Introduction
PD, the second most common neurodegenerative
disorder after Alzheimer's disease (AD), is tightly associated with
aging, the morbidity of which is approximate 2% in the older. PD
impacts basal ganglia in patients who typically experience
bradykinesia, rigidity, tremor and disturbed balance (1). The precise mechanisms of PD remain
elusive, previous studies suggested mitochondrial dysfunction
(2), neuro-inflammation (3) as well as oxidative stress (4) were involved in this process.
Neurological degeneration can be deteriorated by chronic
inflammation in the central nervous system (CNS) which involves
recruitment of cytotoxic molecules, free radicals and glutamate
that have the potential to provoke neuritic beading, excitotoxic,
apoptotic and necrotic degeneration (5).
In China, astragalus membranaceus was
utilized for patients with chronic diseases and healthy individuals
who wish to further improve vital functions (6). AS-IV, the primary pure saponin which is
isolated from the root of astragalus membranaceus, was an
effective compound with distinct pharmacological effects, including
protecting against ischemic brain injury (6), lung inflammation (7), acute pancreatitis (8) and cardiac trauma (9).
Astrocyte dysfunction and even astrocyte
dysregulation critically affected neuronal survival (10). Traditionally, necrosis was deemed to
be the predominant mechanism of astrocyte death in brain injury
models, moreover, mounting evidences demonstrated that astrocytic
apoptosis might contribute to the pathogenesis of multiple
neurodegenerative disorders, for instance, AD and PD (11,12).
In 1947, MPTP was first synthesized by Lee et
al as an analgesic (13). It
caused Parkinsonism in primates including humans, rodents (less
susceptible) and rats (almost immune). After MPTP administration,
mice were reported to only suffer from cell death in SNPC (14), excitingly, most of the recent studies
indicated the appearance of Parkinsonism-like syndromes (especially
chronically) as well (15).
MPP+, whose prodrug is MPTP, is a neurotoxin which
selectively destroy nigral DA neurons and is also widely used to
establish PD experimental models in vitro (16,17).
MPP+ has been shown to induce a syndrome closely resembling PD in
cellular and animal models (18,19).
To the best of our knowledge, as yet, whether and
how AS-IV displays protective effects on MPTP generated PD in mice
and MPP+ induced PD in astrocytes remain elusive. Our data
suggested that AS-IV may be a promising agent for the prevention
and therapy of PD.
Materials and methods
Animals
Adult male C57BL/6 mice aged 8 weeks were used in
current study. Mice were randomly divided into 6 different groups:
i) ethanol-propylene glycol (10 µl) + negative control (NC) group;
ii) ethanol-propylene glycol (10 µl) + MPTP group; iii) AS-IV (1.5
mg/kg/10 µl) + MPTP group; iv) AS-IV (3 mg/kg/10 µl) + MPTP group;
v) AS-IV (6 mg/kg/10 µl) + MPTP group and vi) AS-IV (6 mg/kg/10 µl)
group, with 10 mice in each group. Mice were housed in a
temperature-controlled room with a 12-hour light/dark cycle and
were free to food/water.
PD model was obtained by MPTP (30 mg/kg/10 µl, i.p.)
injection for consecutive 5 days. As for AS-IV, it was dissolved in
ethanol-propylene glycol (50:50 v/v), and injected once a day 30
min before MPTP injection. Eight hours after MPTP administration,
behavioral tests were carried out at 1 day before MPTP injection,
and at 1th/4th/7th/10th day after MPTP injection, respectively.
Mice were handled according to the National Institutes of Health
(NIH, Bethesda, MD) Guide for the Care and Use of Laboratory
Animals (NIH publication 80–23, revised 1996). Experiments were
approved by the Institutional Animal Care and Use Committee (IACUC)
of Nanjing Medical University.
Pole test
A ball with the diameter of 2.5 cm was fixed at the
top of the wooden pole which was at the length of 50 cm and at the
thickness of 1 cm. Mice were placed on the ball to evaluate the
different time spending on getting down from the ball. Results were
re-tested when mice climbed to the reverse direction or stopped.
Each mice was tested for 2–3 times one day. We carried out this
test in accordance to a previous reported study (20).
Traction test
Mice were suspended on a horizontal with a distance
of 30 cm to a platform for observing their hang time. Criteria were
as followed: 0–4 sec recorded as 0 score, 5–9 sec recorded as 1
score, 10–14 sec recorded as 2 score, 15–19 sec recorded as 3
score, 20–24 sec recorded as 4 score, 25–29 sec recorded as 5
score, >30 sec recorded as 6 score. They were recorded as
previously performed (21).
Swim test
Mice were placed in a 20×30×20 cm pool with the
temperature of 28–30°C, swim situation within 1 min was recorded.
Criteria were as followed: 3.0, swims successively; 2.5, swims for
the most time; 2.0, floating time is longer than 30 sec; 1.5; swims
occasionally; 1, swims occasionally and floating for almost all the
time. This test was carried out as previously described (22).
Cell culture
Primary astrocytes were derived from 1–5 day
postnatal mice. In brief, the cerebral cortices were minced in the
medium which contained 20 µg/ml DNase and 0.3% bovine serum albumin
(BSA).
Tissues were digested in 0.25% trypsin solution at
37°C for 30 min. The suspension was filtered through 70 um nylon
filter, pelleted by centrifugation to remove trypsin. Afterwards,
pellets were re-suspended in 10% (v/v) fetal bovine serum (FBS) and
1% penicillin/1% streptomycin containing Dulbecco's modified
Eagle's medium/F12 (DMEM/F12), followed by transferation to flasks
and incubation under the conditions of 37°C, 5% CO2 and
90% relative humidity. When cells reached confluence, flasks were
gently shaken to remove microglia cells and oligodendrocytes.
Astrocytes were rinsed with phosphate buffered saline (PBS) for
three times. Thereafter, astrocytes were trypsinized and loosened
by patting the flasks, thereafter, they were placed in a new flasks
and cultured in DMEM/F12 (15% FBS, L-glutamine and 500 ng/ml
insulin) until confluent.
MPP+ (4 mM/l) was used in primary astrocyte to
obtain cellular model of PD. Different concentrations of AS-IV (10,
20 and 40 µM/l) were administrated 2 h prior to MPP+, at 24 h
following MPP+ treatment, astrocytes were used for following
experiments.
MTT assay
Cell viability was measured by MTT assay.
Approximately 200 µl cells at the concentration of
1×104/ml were seeded into 96-well plates. After
incubation of cells for 24 h, 20 µl of 5 mg/ml MTT solution was
added to each well and the plate was further incubated at 37°C for
another 4 h. Afterwards, wells were rinsed with PBS for 3 times,
and 150 µl DMSO was added into each well. The microtitre plate was
placed on a shaker to dissolve the dye thoroughly. Absorbance at
450 nm was read using a Bio-Rad iMark plate reader.
Annexin-V Fluorescein (FITC)
Astrocytic apoptosis was assessed by FITC apoptosis
detection kit (Oncogene Research Products, San Diego, CA, USA)
according to manufacturer's instructions. Cell samples were
analyzed by flow cytometry apparatus (Becton Dickinson FACSVantage
SE, San Jose, CA, USA). Dual analysis was adopted in the present
study, necrotic cells were propidium iodide (PI)-positive, early
apoptotic cells were Annexin V-FITC-positive, while cells at the
state of late apoptosis were double-positive for Annexin V-FITC and
PI. Cells that were stained with neither Annexin V-FITC nor PI were
classified as live cells.
Western blotting
Expression levels of glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), p-JNK, caspase-3 and Bax/Bcl-2 were
evaluated by western blot. Briefly, astrocyte extract lysates were
washed with pre-cold PBS and homogenized in RIPA lysis buffer which
contained a cocktail of protease inhibitors and phosphatase
inhibitors (Roche Diagnostics, Shanghai, China). Samples were
separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and electro-transferred onto
polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, USA).
Afterwards, PVDM membranes were blocked in 5% bull serum albumin
(BSA) for 1 h at room temperature, and incubated overnight at 4°C
with the corresponding primary antibodies. After washing with
Tris-Buffered Saline and Tween-20 (TBST), PVDF membranes were
incubated with horse radish peroxidase (HRP)-conjugated secondary
antibody for 1 h at room temperature. GAPDH performed as a loading
control.
Statistical analysis
Differences among groups were tested with two-way
ANOVA. Data were presented as mean ± standard deviation (SD).
Significance is determined on a criterion of P<0.05.
Results
Pole test manifests that pretreatment
of AS-IV attenuates MPTP-induced moving deficiency
MPTP was utilized for the establishment of PD model
in vivo. There was no significant difference in climbing
time between MPTP group and NC group at one day before/after
modeling. However, from 4th-10th day after MPTP injection, mice
displayed significantly longer time on climbing than that in NC
group. Moreover, AS-IV pretreatment remarkably attenuated
MPTP-induced extending of climbing time. Data were showed in
Table I. Mice treated with AS-IV (6
mg/kg/10 µl) did not exhibit significant change in climbing time
(data were not shown).
 | Table I.AS-IV pretreatment attenuates
MPTP-induced deficiency in ability of moving. |
Table I.
AS-IV pretreatment attenuates
MPTP-induced deficiency in ability of moving.
| Time point | Ethanol-propylene
glycol + NC | Ethanol-propylene
glycol + MPTP | AS-IV1.5 mg/kg ±
MPTP | AS-IV3.0 mg/kg ±
MPTP | AS-IV 6 mg/kg ±
MPTP |
|---|
| 1 day before PD
(Score) | 5.53±0.91 | 5.52±1.50 | 5.03±1.32 | 5.10±1.30 | 5.35±1.24 |
| 1st day after PD
(Score) | 5.48±0.86 | 5.78±1.53 | 5.27±1.55 | 6.17±1.43 | 5.77±1.35 |
| 4th day after PD
(Score) | 5.61±0.73 |
7.94±1.87a |
7.52±1.83b |
7.22±1.69b |
7.07±1.23c |
| 7th day after PD
(Score) | 5.36±0.81 |
7.31±1.36a |
7.15±1.38b |
6.99±1.32b |
6.81±1.34c |
| 10th day after PD
(Score) | 5.68±0.98 |
7.13±1.45a |
6.83±1.48b |
6.66±1.54b |
6.55±1.41c |
Traction test demonstrates that
pretreatment of AS-IV ameliorates MPTP-induced suspension
deficiency
No significant difference was found in suspension
score between MPTP group and NC group at one day before/after
modeling. Nevertheless, compared with NC group, mice in MPTP group
displayed lower suspension score from 4th to 10th day after
modelling which was remarkably reversed by AS-IV pretreatment. Data
were displayed in Table II. Mice
treated with AS-IV (6 mg/kg/10 µl) did not exhibit significant
change in suspension score (data were not shown).
 | Table II.AS-IV pretreatment ameliorates
MPTP-induced suspension deficiency. |
Table II.
AS-IV pretreatment ameliorates
MPTP-induced suspension deficiency.
| Time point | Ethanol-propylene
glycol + NC | Ethanol-propylene
glycol + MPTP | AS-IV1.5 mg/kg ±
MPTP | AS-IV3.0 mg/kg ±
MPTP | AS-IV6 mg/kg ±
MPTP |
|---|
| 1 day before PD
(Score) | 2.83±0.41 | 2.83±0.65 | 2.83±0.52 | 2.85±0.56 | 2.86±0.64 |
| 1st day after PD
(Score) | 3.00±0.36 |
3.02±0.86a |
2.93±0.55b |
2.91±0.63b |
2.96±0.75c |
| 4th day after PD
(Score) | 2.90±0.46 |
2.20±0.83a |
2.40±0.83b |
2.60±0.69b |
2.87±0.23c |
| 7th day after PD
(Score) | 2.92 ±0.48 |
2.00±0.39a |
2.30±0.36b |
2.42±0.32b |
2.49±0.34c |
| 10th day after PD
(Score) | 2.94±0.48 |
2.41±0.53a |
2.50±0.45b |
2.63±0.54b |
2.74±0.41c |
Swim test indicates that pretreatment
of AS-IV ameliorates MPTP-induced swim deficiency
At one day before/after modeling, no significant
difference was found in swim score between MPTP group and NC group.
Whereas, from 4th-10th day after MPTP injection, mice exhibited
lower swimming score in comparison with NC group. Moreover, AS-IV
pretreatment remarkably reversed MPTP-induced swimming score
downregulation. Data were exhibited in Table III. Mice treated with AS-IV (6
mg/kg/10 µl) did not exhibit significant change in swim score (data
were not shown).
 | Table III.AS-IV pretreatment ameliorates
MPTP-induced swim deficiency. |
Table III.
AS-IV pretreatment ameliorates
MPTP-induced swim deficiency.
| Time point | Ethanol-propylene
glycol + NC | Ethanol-propylene
glycol + MPTP | AS-IV1.5 mg/kg ±
MPTP | AS-IV3.0 mg/kg ±
MPTP | AS-IV6 mg/kg ±
MPTP |
|---|
| 1 day before PD
(Score) | 3.04±0.11 | 3.03±0.05 | 3.03±0.07 | 3.05±0.06 | 3.06±0.04 |
| 1st day after PD
(Score) | 3.20±0.16 |
3.22±0.16a |
3.13±0.25b |
3.11±0.33b |
3.12±0.25c |
| 4th day after PD
(Score) | 3.10±0.06 |
2.42±0.13a |
2.75±0.33b |
2.86±0.29b |
2.89±0.23c |
| 7th day after PD
(Score) | 3.12 ±0.08 |
2.23±0.09a |
2.43±0.31b |
2.54±0.30b |
2.59±0.20c |
| 10th day after PD
(Score) | 3.14±0.12 |
2.35±0.12a | 2.77±
0.35b |
2.73±0.24b |
2.83±0.21c |
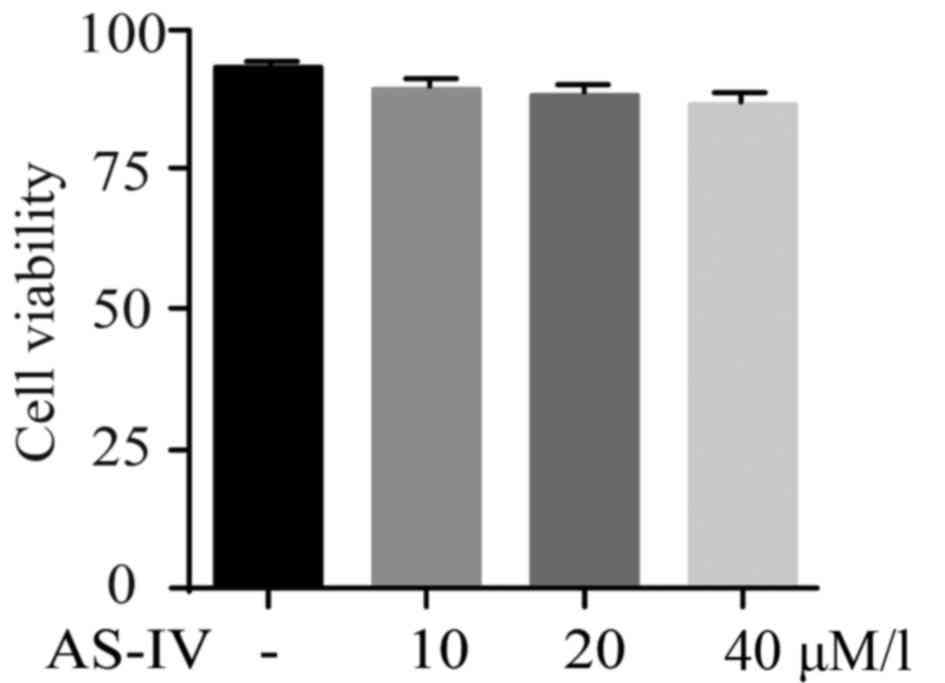
AS-IV shows no cytotoxicity on primary
astrocytes
MPP+ was utilized for the establishment of PD model
in vitro. Influence of AS-IV on cultured astrocytes was
tested by MTT assay. Results revealed that AS-IV alone did not
affect the cell viability of astrocytes as shown in Fig. 1.
AS-IV attenuates MPP+-induced cell
apoptosis of astrocytes
In comparison with NC group, astrocytes
administrated with MPP+ have exhibited predominantly elevated
apoptotic cell number (Fig. 2A and
B), which was significantly reversed by co-administration of
AS-IV (Fig. 2C-E). Consistently, the
corresponding statistical data of cell apoptosis rate were
displayed as in Fig. 2F.
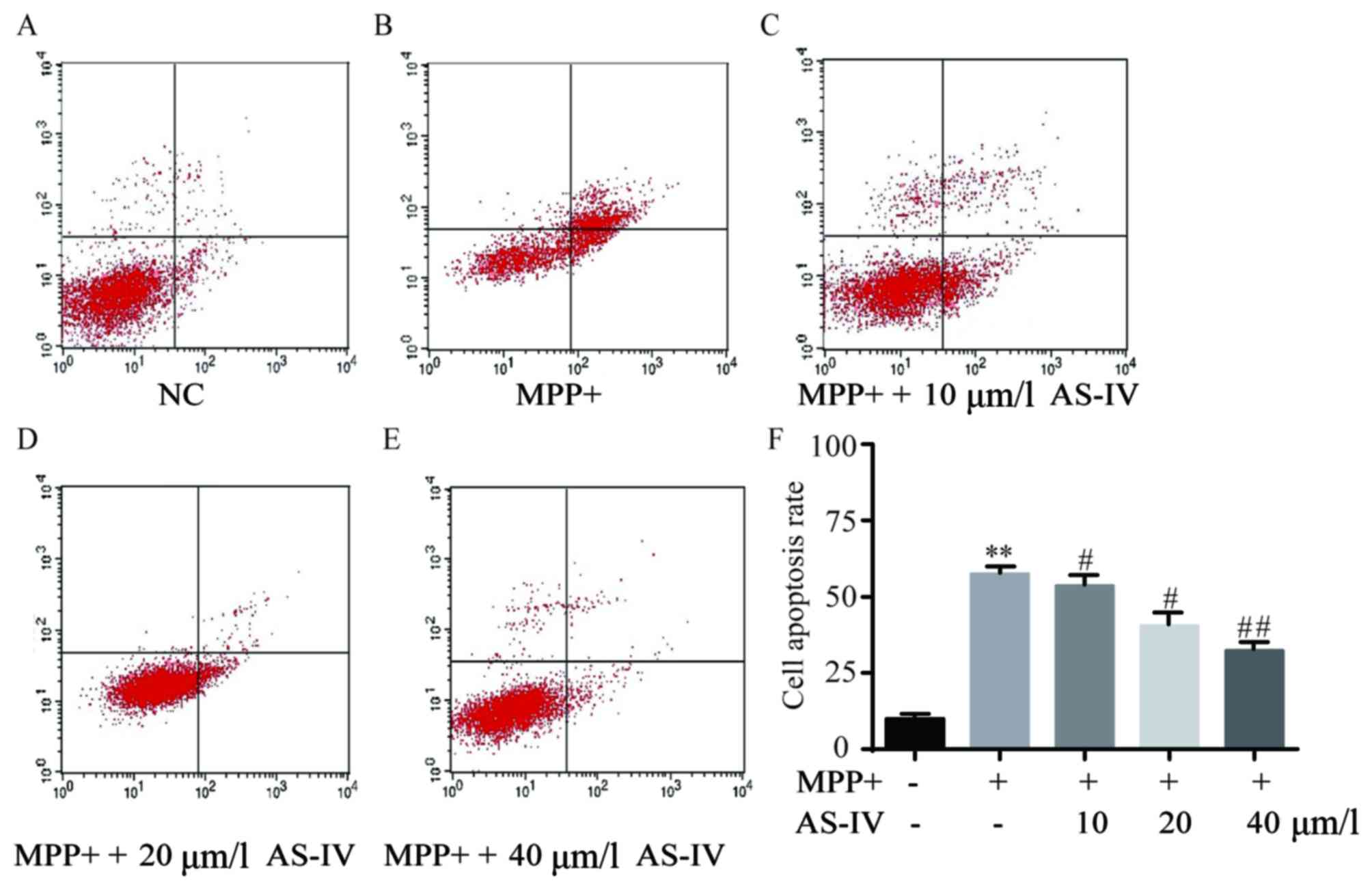 | Figure 2.AS-IV attenuates MPP+-induced
astrocyte cell apoptosis. In comparison with NC group, astrocytes
that were administrated with MPP+ exhibited predominantly elevated
cell apoptosis (A and B), which was significantly reversed by
co-administration with AS-IV dose-dependently (C-E). Consistently,
the corresponding statistical data of cell apoptosis rate were
displayed (F). **P<0.01 MPP+ group vs. NC group,
#P<0.05, ##P<0.01 AS-IV groups vs. MPTP
group, respectively. NC, negative control; AS-IV, astragaloside-IV;
MPP+, 1-methyl-4-phenylpyridnium ion; MPTP, 1-methyl-4-phenyl-1, 2,
3, 6-tetrahydropyridine. |
AS-IV rescues MPP+-induced cell
viability reduction of astrocytes
Influence of AS-IV on cell viability of cultured
astrocytes was evaluated by MTT assay. Results revealed that AS-IV
significantly improved the downregulated astrocyte cell viability
which was generated by MPP+ (Fig.
3).
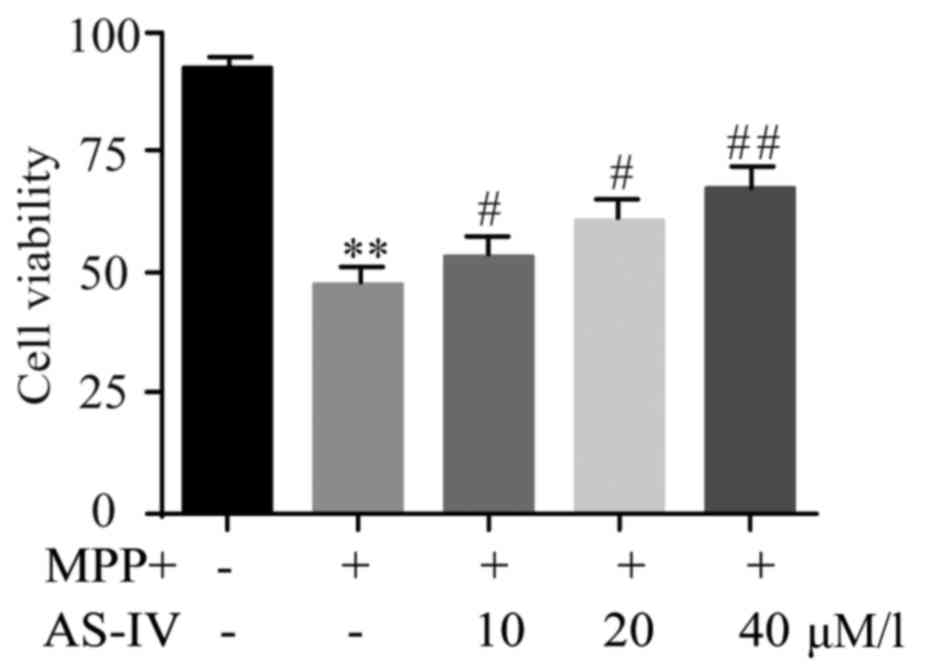 | Figure 3.AS-IV rescues MPP+-induced astrocyte
cell viability reduction. AS-IV significantly improved the
downregulated astrocyte cell viability which was generated by MPP+.
**P<0.01 MPP+ group vs. NC group, #P<0.05,
##P<0.01 AS-IV groups vs. MPTP group, respectively.
NC, negative control; AS-IV, astragaloside-IV; MPP+,
1-methyl-4-phenylpyridnium ion; MPTP, 1-methyl-4-phenyl-1, 2, 3,
6-tetrahydropyridine. |
AS-IV reduces MPP+-induced elevation
of p-JNK in astrocytes
The protein level of p-JNK in different groups was
assessed by western blot. AS-IV pretreatment dose-dependently
inhibited over-expression of p-JNK caused by MPP+ (Fig. 4A). The statistical data were
presented and demonstrated that AS-IV notably repressed the
upregulation of p-JNK that was induced by MPP+ (Fig. 4B).
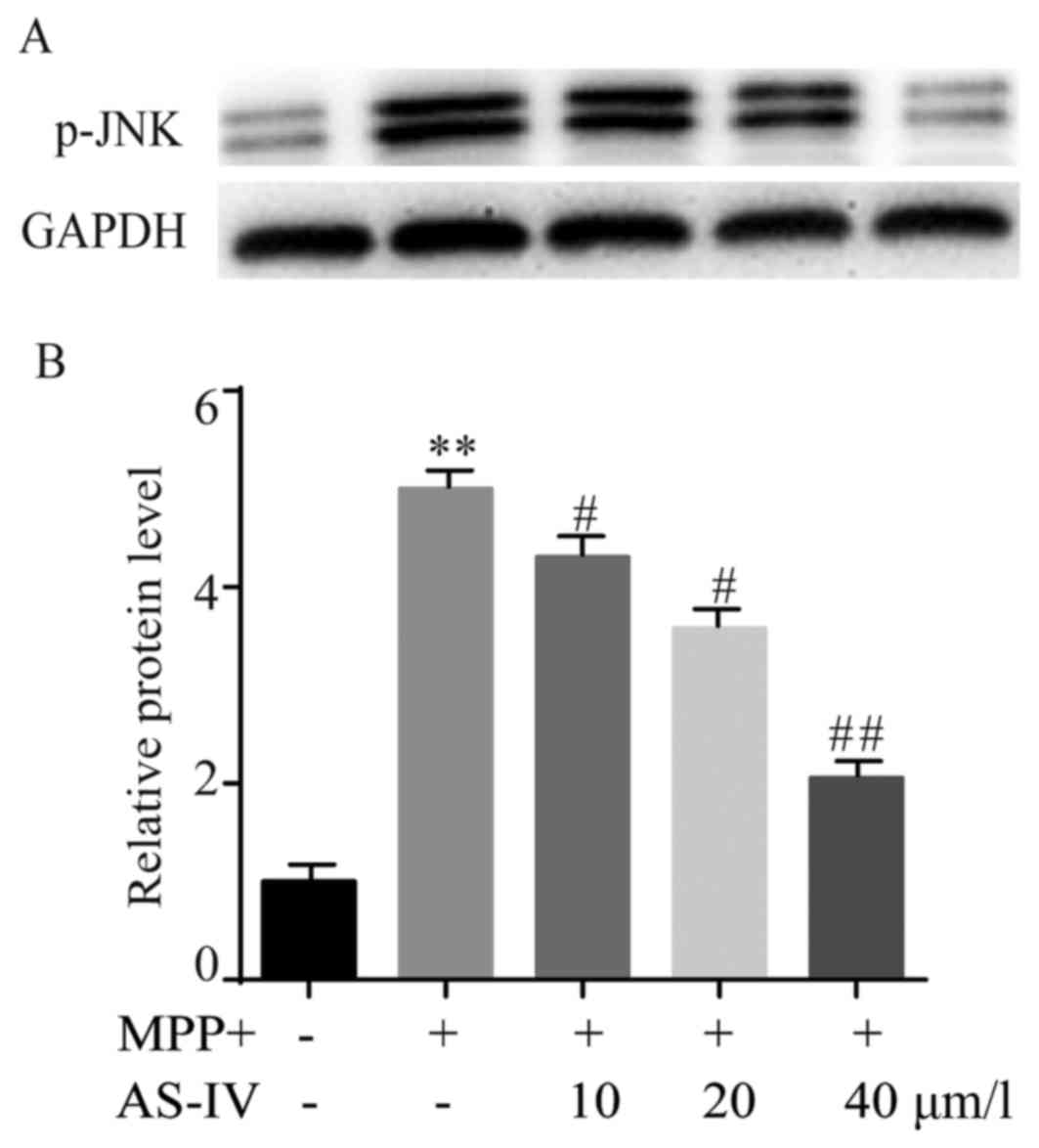 | Figure 4.AS-IV reduces MPP+-induced elevation
of p-JNK in astrocytes. AS-IV inhibited MPP+ induced
over-expression of p-JNK in a dose-dependent manner (A). The
statistical data verified that AS-IV notably repressed MPP+
generated upregulation of p-JNK (B). **P<0.01 MPP+ group vs. NC
group, #P<0.05, ##P<0.01 AS-IV group
vs. MPTP group. NC, negative control; AS-IV, astragaloside-IV;
MPP+, 1-methyl-4-phenylpyridnium ion; p-JNK, phosphorylated-Jun
N-terminal kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase;
MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. |
AS-IV represses MPP+-induced rise of
Bax/Bcl-2 ratio in astrocytes
In MPP+ group, Bax protein level was significantly
higher than NC group, while Bcl-2 manifested an opposite change
profile. Surprisingly, both MPP+-induced upregulation of Bax and
downregulation of Bcl-2 were reversed by AS-IV (Fig. 5A). After MPP+ treatment, Bax/Bcl-2
ratio was obviously higher than NC group, which was remarkably
attenuated by AS-IV as in Fig.
5B.
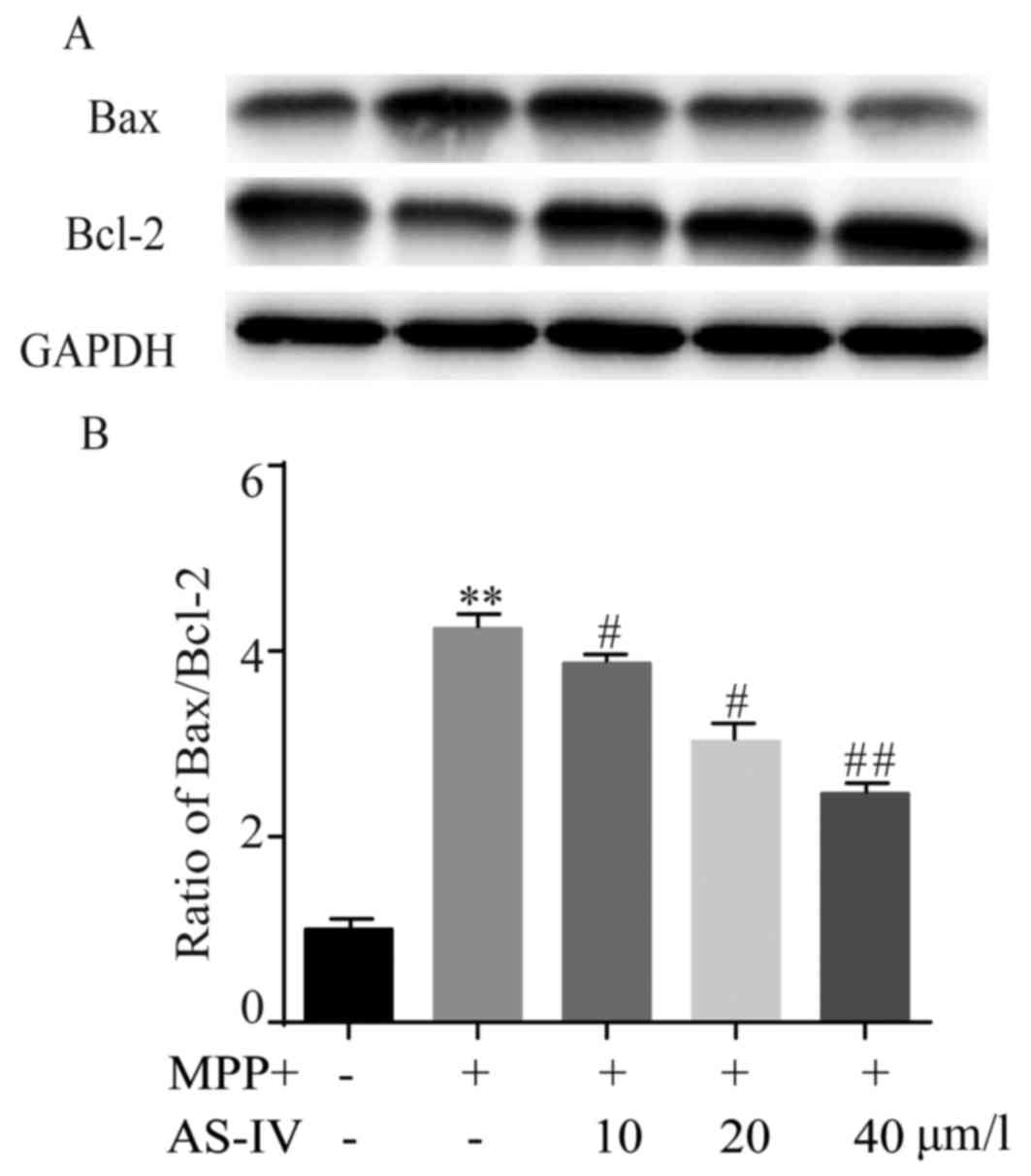 | Figure 5.AS-IV represses MPP+-induced rise of
Bax/Bcl-2 ratio in astrocytes. In MPP+ group, Bax protein level was
significantly higher and Bcl-2 protein level was lower compared to
NC group. MPP+-induced upregulation of Bax and downregulation of
Bcl-2 were reversed by AS-IV (A). After MPP+ treatment, Bax/Bcl-2
ratio was obviously higher than that in NC group, which was
remarkably attenuated by AS-IV (B). **P<0.01 MPP+ group vs. NC
group, #P<0.05, ##P<0.01 AS-IV groups
vs. MPTP group, respectively. NC, negative control; AS-IV,
astragaloside-IV; MPP+, 1-methyl-4-phenylpyridnium ion; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; Bax, Bcl-2-associated X
protein; MPTP, 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. |
AS-IV attenuates MPP+-induced cleaved
caspase-3 activation in astrocytes
MPP+ elevated the immunoreactivity of cleaved
caspase-3 significantly. Astrocytes that were co-administrated with
AS-IV (20, 40 µM/l) exhibited a significant lower caspase-3
activity than those treated with MPP+ (Fig. 6A). The statistical data were
consistent with the result of western blot (Fig. 6B).
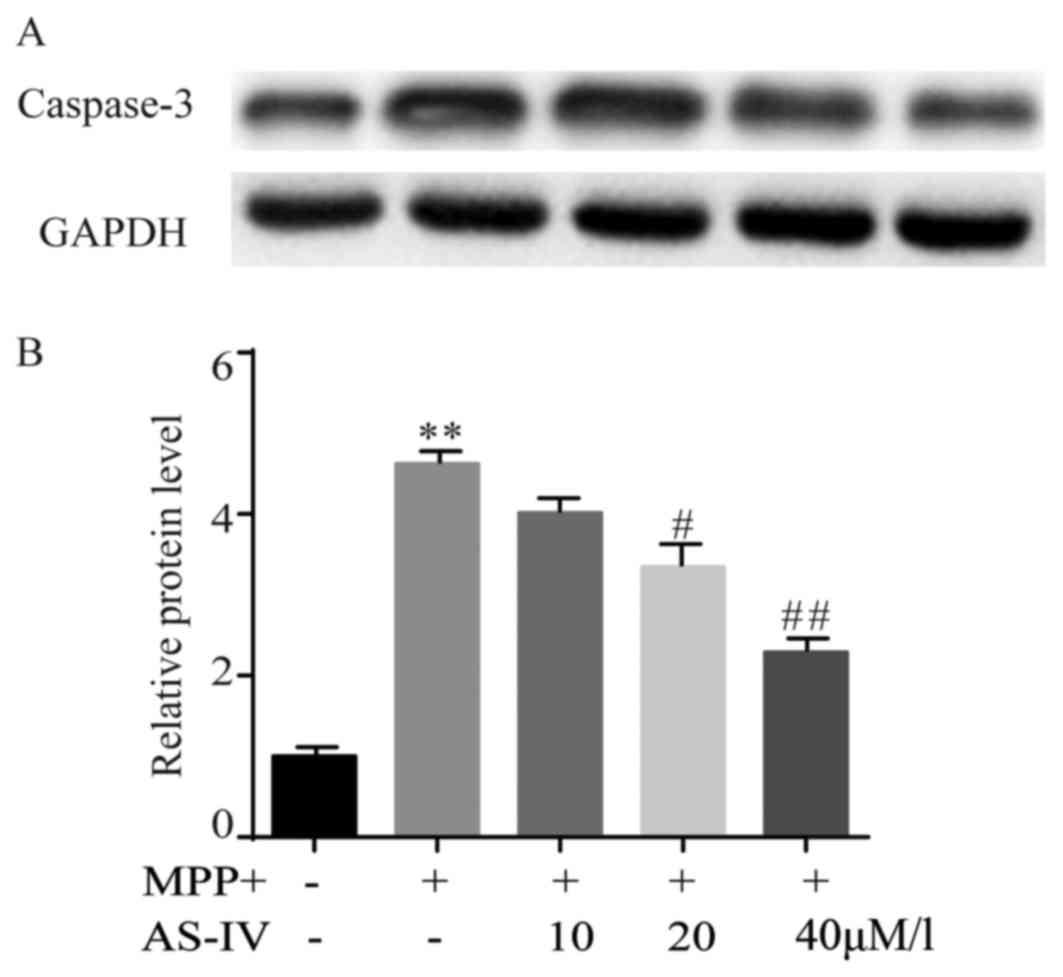 | Figure 6.AS-IV attenuates MPP+-induced cleaved
caspase-3 activation in astrocytes. MPP+ elevated the
immunoreactivity of cleaved caspase-3 significantly in comparison
with NC group. AS-IV (20, 40 uM/l) exhibited a significant lower
caspase-3 activity compared to MPP+ group (A). The corresponding
statistical data were consistent with the result of western blot
(B). **P<0.01 MPP+ group vs. NC group, #P<0.05,
##P<0.01 AS-IV group vs. MPTP group. NC, negative
control; AS-IV, astragaloside-IV; MPP+ 1-methyl-4-phenylpyridnium
ion; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MPTP,
1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. |
Discussion
We found that after injection of MPTP within 6 h,
mice in model group presented acute responses, including
piloerection and fremitus. While at 4th-10th day after PD model
establishment, mice exhibited dyskinesia (chronic responses), for
instance, expansion of climbing time and decline of
suspension/swimming time, which were consistent with previous
reported studies (20–22). Taken together, these behavioral tests
demonstrated the successful obtainment of PD model in vivo.
And AS-IV dose-dependently attenuated the responses of PD mice,
moreover, 6 mg/kg AS-IV significantly improved the aforementioned
behavioral deficiencies. Mice injected with only 6 mg/kg AS-IV did
not exhibit obvious behavioral changes, which suggested that
protective effects of AS-IV on PD mice have been dependent on
changes of intracellular signaling pathways.
We are eager to explore the effects of MPP+ on PD
model in vitro. We first investigated whether AS-IV alone
showed cytotoxicity on astrocytes. MTT assay was carried out and
verified that AS-IV (10, 20 and 40 µM) did not exhibit serious
toxic effects in vitro. Thereafter, we performed the
experiments as followed.
Effects of AS-IV on MPP+ induced astrocyte cell
apoptosis was conducted by FITC. After the establishment of PD
model in vitro, higher cell apoptosis rate was found in MPP+
group compared with NC group, which was dose-dependently and
significantly rescued by co-administration of 20 or 40 µM/l AS-IV.
These data suggested that AS-IV served as a protective role against
MPP+-induced cytotoxicity in astrocytes.
Meanwhile, we evaluated the influence of AS-IV on
cell viability of astrocytes. MTT assay indicated significantly
lower cell viability in MPP+ group than that in NC group, and AS-IV
(10, 20 and 40 µM/l) co-administration elevated MPP+ induced
downregulation of cell viability a dose-dependent manner.
JNK signaling pathway was known to be implicated in
numerous kinds of stress-mediated apoptosis, for instance, nerve
growth factor withdrawal, excitotoxic stress and oxidative stress
(23,24). And elevation of p-JNK (activated JNK)
was discovered in SNc of MPTP-treated PD mice (25,26).
Mounting evidences manifested the involvement of JNK in the process
of cell apoptosis (27). We did
western blot to assess protein level of p-JNK after MPP+ or
MPP+/AS-IV treatments. Interestingly, compared with NC group, MPP+
upregulated p-JNK protein level, indicating the response of p-JNK
to MPP+ in astrocytes, co-treatment of AS-IV (10, 20 and 40 µM/l)
downregulated MPP+-induced elevation of p-JNK. Thus, the data
suggested that AS-IV might exert an anti-apoptotic effect via
suppressing JNK apoptotic pathway.
Tumor-suppressor protein p53 was discovered to be
activated following exposure to MPTP (28,29).
Furthermore, JNK signaling pathway collaborated with p53 in
activating Bax and resulting in Bax-mediated cell apoptosis.
Members of Bcl-2 family were reported to participate in the process
of MPP+ generated cell death (30).
Moreover, studies also indicated the importance of Bax/Bcl-2 ratio
in determining cell fate (31). We
conducted western blot to explore whether Bax and Bcl-2 were
affected by MPP+ in vitro. Results showed that, in MPP+
group, Bax protein level was significantly higher, while Bcl-2
protein level was significantly lower compared to NC group, both of
which were reversed by AS-IV. Compared with NC group, obviously
higher Bax/Bcl-2 ratio was found in MPP+ group which was in
consistent with previous studies (32), and the elevated ratio was remarkably
attenuated by AS-IV. These data indicated that the decline of
Bax/Bcl-2 ratio by AS-IV might due to repression of p-JNK protein
level, an upstream regulator of Bax/Bcl-2.
In apoptotic cells, caspase-3 could be activated by
extrinsic (death ligand) and intrinsic (mitochondrial) pathways
(33,34). A recent study reported that in
SH-SY5Y cells, AS-IV significantly reversed MPP+-induced elevated
activity of caspase-3 (35). We
carried out western blot to investigate the effects of AS-IV on
caspase-3 in astrocytes. Results indicated that MPP+ elevated the
immunoreactivity of cleaved caspase-3 significantly. And astrocytes
that were co-administrated with AS-IV (20, 40 µM/l) exhibited a
significant lower caspase-3 activity than those treated with
MPP+.
Taken together, we proposed that AS-IV might be a
neuroprotective agent for PD via repressing the activation of
JNK/Bax/Bcl2/caspase-3 signaling pathway.
Acknowledgements
We are appreciated for the kind help on experiments
from Feng Xiao and Guanliang Cheng (Department of Neurology,
Huai'an First People's Hospital, Nanjing Medical University).
References
|
1
|
Franco-Iborra S, Vila M and Perier C: The
Parkinson disease mitochondrial hypothesis: Where are we at?
Neuroscientist. 22:266–277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camilleri A and Vassallo N: The centrality
of mitochondria in the pathogenesis and treatment of Parkinson's
disease. CNS Neurosci Ther. 20:591–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanchez-Guajardo V, Tentillier N and
Romero-Ramos M: The relation between α-synuclein and microglia in
Parkinson's disease: Recent developments. Neuroscience. 302:47–58.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie A, Gao J, Xu L and Meng D: Shared
mechanisms of neurodegeneration in Alzheimer's disease and
Parkinson's disease. Biomed Res Int. 2014:6487802014. View Article : Google Scholar
|
|
5
|
Takeuchi H, Mizuno T, Zhang G, Wang J,
Kawanokuchi J, Kuno R and Suzumura A: Neuritic beading induced by
activated microglia is an early feature of neuronal dysfunction
toward neuronal death by inhibition of mitochondrial respiration
and axonal transport. J Biol Chem. 280:10444–10454. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Y, Qin Z, Hong Z, Zhang X, Ding D, Fu
JH, Zhang WD and Chen J: Astragaloside IV protects against ischemic
brain injury in a murine model of transient focal ischemia.
Neurosci Lett. 363:218–223. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu YY, Zhu JX, Bian T, Gao F, Qian XF, Du
Q, Yuan MY, Sun H, Shi LZ and Yu MH: Protective effects of
astragaloside IV against ovalbumin-induced lung inflammation are
regulated/mediated by T-bet/GATA-3. Pharmacology. 94:51–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu L, Yin G, Cheng L, Fan Y, Xiao W, Yu
G, Xing M, Jia R, Sun R, Ma X, et al: Astragaloside IV ameliorates
acute pancreatitis in rats by inhibiting the activation of nuclear
factor-κB. Int J Mol Med. 35:625–636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang WD, Zhang C, Liu RH, Li HL, Zhang
JT, Mao C, Moran S and Chen CL: Preclinical pharmacokinetics and
tissue distribution of a natural cardioprotective agent
astragaloside IV in rats and dogs. Life Sci. 79:808–815. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seifert G, Schilling K and Steinhauser C:
Astrocyte dysfunction in neurological disorders: A molecular
perspective. Nat Rev Neurosci. 7:194–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kobayashi K, Hayashi M, Nakano H, Fukutani
Y, Sasaki K, Shimazaki M and Koshino Y: Apoptosis of astrocytes
with enhanced lysosomal activity and oligodendrocytes in white
matter lesions in Alzheimer's disease. Neuropathol Appl Neurobiol.
28:238–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szydlowska K, Zawadzka M and Kaminska B:
Neuroprotectant FK506 inhibits glutamate-induced apoptosis of
astrocytes in vitro and in vivo. J Neurochem. 99:965–975. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee J, Ziering A, Heineman SD and Berger
L: Piperidine derivatives; 2-phenyl- and 2-phenylalkyl-piperidines.
J Org Chem. 12:885–893. 1947. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pereira E A C and Aziz T Z: Parkinson's
disease and primate research: past, present, and future.
Postgraduate medical journal. 82:293–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Porras G, Li Q and Bezard E: Modeling
Parkinson's disease in primates: the MPTP model. Cold Spring Harbor
perspectives in medicine. 2:a0093082012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seniuk NA, Tatton WG and Greenwood CE:
Dose-dependent destruction of the coeruleus-cortical and
nigral-striatal projections by MPTP. Brain Res. 527:7–20. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hantraye P, Varastet M, Peschanski M,
Riche D, Cesaro P, Willer JC and Maziere M: Stable parkinsonian
syndrome and uneven loss of striatal dopamine fibres following
chronic MPTP administration in baboons. Neuroscience. 53:169–178.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Przedborski S and Jackson-Lewis V:
Mechanisms of MPTP toxicity. Mov Disord. 13 Suppl 1:S35–S38.
1998.
|
|
19
|
Dauer W and Przedborski S: Parkinson's
disease: Mechanisms and models. Neuron. 39:889–909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogawa N, Hirose Y, Ohara S, Ono T and
Watanabe Y: A sireple quantitative hradykinesia test in MPIP
treated mice. Res Commun Chem Pathol Pharmacol. 50:435–441.
1985.PubMed/NCBI
|
|
21
|
Kubara H, Higuchi Y and Tadokoro S:
Effects of central depressants on rota-rod and action performances
in mice. Jpn J Pharmacol. 27:117–126. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Donnan GA, Willjs GL, Kaczmarczyk SJ and
Rowe P: Motor function in the l,
methyl-4-phenyl-l,2,3,6-tetrahydropyridine treated mouse. J Neurol
Sci. 77:185–191. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dickens M, Rogers JS, Cavanagh J, Raitano
A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL and Davis RJ: A
cytoplasmic inhibitor of the JNK signal transduction pathway.
Science. 277:693–696. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saporito MS, Thomas BA and Scott RW: MPTP
activates c-Jun NH(2)-terminal kinase (JNK) and its upstream
regulatory kinase MKK4 in nigrostriatal neurons in vivo. J
Neurochem. 75:1200–1208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia XG, Harding T, Weller M, Bieneman A,
Uney JB and Schulz JB: Gene transfer of the JNK interacting
protein-1 protects dopaminergic neurons in the MPTP model of
Parkinson's disease. Proc Natl Acad Sci USA. 98:pp. 10433–10438.
2001, View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lotharius J, Falsig J, van Beek J, Payne
S, Dringen R, Brundin P and Leist M: Progressive degeneration of
human mesencephalic neuron-derived cells triggered by
dopamine-dependent oxidative stress is dependent on the
mixed-lineage kinase pathway. J Neurosci. 25:6329–6342. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Findley HW, Gu L, Yeager AM and Zhou M:
Expression and regulation of Bcl-2, Bcl-xl, and Bax correlate with
p53 status and sensitivity to apoptosis in childhood acute
lymphoblastic leukemia. Blood. 89:2986–2993. 1997.PubMed/NCBI
|
|
29
|
Mandir AS, Przedborski S, JacksonLewis V,
Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, Hoffman BE, Guastella
DB, Dawson VL and Dawson TM: Poly(ADP-ribose) polymerase activation
mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine
(MPTP)-induced parkinsonism. Proc Natl Acad Sci USA. 96:pp.
5774–5779. 1999, View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O'Malley KL, Liu J, Lotharius J and Holtz
W: Targeted expression of BCL-2 attenuates MPP+ but not 6-OHDA
induced cell death in dopaminergic neurons. Neurobiol Dis.
14:43–51. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blum D, Torch S, Lambeng N, Nissou M,
Benabid AL, Sadoul R and Verna JM: Molecular pathways involved in
the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the
apoptotic theory in Parkinson's disease. Prog Neurobiol.
65:135–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salvesen GS: Caspases: Opening the boxes
and interpreting the arrows. Cell Death Differ. 9:3–5. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ and
Los M: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang ZG, Wu L, Wang JL, Yang JD, Zhang J,
Zhang J, Li LH, Xia Y, Yao LB, Qin HZ and Gao GD: Astragaloside IV
prevents MPP+-induced SH-SY5Y cell death via the inhibition of
Bax-mediated pathways and ROS production. Mol Cell Biochem.
364:209–216. 2012. View Article : Google Scholar : PubMed/NCBI
|