Introduction
Colorectal cancer (CRC) is one of the most common
epithelial cancers (1).
Epidemiologically, CRC is the third most prevalent cancer worldwide
with a high mortality rate in males and females (2). Although CRC management and therapy are
performed by screening, surgery, adjuvant irradiation and
chemotherapy, CRC remains one of the most life-threatening
malignancies, particularly when it reaches the advanced stages
(3,4). Approximately 50% of patients with CRC
develop metastasis, which is usually incurable and fatal (5). The majority of patients with CRC and
distant metastasis are not suitable candidates for conventional
intervention and exhibit a poor 5-year survival rate of <10%
(6). From a therapeutic perspective,
the identification of molecular mechanisms underlying the
metastatic progression of CRC may contribute to the reduction of
morbidity and mortality (7). In
addition, the discovery of effective and safe compounds for the
treatment of CRC is urgently required in order to reduce morbidity
and mortality rates.
Tumor metastasis is a complex process, and is highly
regulated by multiple mechanisms, including aberrant activation of
the phosphoinositide 3-kinase (PI3K)/AKT and transforming growth
factor (TGF)-β/Smad pathways (8–11).
Furthermore, a large number of studies have shown that matrix
metalloproteinase (MMP) overexpression is involved in numerous
malignant tumors, including esophageal cancer, breast cancer, liver
cancer and rectal cancer (12–16).
MMPs serve a very important role in tumor invasion and metastasis
(12). In addition,
epithelial-mesenchymal transition (EMT) is closely associated with
tumor occurrence and metastasis (12,17).
Furthermore, the expression of MMP family-related factors or
N-cadherin/E-cadherin is regulated by the aforementioned pathways
in an interactive manner (18,19). As
a result, targeting PI3K/AKT and TGF-β/Smad pathways may represent
a novel therapeutic method to prevent metastasis without causing
side effects.
Traditional Chinese medicine (TCM) is of interest to
researchers as it induces relatively few side effects and has been
clinically used for thousands of years as an important alternative
remedy for a variety of diseases (20–23).
TCMs are considered to be multi-component and multi-targeted agents
that exert their therapeutic functions holistically (24). Scutellaria barbata D. Don (SB)
is a medicinal herb widely distributed in northeast Asia. In TCM,
SB is a well-known herb considered to be useful for heat-clearing,
detoxification, promotion of blood circulation and removal of blood
stasis (25). SB has long been used
as an important component in several TCM formulas for the clinical
treatment of various types of cancer. SB extracts have been shown
to inhibit the growth of numerous cancer cell types (26–30). In
a previous study, it was reported that SB promotes cancer cell
apoptosis via activation of the mitochondrial-dependent pathway
(31). However, studies in which the
anticancer effect of SB and its mechanisms are elucidated,
particularly studies relating to metastasis, are lacking. In the
present study, the effects of SB on the migration and invasion
abilities of HCT-8 human colorectal carcinoma cells and their
regulation through PI3K/AKT and TGF-β/Smad signaling pathways were
evaluated.
Materials and methods
Materials and reagents
RPMI-1640 medium, fetal bovine serum (FBS),
penicillin-streptomycin and trypsin-EDTA were obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA). Rabbit polyclonal
antibodies against AKT (cat. no. 10176-2-AP) and PI3K (cat. no.
13329-1-AP) were purchased from Proteintech Group (Wuhan, China).
Rabbit polyclonal antibodies against phospho (p)-AKT (cat. no.
sc-135650) and p-PI3K (cat. no. sc-12929), and goat polyclonal
antibodies against phosphatase and tensin homolog (PTEN) (cat. no.
sc-6818) were purchased from Santa Cruz Biotechnology (Shanghai)
Co., Ltd. (Shanghai, China). Rabbit polyclonal antibodies against
MMP1 (cat. no. D120093), 2 (cat. no. D161446), 9 (cat. no. D120097)
and 13 (cat. no. D120098)] were purchased from Sangon Biotech Co.,
Ltd. (Shanghai, China), and MMP3/10 (cat. no. sc-30070) was
purchased from Santa Cruz Biotechnology (Shanghai) Co., Ltd. Mouse
monoclonal antibodies against E-cadherin (cat. no. ab76055) and
N-cadherin (cat. no. ab98952) were purchased from Abcam (Hong Kong)
Ltd. (Hong Kong, China). Rabbit polyclonal antibodies against
TGF-β1 (cat. no. 3711S), Smad4 (cat. no. 38454S), Smad2/3 (cat. no.
8685S) and β-actin (cat. no. 4967), and horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. no. 7074) were provided
by Cell Signaling Technology, Inc. (Beverly, MA, USA). Transwell
chambers were obtained from Corning Life Sciences (Tewksbury, MA,
USA). BD BioCoat Matrigel Invasion Chamber was purchased from BD
Biosciences (San Jose, CA, USA). All other chemicals were obtained
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) unless
otherwise stated.
Ethanol extract of SB (EESB)
preparation
Authentic plant material was purchased from Guo Yi
Tang Chinese herbal medicine store (Fujian, China). The original
herb was identified as SB by Dr Wei Xu at the Department of
Pharmacology, Fujian University of Traditional Chinese Medicine
(Fuzhou, China). The plants were dried and cut into small pieces,
and EESB was obtained as previously described (32). EESB stock solutions were prepared by
dissolving EESB powder in PBS at a concentration of 500 mg/ml, and
stored at −20°C. EESB working concentrations were obtained by
diluting the stock solution in the culture medium.
Cell culture
HCT-8 human colorectal carcinoma cells were obtained
from Nanjing KeyGen Biotech. Co., Ltd. (Nanjing, China). Cells were
grown in RPMI-1640 medium containing 10% (v/v) FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin in a 37°C humidified
incubator with 5% CO2. Cells were digested at room
temperature for 3 min using trypsin-EDTA and subcultured when
80–90% confluency was reached.
MTT assay
Cell viability was assessed using an MTT
colorimetric assay. Cells were harvested, re-suspended at a final
concentration of 1×105 cells/ml and then seeded into
96-well plates at a volume of 100 µl/well. After 12 h incubation at
37°C, cells were treated with EESB at different concentrations (0,
0.125, 0.25, 0.5, 1, 1.5 and 2 mg/ml) and incubated for 24 or 48 h.
Subsequently, 100 µl MTT (0.5 mg/ml) was added to each well. The
plates were incubated at 37°C for 4 h, and 100 µl DMSO was added to
dissolve the purple formazan crystals. The absorbance was read at
570 nm using an ELISA reader (Model ELx800; BioTek Instruments,
Inc., Winooski, VT, USA).
Microscopic observation of cell
density
HCT-8 cells were seeded into 6-well plates at a
density of 5×105 cells/well in 2 ml medium. Cells were
treated with EESB at different concentrations (0, 0.125, 0.25 and
0.5 mg/ml) and incubated for 24 h. Cell density was observed using
a phase-contrast microscope (Leica Microsystems GmbH, Wetzlar,
Germany). Images were captured at a magnification of ×200.
Cell migration and invasion analysis
using Transwell assays
Migration assays were performed using Transwell cell
culture chambers with 8-µm pore filters (Corning Life Sciences).
Following treatment with EESB at different concentrations (0,
0.125, 0.25 and 0.5 mg/ml) for 24 h, HCT-8 cells were trypsinized
and resuspended in serum-free RPMI-1640. A total of
5×104 cells in 200 µl serum-free RPMI-1640 were plated
in the upper chamber. RPMI-1640 media containing 10% (v/v) FBS was
placed in the lower chamber as a chemoattractant. Cells were
allowed to migrate for 12 h, and the non-migrated cells were
removed from the upper surface of the Transwell membranes using a
cotton swab. Membranes were fixed with ice-cold 4% paraformaldehyde
for 10 min and stained using crystal violet at room temperature for
15 min. The average number of migrating cells per field was
assessed by counting three random fields under a phase-contrast
microscope (Leica) at a magnification of ×200. The procedure for
the cell invasion assay was the same as that described for the
migration assay, with the exception that the upper chamber was
coated with Matrigel Matrix (BD Biosciences).
Western blot analysis
HCT-8 cells were seeded into 25-cm2
flasks at a density of 1.25×106 cells/flask in 5 ml
medium. Following incubation for 12 h, cells were treated with EESB
at different concentrations (0, 0.125, 0.25 and 0.5 mg/ml) and
incubated for 24 h. The treated cells were lysed using Pierce
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) containing EASYpack protease and PhosSTOP phosphatase
inhibitor cocktails (both Roche Diagnostics, Basel, Switzerland).
The lysates were then centrifuged at 17,000 × g for 20 min at 4°C,
and the total protein concentration was determined by BCA assay.
Equal amounts of total proteins (50 mg) were resolved using 10%
SDS-PAGE gels and then electroblotted onto nitrocellulose
membranes. The membranes were blocked with 5% nonfat dry milk at
room temperature for 2 h, and treated with primary antibodies
against E-cadherin (1:1,000), N-cadherin (1:1,000), TGF-β1
(1:1,000), Smad2/3 (1:1,000), Smad4 (1:1,000), AKT (1:500), p-AKT
(1:500), PTEN (1:500), PI3K (1:500), p-PI3K (1:500), MMP1
(1:1,000), MMP2 (1:1,000), MMP3/10 (1:1,000), MMP9 (1:1,000), MMP13
(1:1,000) and β-actin (1:1,000) overnight at 4°C. Subsequently, the
membranes were incubated with HRP-conjugated secondary antibody at
room temperature for 1 h and the protein bands were detected using
an enhanced chemiluminescence detection reagent, SuperSignal West
Pico Chemiluminescent substrate (Thermo Fisher Scientific, Inc.).
β-actin was used as the internal control. Images were taken using a
ChemiDoc XRS+ imaging system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Image Lab™ software (version 3.0; Bio-Rad Laboratories,
Inc.) was used for densitometric analysis and quantification of the
western blots.
Statistical analysis
All data were obtained as the mean of three
experiments. Statistical analysis was performed using SPSS software
(version 17.0) for Windows (SPSS, Inc., Chicago, IL, USA) using
one-way analysis of variance, followed by Fisher's least
significant difference and Dunnett's tests. Data are expressed as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Effect of EESB on HCT-8 cell
viability
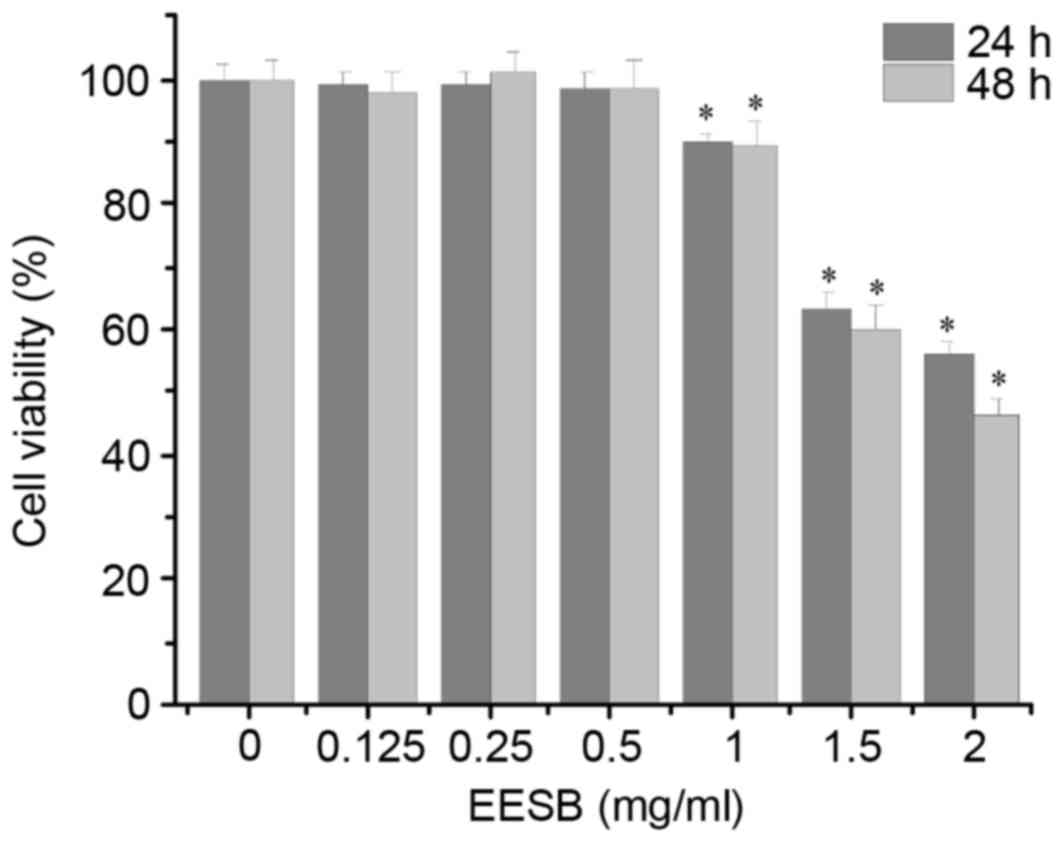
As shown in Fig. 1,
EESB at low concentrations (0.125, 0.25 and 0.5 mg/ml) did not
exhibit a significant effect on HCT-8 cell proliferation, while
EESB at high concentrations (1, 1.5 and 2 mg/ml) significantly
inhibited HCT-8 cell growth compared with that of the untreated
cells. On the basis of these results, EESB concentrations of 0.125,
0.25 and 0.5 mg/ml were selected for the subsequent experiments. To
further verify that the selected concentrations of EESB were not
cytotoxic, the effect of EESB on HCT-8 cell density was analyzed
under a microscope. As shown in Fig.
2, as the drug concentration increased from 0 to 0.5 mg/ml,
there was no clear change in cell density, which indicated that
these low doses of EESB had no marked effect on cell growth.
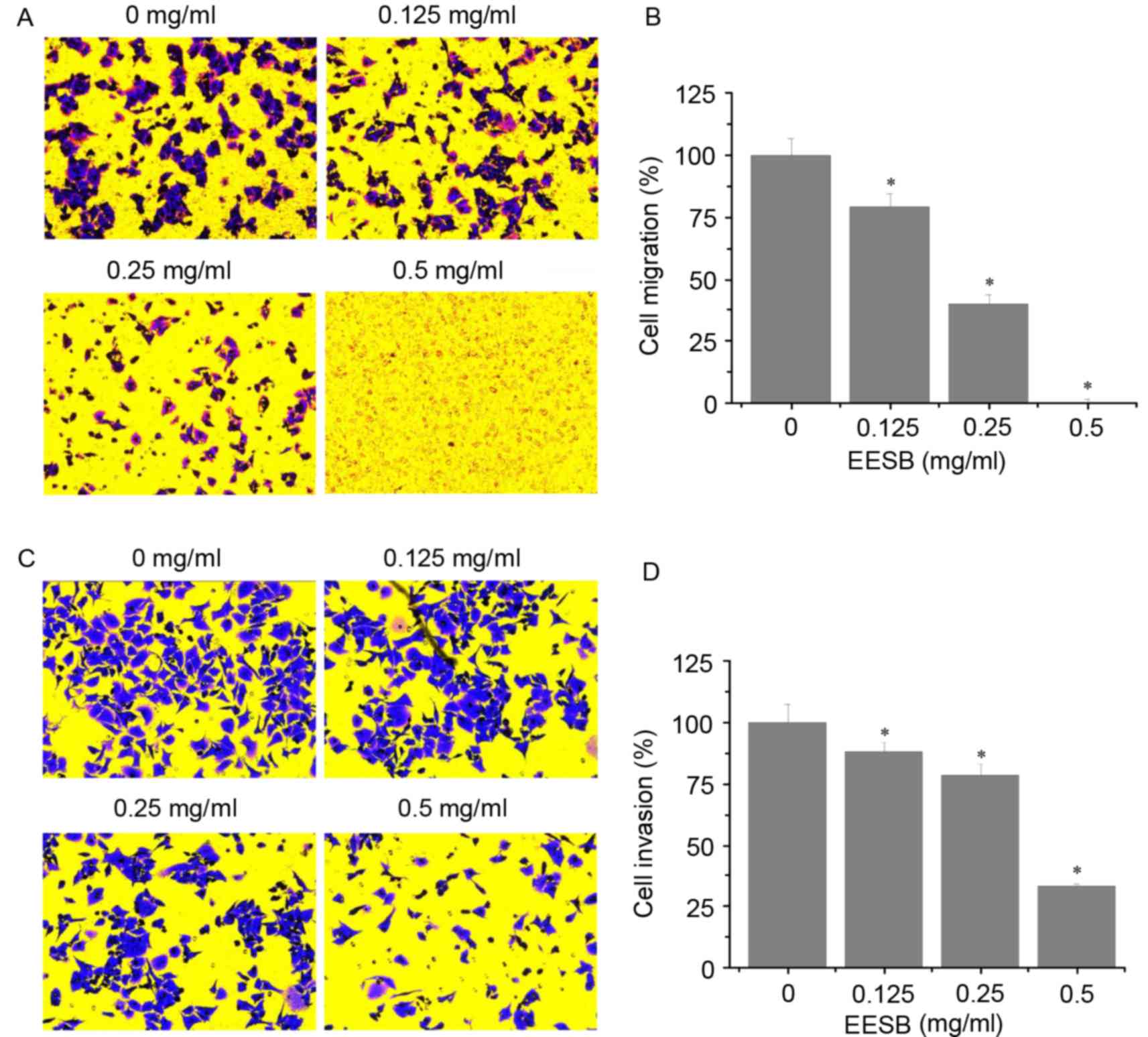
Effect of EESB on HCT-8 cell migration
and invasion
As shown in Fig. 3A and
B, EESB significantly reduced the number of migrated cells
compared with the untreated control. Similarly, EESB treatment
significantly reduced cell invasion through the Matrigel membrane
compared with the untreated control (Fig. 3C and D). The inhibitory effects on
migration and invasion were concentration-dependent. The number of
migrated cells was reduced by 20.89% using 0.125 mg/ml EESB and by
99.89% using 0.5 mg/ml EESB, as compared with the untreated control
cells (Fig. 3B). The invasion assay
results showed that EESB treatment for 24 h reduced the HCT-8 cell
invasion rate by 11.86% when 0.125 mg/ml EESB was used and by
66.90% when 0.5 mg/ml EESB was used, as compared with the untreated
control cells (Fig. 3D).
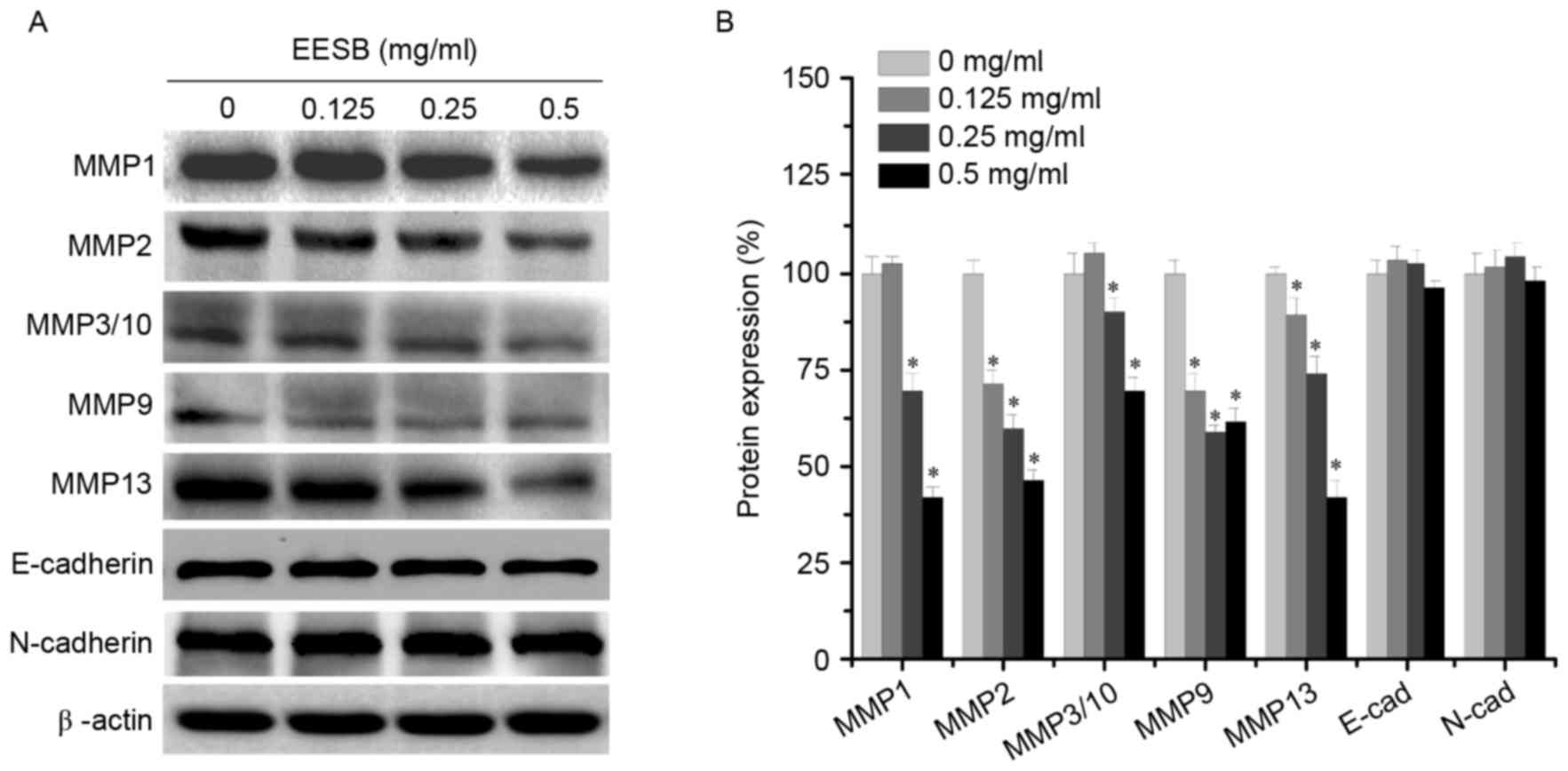
Effect of EESB on MMP and
E-/N-cadherin expression
To elucidate the antimetastatic mechanisms of EESB,
the expression levels of MMPs (MMP1, MMP2, MMP3/10, MMP9 and MMP13)
and the EMT-regulated factors (E-cadherin and N-cadherin) were
analyzed using western blotting. As shown in Fig. 4, EESB significantly inhibited the
expression of MMP1, MMP2, MMP3/10, MMP9 and MMP13 to different
extents, but exerted only a slight effect on the expression of the
mesenchymal marker N-cadherin and the epithelial marker E-cadherin.
These results indicated that EESB inhibited HCT-8 metastasis via
the suppression of MMP expression but not via EMT.
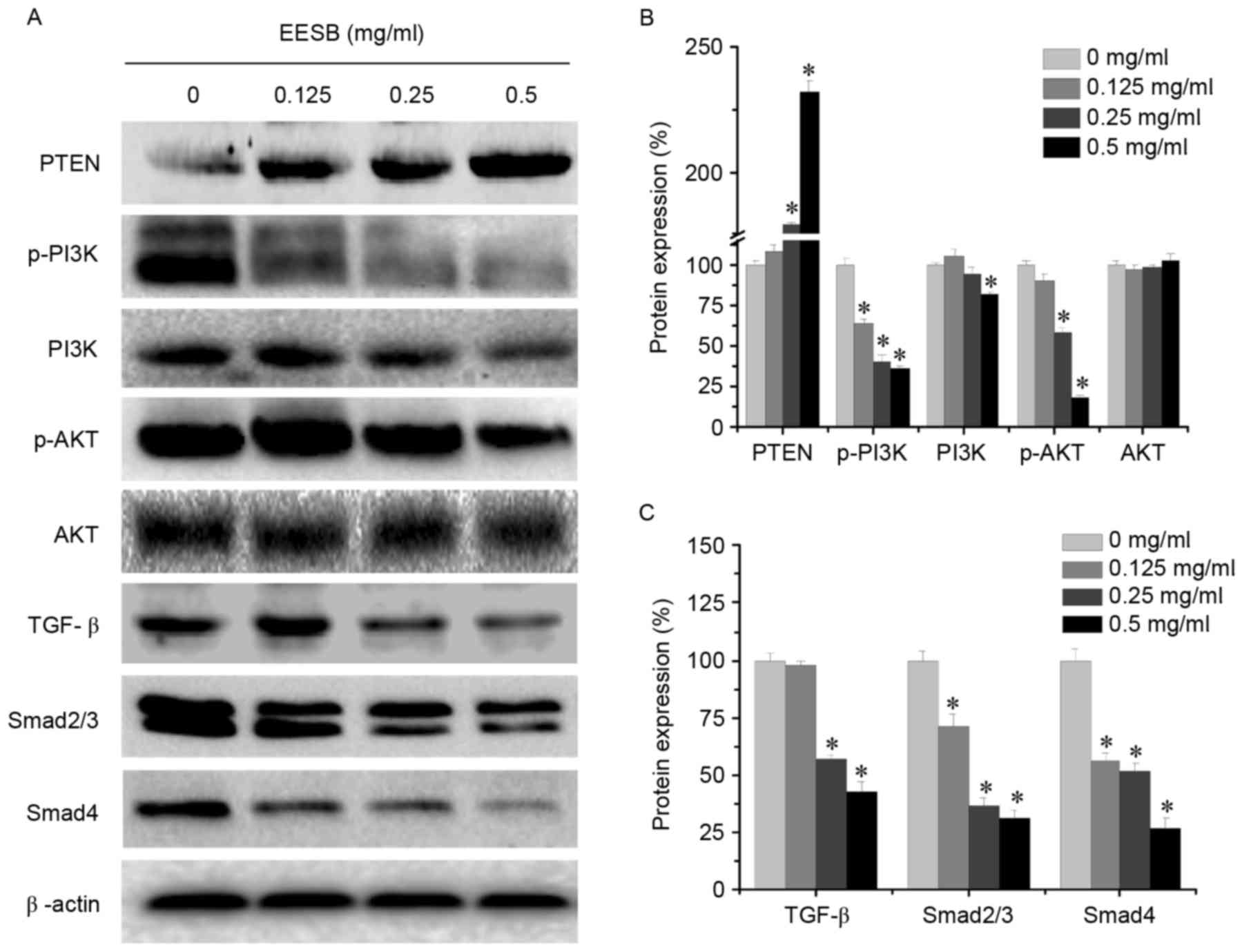
Effect of EESB on PI3K/AKT and
TGF-β/Smad signaling pathways
EESB markedly suppressed the activation of the
PI3K/AKT pathway by significantly downregulating p-PI3K, PI3K and
p-AKT protein levels (Fig. 5). In
addition, PTEN, which is a tumor suppressor and PI3K/AKT upstream
factor (33), was significantly
upregulated following EESB treatment. Furthermore, EESB treatment
significantly inhibited the expression of TGF-β, Smad2/3 and Smad4.
These results suggested that the antimetastatic effect of EESB on
CRC may be partly mediated by suppression of the PI3K/AKT and
TGF-β/Smad signaling pathways.
Discussion
CRC remains a potentially lethal disease with a poor
prognosis, mostly due to metastasis in the majority of patients.
Multi-drug combination therapies have been developed leading to
significantly improved patient response and overall survival.
However, resistance to these drugs is inevitable and continues to
be a notable problem (34,35). Therefore, novel agents, including
natural products, are currently being considered for more efficient
cancer treatment. TCM, with its relatively high safety and long
history of pharmacological applications, has attracted attention in
the field of cancer treatment (20,23). SB
is a herb used in TCM formulations, where it is considered to have
‘heat-clearing and detoxifying’ actions, and has many reported
applications in cancer treatment (36–41).
Similar to other medicinal herbs, SB is a multi-targeted agent that
is considered to exert its therapeutic function holistically
(27,31,32,42);
thus, it may be a good candidate as an anticancer drug. However,
the specific mechanism of its anticancer effect, particularly its
antimetastatic ability, is not yet clear.
The process of tumor metastasis is complex. One of
the most studied mechanisms relates to MMP involvement. MMPs are a
group of important proteases that degrade extracellular matrix
(ECM). Numerous studies have shown that MMPs are associated with
tumor growth, metastasis and invasion (43–45). The
main members of the family may be divided into five groups
according to their domain, enzyme and substrate specificity as
follows: Collagenase (MMP1, MMP8 and MMP13), gelatinase (MMP2 and
MMP9), matrix soluble elements (MMP3, MMP7, MMP10 and MMP11) and
matrix dissolution factor and membrane type (MT) metalloproteinase
(MT1-MMP, MT2-MMP and MT3-MMP) (46). It has been reported that MMP1
expression is increased significantly in gastric cancer, which
destroys the basement membrane, and promotes tumor
lymphangiogenesis, tumor invasion and metastasis (47). MMP2 has been demonstrated to be
closely associated with migration and invasion in several types of
tumors, including breast cancer, ovarian cancer and lung cancer
(48,49). MMP9 expression was identified to be
increased in osteosarcoma, lung cancer, pancreatic cancer and CRC
tissues to different extents, which was positively correlated with
tumor metastasis (48–50). Similarly, it has been observed that
MMP3 and MMP10 expression levels in lung cancer, esophageal cancer,
liver cancer and endometrial adenocarcinoma tissues are higher than
those in normal tissues, and that their expression has an
association with tumor invasion, metastasis and proliferation
(51–55). In addition, a review of a number of
studies has demonstrated that MMP13 expression in malignant tumors,
such as colon cancer, breast cancer, non-small cell lung cancer and
oral squamous cell carcinoma, is closely associated with tumor
occurrence, development, invasion and metastasis (56). On the basis of this evidence, MMPs
have a close association with tumor invasion and metastasis,
serving a very important role. In the present study, EESB
downregulated the expression of the MMPs, to different extents,
suggesting that EESB may inhibit CRC metastasis by inhibiting the
expression of these specific MMPs to balance the ECM
environment.
EMT is an important phenomenon in the occurrence and
development of tumors and is also an important mechanism allowing
tumor invasion and metastasis (57,58).
E-cadherin and N-cadherin are two important factors in the
maintenance of the EMT balance; EMT regulation is influenced by
these two cadherins (17). However,
in the present study, EESB showed only a weak effect on the
expression of the mesenchymal marker N-cadherin and epithelial
marker E-cadherin, suggesting that EESB inhibits CRC metastasis
thorough mechanisms other than EMT.
In addition to the aforementioned factors, numerous
pathways also contribute to tumor metastasis. For example,
activation of the PI3K/AKT signaling pathway accelerates
angiogenesis, tumor invasion and metastasis through the disturbance
of tumor-suppressor PTEN or other causal factors, which is
important in the occurrence and development of malignant tumors
(59). Furthermore, the PI3K/AKT
signaling pathway is involved in regulating the expression of MMP-2
and MMP-9 in a variety of tumor tissues and cells, to regulate
multidrug resistance, as well as tumor invasion and metastasis
(60). Furthermore, TGF-β promotes
tumor metastasis by increasing tumor angiogenesis, immune
suppression, and the production and deposition of ECM (61). Previous studies have shown that TGF-β
may cause tumor metastasis through activation of the Smad pathway,
and Smad2 and Smad4 are important proteins that regulate the
transcription and the antiproliferative response of TGF-β, and
regulate the expression of genes downstream of TGF-β that are
involved in tumor metastasis (62,63).
Furthermore, TGF-β may mediate tumor cell invasion by regulating
ECM-degrading proteinases (64).
Among the increasing number of ECM-degrading proteinases implicated
in tumor cell invasion, the majority of the attention has been
focused on the MMP family and the plasminogen activator system
(65,66). TGF-β1 has been suggested to activate
the Smad signaling pathway, and significantly promote the
expression of MMPs and other invasion and metastasis-related genes
in highly invasive breast cancer cells, thereby enhancing the
ability of the cells to invade and metastasize (67,68). In
the present study, EESB decreased the expression of proteins in the
PI3K/AKT and TGF-β/Smad2/3 pathways, and upregulated the expression
of the tumor-suppressor PTEN, thus indicating that the inhibitory
effect of EESB on metastatic CRC cells may be mediated by the
suppression of certain members of the MMP family, and PI3K/AKT and
TGF-β/Smad signaling pathways.
In conclusion, EESB exerted significant
antimetastatic effects on CRC cells by inhibition of their
migration and invasion ability, and via the regulation of PI3K/AKT
and TGF-β/Smad signaling pathways. These mechanisms are potentially
those by which EESB exhibits its effectiveness in cancer
treatment.
Acknowledgements
The present study was sponsored by the Research Fund
for the Doctoral Program of Higher Education of China (grant no.
20133519110003), the Project Funding for the Training of Young and
Middle-aged Backbone Personnel of Fujian Provincial Health and
Family Planning Commission (grant no. 2016-ZQN-67), and the
Developmental Fund of Chen Keji Integrative Medicine (Fujian,
China; grant nos. CKJ2014013 and CKJ2015007).
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
EESB
|
ethanol extract of Scutellaria
barbata D. Don
|
|
TCM
|
traditional Chinese medicine
|
|
ECM
|
extracellular matrix
|
|
MMP
|
matrix metalloproteinase
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Tenesa A and Dunlop MG: New insights into
the aetiology of colorectal cancer from genome-wide association
studies. Nat Rev Genet. 10:353–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Grávalos C, Cassinello J, Fernández-Rañada
I and Holgado E: Role of tyrosine kinase inhibitors in the
treatment of advanced colorectal cancer. Clin Colorectal Cancer.
6:691–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E and Oliveira J: ESMO
Guidelines Working Group: Advanced colorectal cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20 Suppl 4:S61–S63. 2009. View Article : Google Scholar
|
|
6
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surg. 244:254–259. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: Historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shen A, Lin W, Chen Y, Liu L, Chen H,
Zhuang Q, Lin J, Sferra TJ and Peng J: Pien Tze Huang inhibits
metastasis of human colorectal carcinoma cells via modulation of
TGF-β1/ZEB/miR-200 signaling network. Int J Oncol. 46:685–690.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu T, Jing C, Shi Y, Miao R, Peng L, Kong
S, Ma Y and Li L: microRNA-20a enhances the
epithelial-to-mesenchymal transition of colorectal cancer cells by
modulating matrix metalloproteinases. Exp Ther Med. 10:683–688.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alam SK, Yadav VK, Bajaj S, Datta A, Dutta
SK, Bhattacharyya M, Bhattacharya S, Debnath S, Roy S, Boardman LA,
et al: DNA damage-induced ephrin-B2 reverse signaling promotes
chemoresistance and drives EMT in colorectal carcinoma harboring
mutant p53. Cell Death Differ. 23:707–722. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo S: Research progress in the mechanism
of colorectal cancer metastasis. J Mudanjiang Med College.
29:65–67. 2008.
|
|
13
|
Juchniewicz A, Kowalczuk O, Milewski R,
Laudański W, Dzięgielewski P, Kozłowski M and Nikliński J: MMP-10,
MMP-7, TIMP-1 and TIMP-2 mRNA expression in esophageal cancer. Acta
Biochim Pol. 64:295–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun EJ, Song KS, Shin S, Kim S, Heo JY,
Kweon GR, Wu T, Park JI and Lim K: Docosahexaenoic acid suppresses
breast cancer cell metastasis by targeting
matrix-metalloproteinases. Oncotarget. 7:49961–49971. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dou CY, Cao CJ, Wang Z, Zhang RH, Huang
LL, Lian JY, Xie WL and Wang LT: EFEMP1 inhibits migration of
hepatocellular carcinoma by regulating MMP2 and MMP9 via ERK1/2
activity. Oncol Rep. 35:3489–3495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fuksiewicz M, Kotowicz B, Rutkowski A and
Kowalska M: The matrix metalloproteinase-7 and pro-enzyme of
metalloproteinase-1 as a potential marker for patients with rectal
cancer without distant metastasis. Tumour Biol. 36:3629–3635. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang M, Zhuo N and Guo Z: Molecular
mechanism of epithelial-mesenchymal transition and its role in
tumor metastasis. Chin Med Herald. 11:163–165. 2014.
|
|
18
|
Wei F, Shen Q and Liu C: Phenethyl
isothiocyanate inhibits PI3K/NF-kB to down-regulate MMP-9
expression in human colon cancer cells. Med Sci J Central South
China. 42:351–354. 2014.
|
|
19
|
Liang S, Lv Y and Wang X: Study of the
correlation and expression of focal adhesion kinase and matrix
metalloproteinase-9 in colorectal carcinoma. J Colorectal Anal
Surger. 14:157–160. 2008.
|
|
20
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao J, Jiang P and Zhang W: Molecular
networks for the study of TCM pharmacology. Brief Bioinform.
11:417–430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract induces
apoptosis via activation of the mitochondrion-dependent pathway in
human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
23
|
Demain AL and Zhang L: Natural Products
and Drug Discovery. Can thousands of years of ancient medical
knowledge lead us to new and powerful drug combinations in the
fight against cancer and dementia? EMBO Rep. 10:194–200. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shen AL, Hong F, Liu LY, Lin JM, Zhuang
QC, Hong ZF and Peng J: Effects of Pien Tze Huang on angiogenesis
in vivo and in vitro. Chin J Integr Med. 18:431–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Read BE: The Chinese Pharmacopoeia. Can
Med Assoc J. 23:568–570. 1930.PubMed/NCBI
|
|
26
|
Cha YY, Lee EO, Lee HJ, Park YD, Ko SG,
Kim DH, Kim HM, Kang IC and Kim SH: Methylene chloride fraction of
Scutellaria barbata induces apoptosis in human U937 leukemia cells
via the mitochondrial signaling pathway. Clin Chim Acta. 348:41–48.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin J, Chen Y, Cai Q, Wei L, Zhan Y, Shen
A, Sferra TJ and Peng J: Scutellaria barbata D Don inhibits
colorectal cancer growth via suppression of multiple signaling
pathways. Integr Cancer Ther. 13:240–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suh SJ, Yoon JW, Lee TK, Jin UH, Kim SL,
Kim MS, Kwon DY, Lee YC and Kim CH: Chemoprevention of Scutellaria
bardata on human cancer cells and tumorigenesis in skin cancer.
Phytother Res. 21:135–141. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin X, Zhou J, Jie C, Xing D and Zhang Y:
Anticancer activity and mechanism of Scutellaria barbata extract on
human lung cancer cell line A549. Life Sci. 75:2233–2244. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee TK, Lee DK, Kim DI, Lee YC, Chang YC
and Kim CH: Inhibitory effects of Scutellaria barbata D. Don on
human uterine leiomyomal smooth muscle cell proliferation through
cell cycle analysis. Int Immunopharmacol. 4:447–454. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei L, Chen Y, Lin J, Zhao J, Chen X, Xu
W, Liu X, Sferra T and Peng J: Scutellaria barbata D. Don induces
apoptosis of human colon carcinoma cell through activation of the
mitochondrion-dependent pathway. J Med Plants Res. 5:1962–1970.
2011.
|
|
32
|
Wei L, Lin J, Wu G, Xu W, Li H, Hong Z and
Peng J: Scutellaria barbata D. Don induces G1/S arrest via
modulation of p53 and Akt pathways in human colon carcinoma cells.
Oncol Rep. 29:1623–1628. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Georgescu MM: PTEN tumor suppressor
network in PI3K-Akt pathway control. Genes Cancer. 1:1170–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodel C, Hofheinz R and Liersch T: Rectal
cancer: State of the art in 2012. Curr Opin Oncol. 24:441–447.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan P, Lu B and Bao W: Analysis on the
clinical application of Scutellaria barbata D. Don in the
anti-cancer therapy. Jiangxi Tradit China Med. 37:57–58. 2006.
|
|
37
|
Dai ZJ, Liu XX, Tang W, Xue Q, Wang XJ, Ji
ZZ, Kang HF and Diao Y: Antitumor and immune-modulating effects of
Scutellaria barbata extract in mice bearing hepatocarcinoma H22
cells-derived tumor. Nan Fang Yi Ke Da Xue Xue Bao. 28:1835–1837.
2008.(In Chinese). PubMed/NCBI
|
|
38
|
Goh D, Lee YH and Ong ES: Inhibitory
effects of a chemically standardized extract from Scutellaria
barbata in human colon cancer cell lines, LoVo. J Agric Food Chem.
53:8197–8204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marconett CN, Morgenstern TJ, San Roman
AK, Sundar SN, Singhal AK and Firestone GL: BZL101, a phytochemical
extract from the Scutellaria barbata plant, disrupts proliferation
of human breast and prostate cancer cells through distinct
mechanisms dependent on the cancer cell phenotype. Cancer Biol
Ther. 10:397–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wong BY, Nguyen DL, Lin T, Wong HH,
Cavalcante A, Greenberg NM, Hausted RP and Zheng J: Chinese
medicinal herb Scutellaria barbata modulates apoptosis and cell
survival in murine and human prostate cancer cells and tumor
development in TRAMP mice. Eur J Cancer Prev. 18:331–341. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao Z, Holle L, Song W, Wei Y, Wagner TE
and Yu X: Antitumor and anti-angiogenic activities of Scutellaria
barbata extracts in vitro are partially mediated by inhibition of
Akt/protein kinase B. Mol Med Rep. 5:788–792. 2012.PubMed/NCBI
|
|
42
|
Zhang L, Cai Q, Lin J, Fang Y, Zhan Y,
Shen A, Wei L, Wang L and Peng J: Chloroform fraction of
Scutellaria barbata D. Don promotes apoptosis and suppresses
proliferation in human colon cancer cells. Mol Med Rep. 9:701–706.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Herszényi L, Hritz I, Lakatos G, Varga MZ
and Tulassay Z: The behavior of matrix metalloproteinases and their
inhibitors in colorectal cancer. Int J Mol Sci. 13:13240–13263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Littlepage LE, Sternlicht MD, Rougier N,
Phillips J, Gallo E, Yu Y, Williams K, Brenot A, Gordon JI and Werb
Z: Matrix metalloproteinases contribute distinct roles in
neuroendocrine prostate carcinogenesis, metastasis, and
angiogenesis progression. Cancer Res. 70:2224–2234. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laurie AS, Sandra JT and William GS:
Matrix metalloproteinases: Changing roles in tumor progression and
metastasis. Am J Pathol. 181:1895–1899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gao Y and Wang S: Relationship between
matrix metalloproteinases and their inhibitors and their
relationship with invasion and metastasis of malignant tumors. J
New Med. 42:341–343. 2011.
|
|
47
|
Liu T, Ma Y and Zhang R: Advance research
on relationship between matrix metalloproteinases and invasion and
metastasis of malignant tumors. J Jilin Univ. 30:662–664. 2004.
|
|
48
|
Meng F, Liu X and Qi S: Correlation of
matrix metalloproteinases-2 and-9 in ovarian cancer. Chin J
Gerontology. 33:3505–3506. 2013.
|
|
49
|
Ming S, Sun T and Xiao W: Role of matrix
metalloproteinases −2, −9 and its inhibitor 1 in the invasion and
metastasis of lung cancer. Chin J Respirator Crit Care. 4:198–202.
2005.
|
|
50
|
Zheng H, Shen B, Nie Y and Du Y: The
expression of MMP-9, MMP-13 and TIMP-3 in hepatocellular carcinoma.
Guangdong Med J. 34:1995–1998. 2013.
|
|
51
|
Yan Z, Xu X and Yang G: Abnormal
expression and clinical significance of matrix metalloprteinase 10
in esophagus carcinoma. Ningxia Med J. 27:14–15. 2005.
|
|
52
|
He J, Ding C, He G and Huang Q:
Relationship between expression of ESM-1 and MMP-3 and invasion and
metastasis of human hepatocellular carcinoma. Med Sci J Central
South China. 40:368–372. 2012.
|
|
53
|
Yue X, Zhang Q, Xu A, Xing Y and Zhang F:
Expression and clinical significance of MMP10 and CD105 in patients
with non-small-cell lung carcinoma. Shandong Med J. 49:13–15.
2009.
|
|
54
|
Feng J, Gou W, Liu D and Li X: Expressions
of matrix metalloproteinase-3 and matrix metalloproteinase-10 in
endometrial carcinoma. J Xian Jiaotong Univ (Med Sci). 31:97–101.
2010.
|
|
55
|
Tian J, Xu M and Jing H: Matrix
metalloproteinases 3 and its inhibitory factor 2 expression in lung
squamous carcinoma. West China Med J. 28:369–372. 2013.
|
|
56
|
Ma R and Zhang C: Review of matrix
metalloproteinases-13 and its relationship with invasion and
metastasis of malignant tumor. J Community Med. 10:47–49. 2012.
|
|
57
|
Da C, Liu Y, Zhan Y, Liu K and Wang R:
Nobiletin inhibits epithelial-mesenchymal transition of human
non-small cell lung cancer cells by antagonizing the TGF-β1/Smad3
signaling pathway. Oncol Rep. 35:2767–2774. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L,
Jiang H, Ren J, Cai J and Li Q: Resveratrol suppresses
epithelial-to-mesenchymal transition in colorectal cancer through
TGF-β1/Smads signaling pathway mediated Snail/E-cadherin
expression. BMC Cancer. 15:972015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Guo L and Wang Q: Correlation of
PI3K/AKT/mTOR signal pathway to infiltration and metastasis of
malignant tumor. Modern Oncol. 17:1585–1589. 2009.
|
|
60
|
Guo C, Ke W, Song K, Wang J, Zhou L and Li
K: PI3K/AKT signalling pathway involves in the modulation of
multidrug resistance and metastasis in breast cancer. Prog Mod
Biomed. 12:4809–4812. 2012.
|
|
61
|
Jia BY and Wang YJ: Metastasis associated
signaling pathway in colorectal cancer. Int J Dig Dis. 3:183–185.
2015.
|
|
62
|
Ding P, Song B and Zhu L: Effect of
TGF-β/Smad 4 on the tumor metastasis of colorectal cancer cells.
Chin J Cancer Prev Treat. 18:1518–1520. 2011.
|
|
63
|
Qi Y and Li H: Research of Qilian Fuzheng
capsule's function on anti-lung cancer metastasis by regulating
TGF-β pathway. Chin Archives Tradit Chin Med. 32:2567–2569.
2014.
|
|
64
|
Liotta LA: Tumor invasion and
metastases-role of the extracellular matrix. Rhoads Memorial Award
lecture. Cancer Res. 46:1–7. 1986.PubMed/NCBI
|
|
65
|
Stetler-Stevenson WG, Hewitt R and
Corcoran M: Matrix metalloproteinases and tumor invasion: From
correlation and causality to the clinic. Semin Cancer Biol.
7:147–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Chambers AF and Matrisian LM: Changing
views of the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Perera M, Tsang CS, Distel RJ, Lacy JN,
Ohno-Machado L, Ricchiuti V, Samaranayake LP, Smejkal GB, Smith MG,
Trachtenberg AJ and Kuo WP: TGF-beta1 interactome: Metastasis and
beyond. Cancer Genomics Proteomics. 7:217–229. 2010.PubMed/NCBI
|
|
68
|
Roberts AB and Wakefield LM: The two faces
of transforming growth factor beta in carcinogenesis. Proc Natl
Acad Sci USA. 100:pp. 8621–8623. 2003, View Article : Google Scholar : PubMed/NCBI
|