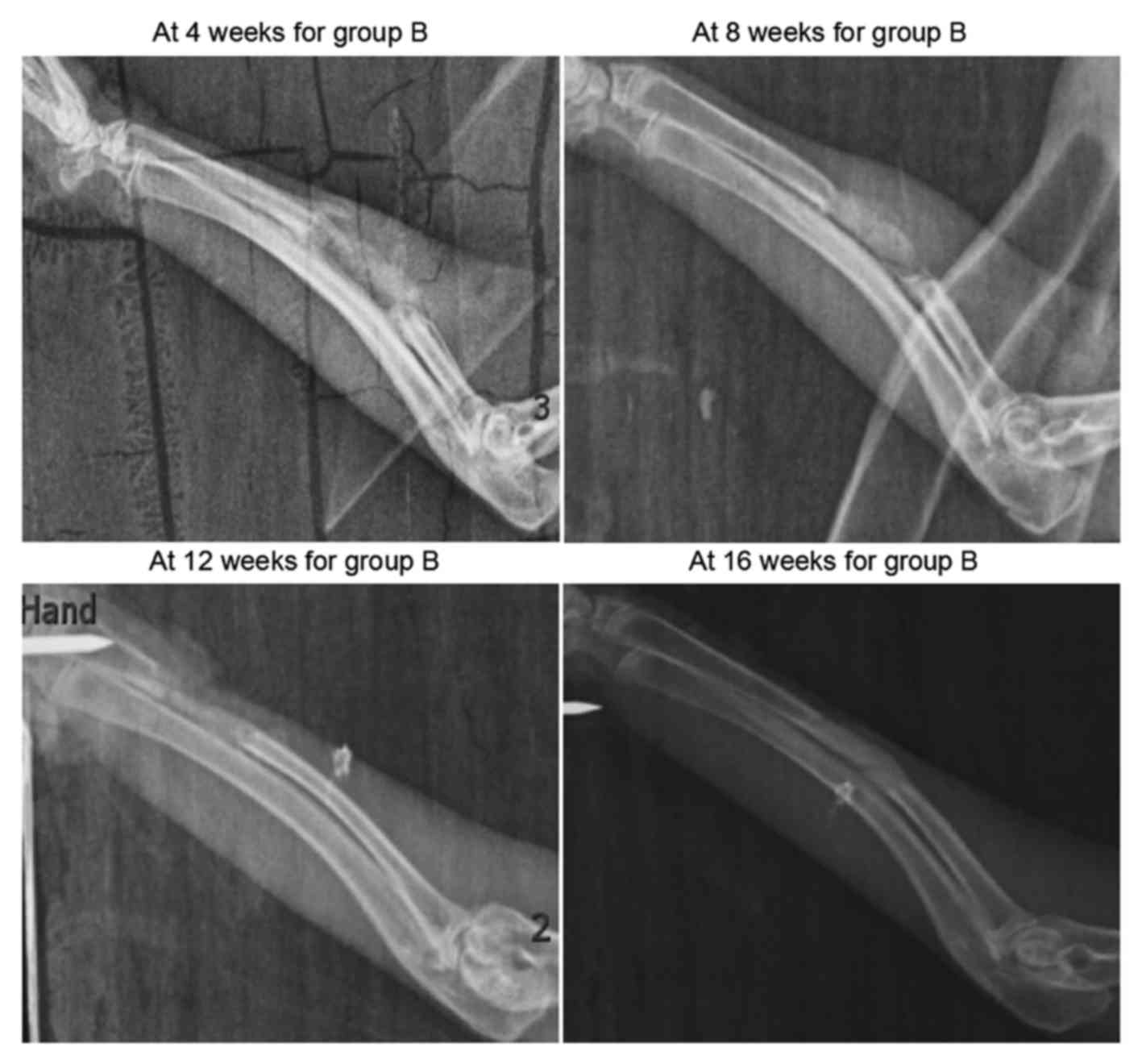
As shown in Fig. 3,
sporadic calcified shadows were observed in the scaffolds in group
B at 4 weeks after surgery. These were clearly darker compared with
the adjacent soft tissue shadows, though their morphology was less
distinct. At 8 weeks, the calcified shadows were enhanced, although
they remained lighter in color compared with normal bone tissues
and were more distinct in morphology in comparison with the earlier
X-ray scan. At 12 and 16 weeks after surgery, X-ray scanning
revealed slightly lighter bone density shadows compared with the
normal bone tissues, and the marrow cavity was partially
unobstructed.
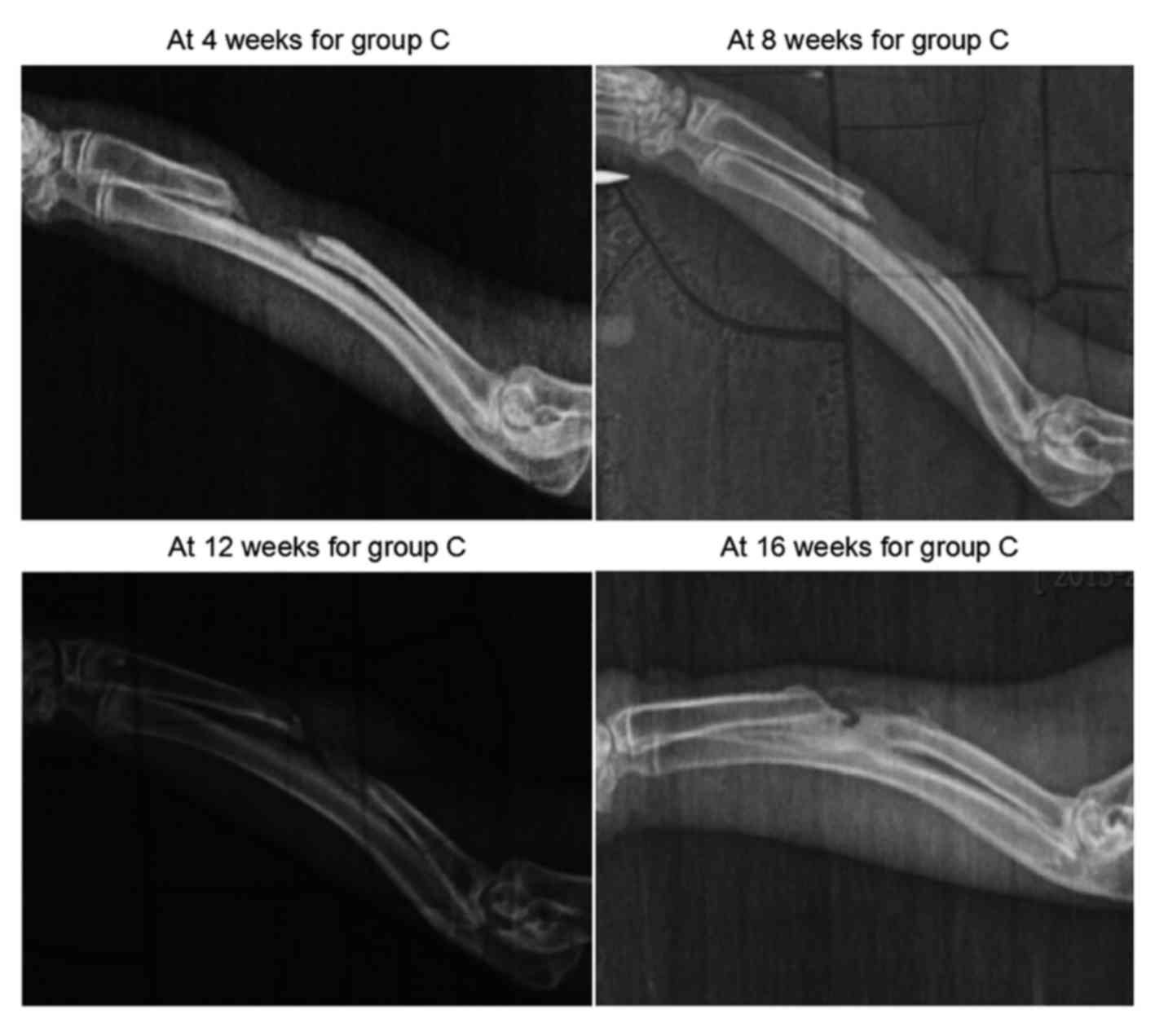
As demonstrated in Fig.
4, new bone tissues grew from the two broken ends at 4 weeks in
group C, however, the length of growth was small. At 8 weeks, the
calcified shadows at the two broken ends were enhanced, and the
growth was insignificant. In the blank region (defect), soft tissue
shadows were observed. At 12 and 16 weeks after surgery, the
elongation at the two broken ends appeared to have stopped, and the
calcified shadows were enhanced to the extent of resembling the
normal bone tissues. As the two broken ends were closed separately,
bone nonunion occurred. Groups A and B both indicated osteogenesis
in early stages, but in group A, higher X-ray bone density was
observed, indicating that group A had a better capacity for
osteogenesis. Group C finally became bone nonunion.
Pathological observation
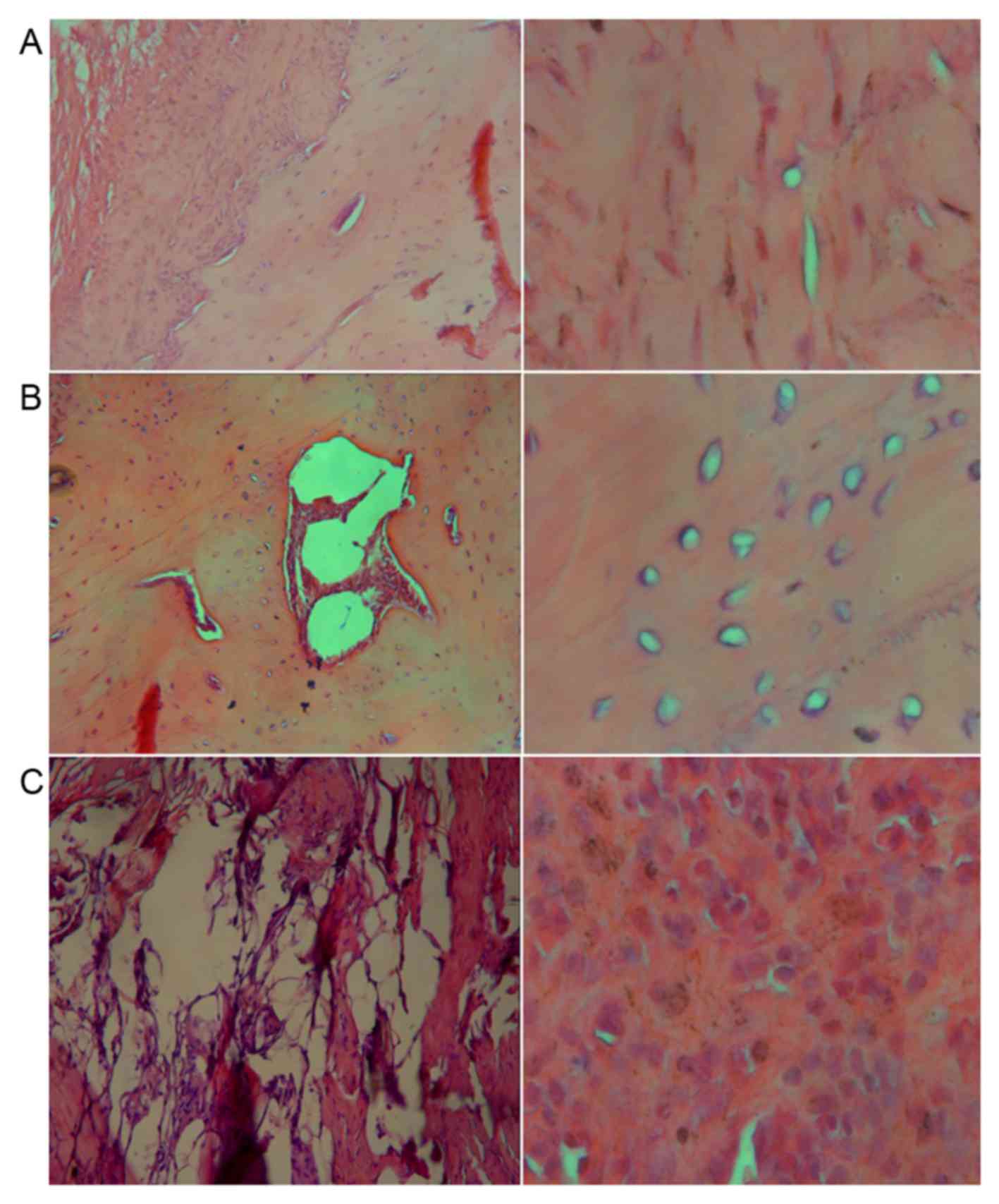
HE staining was performed in each group at 16 weeks.
As seen from Fig. 5A, new bone
tissues were observed in group A. Trabecular bones and long
spindle-shaped osteocytes with deeply stained nuclei were observed
under ×100 magnification. The bone plate was distinct and the
central canal, while bone matrix in lamellar arrangement was
detected on the outside and the osteocytes. Under higher
magnification, long spindle-shaped osteocytes were observed. In
group B, the scaffolds were partially replaced by bone tissues and
the osteocytes were surrounded by a considerable amount of
chondrocytes (Fig. 5B). However, no
trabecular bones or bone plates were observed, and large amounts of
chondrocytes were observed under high magnification (Fig. 5B). Furthermore, in group C, large
amounts of fibrous connective tissues were detected in contrast to
a few bone-like tissues. Round and deeply-stained nuclei of
myofibroblasts were observed under high magnification, which were
larger in size compared with the osteocytes and vacuolated
adipocytes (Fig. 5C). The
Lane-Sandhu histological scores of each group are listed in
Table I. Group A exhibited
significantly higher Lane-Sandhu scores at each time point compared
with groups B and C, indicating that the scaffold in group A
achieved better osteogenesis.
 | Table I.Lane-Sandhu scores in each group
following scaffold implantation. |
Table I.
Lane-Sandhu scores in each group
following scaffold implantation.
| Group | 4 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|
| A |
6.50±0.56a |
8.75±0.48a |
9.85±0.14a |
10.54±0.13a |
| B | 3.95±0.38 | 5.56±0.68 | 7.25±0.51 | 8.54±0.25 |
| C | 2.54±0.31 | 3.58±0.54 | 4.39±0.42 | 6.44±0.41 |
Discussion
Bone tissue engineering involves three key aspects,
including the presence of signaling molecules that induce bone
tissue differentiation, appropriate scaffold materials and the
ideal seed cells. Among these, the use of an appropriate scaffold
material is the most important aspect, and this material is
required to possess good mechanical strength combined with
toughness. In normal bone tissues, the tough layer of connective
tissues provides support. Thus, ensuring high mechanical strength
in artificial scaffolds is essential. In order to repair different
types of bone defects, scaffolds that are sufficiently strong and
allow remodeling are required (18).
Good biocompatibility with the host is essential, which refers to
the absence of immune rejection risk, inflammation or even
carcinogenesis following implantation. Three-dimensional (3D)
scaffolds with reticular structure and large surface area of pores
are preferred since these characteristics are conducive to the
regeneration of bone tissues and vessels. A large surface area
facilitates the growth and adhesion of the stem cells, and results
in a more uniform degradation of the scaffolds. In addition, an
appropriate degradation rate of the scaffolds is crucial (19). Long-term presence of scaffolds in the
host will prevent regeneration and growth of bone tissues; however,
if the scaffold degrades too fast, it cannot provide the support
necessary for the repair of the bone defect (20,21).
Among various materials used in tissue-engineered
bones, natural polymers include collagen, CS and alginates, while
artificial polymers include polylactic acid, polyphosphazenes and
poly-β-hydroxybutyric acid. Furthermore, the main artificial
inorganic materials used are hydroxyapatite, bioactive glass and
β-tricalcium phosphate. Natural polymers are superior in
biocompatibility, but poor in mechanical strength. In addition,
artificial polymers contain ingredients that mimic the inorganic
components of normal bone, leading to the risk of inflammation and
foreign body reaction. Artificial inorganic materials are superior
in plasticity and mechanical strength, but poor in hydrophilicity
and controllability of degradation rates (22). Therefore, in order to combine the
advantages of different materials, composite scaffolds are
constructed for bone tissue engineering (23–26).
In the study by Zhu et al (27), hydroxyapatite/calcium
polyphosphate/poly-L-actic acid scaffolds were prepared from the
raw materials of nHA, calcium polyphosphate and polylactic acid
using a solvent casting, particle filtering method and gas forming
method. The scaffolds had a 3D reticular structure with an average
pore size of 200–400 µm and high modulus of elasticity. The
mechanical performance was enhanced in the composite scaffold
compared with the scaffold composed of a single material (27). Furthermore, Xiao et al
(28) adopted a co-precipitation
method and prepared composite bone cement using nHA, carboxymethyl
CS and sodium alginate, which was then applied to the co-culture
with rabbit bone marrow stromal cells in vitro. The cells
adhered to the scaffold surface and proliferated continuously at
days 2, 4, 6 and 8. Under a scanning electron microscope,
pseudopods were observed in the cells that were adherent to the
material surface, indicating good biocompatibility with the cells
in vitro. In addition, Ye et al (29) prepared 3D scaffolds from PHBV by
electrospinning technology, as well as bone substitute made of
β-tricalcium phosphate alone. Application of the two scaffolds for
the repair of rabbit tibial bone defect indicated that the
composite scaffold had a superior repair effect after 8 weeks,
while the degradation rate of the composite scaffold was more
conducive to the repair (29).
Furthermore, Yang et al (30)
used SF and HA to prepare a composite scaffold for the repair of a
rabbit articular cartilage defect. The articular cartilage was then
harvested and observed to have uniformly and smoothly repaired the
defect. CT examination indicated smooth articular surface and
normal joint space, while observation of pathological specimens
showed the filling of large amounts of chondrocytes and close
binding to the cartilage (30).
Mature trabecular bones and reticular structure were observed with
complete repair of subchondral bone, thus indicating sufficient
support and biocompatibility in vivo. Therefore, as compared
with scaffolds composed of a single material, composite scaffolds
can achieve the desired combination of properties by changing the
proportion of each ingredient and the way of compounding. Although
the SF/CS scaffold prepared in a preliminary study exhibited high
biocompatibility in the repair of rabbit knee joint defect, the
mechanical strength required further improvement (31–33).
Therefore, another ingredient, namely nHA, was introduced in the
scaffold used in the present study.
The SF/CS/nHA composite scaffold constructed in the
current study had a 3D reticular structure with an average pore
size of 100–200 µm and excellent physical and chemical properties.
In order to test its performance, a defect measuring 1.5 cm in
length was induced in the middle segment of rabbit radial bones.
After incising the skin and exposing the radial bone, a large
segmental bone defect was made with the removal of the periosteum.
Surgery was performed using the same approach and procedures in all
rabbits, and the length of the defect was four times larger than
the diameter of the rabbit radial shaft. The severity of the defect
was far beyond the scope of self-repair. The purpose of removing
the periosteum was to prevent osteogenesis from the periosteum.
Within 1 week after scaffold implantation, no reddening, fever or
other signs of infection were observed in all groups. The incisions
healed 2 weeks later, without festering or rejection. After 16
weeks, rabbits in group A did not suffer from any systemic
reactions, such as anorexia, while vomiting and death were not
reported. The aforementioned results of the present study indicated
good biocompatibility of the scaffold. Furthermore, X-ray scans
indicated enhanced calcified shadows over time in group A, with
distinct morphology and margins. The defect region did not differ
from the normal bone tissue on X-ray scans conducted 16 weeks after
surgery, which suggested excellent osteoinductive and osteogenic
effect of the scaffold. However, the calcified shadows in group B
were evidently lighter compared with group A at the same time
points, indicating poor osteogenic capacity. This may be due to the
introduction of nHA in the scaffold used in group A, since
degradation of nHA provides calcium and phosphorus ions that are
required for osteogenesis (34,35). In
group C, the two broken ends of the bones were closed separately
without osseointegration. Under low magnification, there were more
osteoblasts in group A as compared with group B, and typical
structures of bone tissues, such as trabecular bones and fibrous
rings, were also observed. Under high magnification, group A had
typical osteoblasts in the bone lacunae, while there were more
round chondrocytes in group B. These observations provided
convincing evidence that the addition of nHA enhanced the
osteoinductive capacity.
In conclusion, the SF/CS/nHA composite scaffold
demonstrated high biocompatibility and osteoinduction in the repair
of a defect in rabbit radial bone. The degradation rate of the
scaffolds was compatible with the regeneration of the bone tissues.
Therefore, the SF/CS/nHA composite scaffold may qualify as a novel
scaffold material, although the details of the repair mechanism
currently remain unknown.
Acknowledgements
This study was supported by grants from the Guizhou
Province Science and Technology Fund Project [no. SY (2010) 3101],
the Guizhou Social Research Project [no. (2010) 015], and the
Guizhou Province Governor Fund Project [no. (2011) (25)].
References
|
1
|
Xin Lei and SU Jia-can: Current status and
prospect of artificial bone repair materials. J Traumatic Surg.
3:254–257. 2011.
|
|
2
|
Puppi D, Chiellini F, Piras AM and
Chiellini E: Polymeric materials for bone and cartilage repair.
Progress in Polymer Science. 35:403–440. 2010. View Article : Google Scholar
|
|
3
|
Pan Z and Ding JD: Poly
(lactide-co-glycolide) porous scaffolds for tissue engineering and
regenerative medicine. Interface focus. 2:366–377. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park SH, Park DS and Shin JW, Kang YG, Kim
HK, Yoon TR and Shin JW: Scaffolds for bone tissue engineering
fabricated from two different materials by the rapid prototyping
technique: PCL versus PLGA. J Mater Sci Mater Med. 23:2671–2678.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Z, Li M, Yu B, Cao L, Yang Q and Su
J: Nanocalcium-deficient hydroxyapatite-poly
(e-caprolactone)-polyethylene glycol-poly (e-caprolactone)
composite scaffolds. Int J Nanomedicine. 7:3123–3131.
2012.PubMed/NCBI
|
|
6
|
De Santis R, Gloria A, Russo T, D'Amora U,
Zeppetelli S, Dionigi C, Sytcheva A, Herrmannsdörfer T, Dediu V and
Ambrosio L: A basic approach toward the development of
nanocomposite magnetic scaffolds for advanced bonetissue
engineering. J Appl Polym Sci. 122:3599–3605. 2011. View Article : Google Scholar
|
|
7
|
Budiraharjo R, Neoh KG and Kang ET:
Hydroxyapatite-coated carboxymethyl chitosan scaffolds for
promoting osteoblast and stem cell differentiation. J Colloid
Interface Sci. 366:224–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alves da Silva ML, Crawford A, Mundy JM,
Correlo VM, Sol P, Bhattacharya M, Hatton PV, Reis RL and Neves NM:
Chitosan/polyester-based scaffolds for cartilage tissue
engineering: Assessment of extracellular matrix formation. Acta
Biomater. 6:1149–1157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng J, Yu RF, Huang WL, Yuan C and Mo G:
Restoration of cartilage defect with silk fibrin/chitosan
biological scaffold compound by bone marrow mesenchymal stem cells
in elderly rabbits. Chin J Geriatrics. 31:156–160. 2012.
|
|
10
|
Tiyaboonchai W, Chomchalao P, Pongcharoen
S, Sutheerawattananonda M and Sobhon P: Preparation and
characterization of blended Bombyx mori silk fibroin scaffolds.
Fiber Polym. 12:3242011. View Article : Google Scholar
|
|
11
|
Murphy CM, Haugh MG and O'Brien FJ: The
effect of mean pore size on cell attachment, proliferation
andmigration in collagen-glycosaminoglycan scaffolds for bone
tissue engineering. Biomaterials. 31:461–466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu Q, Zhang X, Hu X and Kaplan DL: Green
process to prepare silk fibroin/gelatin biomaterial scaffolds.
Macromol Biosci. 10:289–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang HF, Zhao CY, Fan HS, Zhang H, Pei FX
and Wang GL: Histological and biomechanical study of repairing
rabbit radius segmental bone defect with porous titanium. Beijing
Da Xue Xue Bao. 43:724–729. 2011.(In Chinese). PubMed/NCBI
|
|
14
|
Rahimzadeh R, Veshkini A, Sharifi D and
Hesaraki S: Value of color Doppler ultrasonography and radiography
for the assessment of the cancellous bone scaffold coated with
nano-hydroxyapatite in repair of radial bone in rabbit. Acta Cir
Bras. 27:148–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hao W, Dong J, Jiang M, Wu J, Cui F and
Zhou D: Enhanced bone formation in large segmental radial defects
by combining adipose-derived stem cells expressing bone
morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop.
34:1341–1349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng J, She R, Huang W, Dong Z, Mo G and
Liu B: A silk fibroin/chitosan scaffold in combination with bone
marrow-derived mesenchymal stem cells to repair cartilage defects
in the rabbit knee. J Mater Sci Mater Med. 24:2037–2046. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Y, Huang D, Zhang D, Mou Y and Liu
X: Promotion effect of FTY-720OP on treatment of bone defect with
allograft bone by suppressing osteoclast formation and function.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 30:426–431. 2016.(In
Chinese). PubMed/NCBI
|
|
18
|
Salerno M, Cenni E, Fotia C, Avnet S,
Granchi D, Castelli F, Micieli D, Pignatello R, Capulli M, Rucci N,
et al: Bone-targeted doxorubicin-loaded nanoparticles as a tool for
the treatment of skeletal metastases. Curr Cancer Drug Targets.
10:649–659. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yewle JN, Puleo DA and Bachas LG: Enhanced
affinity bifunctional bisphosphonates for targeted delivery of
therapeutic agents to bone. Bioconjug Chem. 22:2496–2506. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly DJ and Jacobs CR: The role of
mechanical signals in regulating chondrogenesis and osteogenesis of
mesenchymal stem cells. Birth Defects Res C Embryo Today. 90:75–85.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jayasuriya AC and Bhat A: Fabrication and
characterization of novel hybrid organic/inorganic microparticles
to apply in bone regeneration. J Biomed Mater Res A. 93:1280–1288.
2010.PubMed/NCBI
|
|
22
|
Lee JS, Park WY, Cha JK, Jung UW, Kim CS,
Lee YK and Choi SH: Periodontal tissue reaction to customized
nano-hydroxyapatite block scaffold in one-wall intrabony defect: A
histologic study in dogs. J Periodontal Implant Sci. 42:50–58.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Martínez-Vázquez FJ, Perera FH, Miranda P,
Pajares A and Guiberteau F: Improving the compressive strength of
bioceramic robocast scaffolds by polymer infiltration. Acta
Biomater. 6:4361–4368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Crouzier T, Sailhan F, Becquart P, Guillot
R, Logeart-Avramoglou D and Picart C: The performance of BMP-2
loaded TCP/HAP porous ceramics with a polyelectrolyte multilayer
film coating. Biomaterials. 32:7543–7554. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Pol U, Mathieu L, Zeiter S,
Bourban PE, Zambelli PY, Pearce SG, Bouré LP and Pioletti DP:
Augmentation of bone defect healing using a new biocomposite
scaffold: An in vivo study in sheep. Acta Biomater. 6:3755–3762.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao F, Chen Y, Li Z, Wang Y, Shi B, Gong
Z and Cheng X: A novel bioactive three-dimensional beta-tricalcium
phosphate/chitosan scaffold for periodontal tissue engineering. J
Mater Sci Mater Med. 21:489–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu LY, Wang YP, Shi ZL and Zhang HM:
Fabrication and properties of nanometer hydroxyapatite/calcium
polyphosphate/poly (L-lactic acid) composite scaffold for bone
tissue engineering. J Clin Rehabil Tissue Eng Res. 16:431–433.
2012.
|
|
28
|
Xiao HJ, Xue F, He ZM, Jin FQ and Shen YC:
Preparation and characterization of
nano-hydroxyapatite/carboxymethychitosan-sodium alginate bone
cement composite material. J Clin Rehabil Tissue Eng Res.
15:7113–7117. 2011.
|
|
29
|
Ye R, Zhang XF, Yan HN, Pan YF, Huang NP,
Lv LX and Jiang ZL: Hydroxybutyrate-hydroxyvalerate sodium meters
of fiber material with bone defect repair. J Clin Rehabil Tissue
Eng Res. 16:6284–6288. 2012.
|
|
30
|
Yang Y, Xu WY, Zhang Y, Zhang XX, Liu ZB,
Qian H, Huang JP and Cui ZH: Symbol Silk fibroin/hydroxyapatite
combined with bone marrow mesenchymal stem cells for construction
of tissue engineered cartilage. J Clin Rehabil Tissue Eng Res.
15:5339–5342. 2011.
|
|
31
|
Rong Zijie, Yang Lianjun and Zhang Zanjie:
The effect of nano-hydroxyapatite/collagen scaffolds incorporating
ADM-PLGA microspheres in repairing the rabbits bone defects. J
Pract Med. 22:3559–3562. 2014.
|
|
32
|
Ye Peng, Tian Ren-yuan and Ma Li-kun: Silk
fibroin/chitosan/nano hydroxyapatite complicated scaffolds for bone
tissue engineering. Chin J Tissue Eng Res. 29:5270–5274. 2013.
|
|
33
|
Ma Likun, Ye Peng and Jiang Deng: The
cytotoxicity of silk fibroin/chitosan/nanohydroxyapatite bone
tissue engineering scaffolds in vitro. Med J Westchina. 8:975–980.
2014.
|
|
34
|
Zhou H and Lee J: Nanoscale hydroxyapatite
particles for bone tissue engineering. Acta Biomater. 7:2769–2781.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang CY, Lu H, Zhuang Z, Wang XP and Fang
QF: Nano-hydroxyapatite/poly (l-lactic acid) composite synthesized
by a modified in situ precipitation: Preparation and properties. J
Mater Sci Mater Med. 21:3077–3083. 2010. View Article : Google Scholar : PubMed/NCBI
|