Introduction
Myocardial infarction is a major cause of mortality
and morbidity (1). Currently the
main method of treating myocardial infarction is the
re-establishment of blood flow as early as possible to prevent
further injury to the myocardium. However, this may cause
myocardial ischemia/reperfusion (I/R) injury and there are
currently no therapeutic strategies available to treat this
(2). Myocardial I/R injury induces a
series of pathological changes, including cellular apoptosis,
calcium overload and oxidative stress (3,4).
Therefore, it is critical to identify novel therapeutic agents to
reduce I/R injury in patients with myocardial infarction.
Pterostilbene (PTB), a natural phytoalexin found in
blueberries, is a dimethylated analog of resveratrol (5). Previous studies have demonstrated that
PTB exhibits various biological roles, including anti-inflammatory,
anti-oxidation and anti-apoptotic functions (6–8).
Oxidative stress, apoptosis and inflammation serve critical roles
in myocardial I/R injury; thus, PTB is a promising candidate for
the treatment of myocardial I/R injury. However, the effects of PTB
on myocardial I/R injury remain unclear.
The phosphatidylinositol 3′-kinase-protein kinase B
(PI3K/Akt) signaling pathway co-ordinates various intracellular
processes, including regulation of cell proliferation and survival
(9,10). The PI3K/Akt signaling pathway is a
pro-survival signaling pathway that confers protection against
myocardial ischemia (11). However,
it remains unknown whether PTB confers cardioprotection via
activation of the PI3K/Akt signaling pathway. Therefore, the
current study aimed to determine whether PTB reduces myocardial I/R
injury and if so, identify whether PI3K/Akt signaling is activated
by treatment with PTB.
Materials and methods
Animals
A total of 60 adult male Sprague-Dawley rats
(250–300 g; 1.5–2 months) were obtained from the Center of
Experimental Animals in the Xi'an Jiaotong University (Xi'an,
China). Animals were housed in a controlled room with a temperature
of 22°C, 33% humidity, a 12 h light/dark cycle and free access to
food and water. All animals used in the current study were cared
for in accordance with the Guide for the Care and Use of Laboratory
Animals published by the National Research Council (US) Committee
(12) and all procedures were
approved by The Committee of Experimental Animals of the Xi'an
Jiaotong University.
Reagents
PTB, the PI3K inhibitor LY294002 and DAPI were all
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Myocardial myeloperoxidase (MPO; cat. no. A044), creatine kinase-MB
(CK-MB; cat. no. H197) and lactate dehydrogenase (LDH; cat. no.
A020-1) assay kits were all purchased from Nanjing Jiancheng
Bioengineering Research Institute (Nanjing, China). Interleukin-6
(IL-6; cat. no. R6000B) and tumor necrosis factor-α (TNF-α; cat.
no. RTA00) ELISA kits were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). IL-8 ELISA kit (cat. no. kt30449) was
purchased from MSK Bio (Wuhan, China). Terminal deoxynucleotidyl
transferase dUTP nick-end labeling (TUNEL) kits were purchased from
Roche Applied Science (Mannheim, Germany). Antibodies against Akt
(cat. no. 4685), p-Akt (Ser473; cat. no. 4058), Bcl-2 (cat. no.
3498), Bax (cat. no. 14796) and β-actin (cat. no. 4970) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The horseradish peroxidase-conjugated immunoglobulin G secondary
antibody (cat. no. A0208) was purchased from Beyotime Institute of
Biotechnology (Haimen, China).
Myocardial ischemia-reperfusion (MI/R)
model and experimental protocol
Sprague-Dawley rats were anesthetized
intraperitoneally (i.p.) using sodium pentobarbital (Sigma-Aldrich;
Merck KGaA; 40 mg/kg). Myocardial ischemia was induced by
exteriorizing the heart with a left thoracic incision followed by a
slipknot (5-0 silk) around the left anterior descending coronary
artery (LAD). Following 30 min ischemia, the slipknot was released
and the animal underwent 120 min reperfusion. The Sham group
underwent the same procedure without LAD ligation.
Rats were randomly assigned to one of four groups
(n=15): i) A sham group; ii) an MI/R group that received treatment
vehicle with 0.9% sodium chloride i.p.; iii) an MI/R+PTB group that
received PTB (10 mg/kg, i.p.) 10 min prior to reperfusion iv) and
an MI/R+PTB+LY group that received PTB (10 mg/kg, i.p.) 10 min
prior to reperfusion and LY (10 mg/kg, i.p.) every 2 days three
times prior to surgery.
Evaluation of myocardial infarct
size
Tetrazolium chloride (TTC) staining was used to
assess the myocardial infarct size. Following reperfusion, rats
were anaesthetized and euthanized. Rat hearts were then immediately
isolated, washed in PBS and sectioned into transverse slices 5 mm
thick. Following incubation in 1% (0.01 g/ml) TTC at 37°C in PBS
for 15 min, heart slices were photographed with a digital camera to
distinguish between the red-stained viable tissues and the
white-unstained infarcted tissues. Areas of infarct size were
measured digitally using Image Pro Plus 6.0 software (Media
Cybernetics Inc., Rockville, MD, USA).
Evaluation of serum levels of CK-MB
and LDH
Following reperfusion, blood (6 ml) was taken from
the carotid artery of all rats and kept at room temperature for 30
min. Serum was collected by centrifugation at 3,000 × g at 4 °C for
15 min and kept at −70°C for preservation. The CK-MB and LDH assay
kits were used to measure levels of CK-MB and LDH in the serum,
following the manufacturer's instructions. Enzyme activity was
expressed in U/l.
Assay of MPO activity and levels of
the inflammatory cytokines TNF-α, IL-6, IL-8 in the serum
Following reperfusion, the myocardial tissue was
kept at −70°C for preservation. An MPO assay kit was used to detect
MPO levels in the myocardial tissue following the manufacturer's
instructions. Levels of TNF-α, IL-6 and IL-8 were
spectrophotometrically analyzed using ELISA kits following the
manufacturer's instructions.
Determination of myocardial
apoptosis
Myocardial apoptosis was determined using TUNEL
staining. The tissue samples were fixed using 4% formaldehyde at
4°C for, 24 h and embedded in paraffin. The paraffin-embedded
tissue was cut into sections 4–5 µm thick. Sections were incubated
in 50 µl TUNEL mixture (solution A: Solution B=1:9) in a humidified
chamber at 37°C for 1 h. Slides were incubated with DAPI for 5 min
at room temperature in the dark to detect the nuclei. Sections were
covered using cover slips with anti-fade mounting medium (cat. no.
P0126; Beyotime Institute of Biotechnology, Haimen, China).
Sections were observed using fluorescence microscopy in six random
fields of view. The apoptotic index was calculated as the ratio of
the number of TUNEL-positive neurons to the total number of
nuclei.
Western blot analysis
Heart tissues were lysed with RIPA lysis buffer
(cat. no. sc-24948, Santa Cruz Biotechnology, Inc., Dallas, TX,
USA). Following sonication at 4°C for 25 sec at a frequency of 20
kHz, lysates were centrifuged at 14,000 × g at 4°C for 30 min and
protein concentration was detected using the BCA determination
method. Proteins (30 µg/lane) were then separated by SDS-PAGE
(8–15%) and transferred to a polyvinylidene difluoride membrane
(EMD Millipore, Billerica, MA, USA). Following blocking with 5%
skimmed milk in Tris-buffered saline at room temperature for 1 h,
the membrane was incubated with primary antibodies against p-Akt
(1:1,000), Akt (1:1,000), Bcl-2 (1:1,000), Bax (1:1,000) and
β-actin (1:1,000) overnight at 4°C. The membrane was then washed
with PBS and incubated with horseradish peroxidase-conjugated
immunoglobulin G secondary antibody (1:10,000) for 1 h at 37°C.
Blots were developed using a SuperSignal chemiluminescence
detection kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
and immunoblotting was visualized and analyzed using the Quantity
One System 4.62 (Bio-Rad, Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) was used to
perform statistical analysis. Differences among groups were
evaluated by one-way analysis of variance followed by a Dunnett's
t-test for multiple comparisons. P<0.05 was determined to
indicate a statistically significant difference.
Results
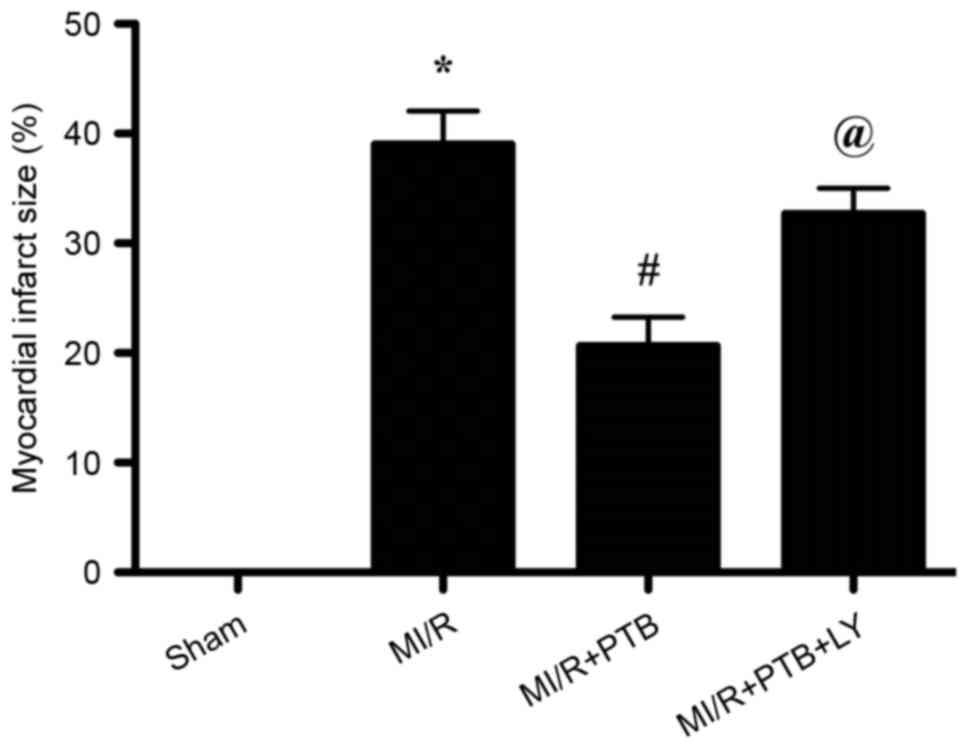
PTB decreases myocardial infarct size
via PI3K/Akt signaling pathway activation
A myocardial infarct was induced all groups apart
from the Sham group (Fig. 1).
Treatment with PTB significantly decreased the myocardial infarct
size compared with the M/IR group (P<0.05; Fig. 1). However, the protective effect of
PTB was reversed by LY treatment, as the myocardial infarct size
was significantly increased in the MI/R+PTB+LY group compared with
the MI/R+PTB group (P<0.05; Fig.
1).
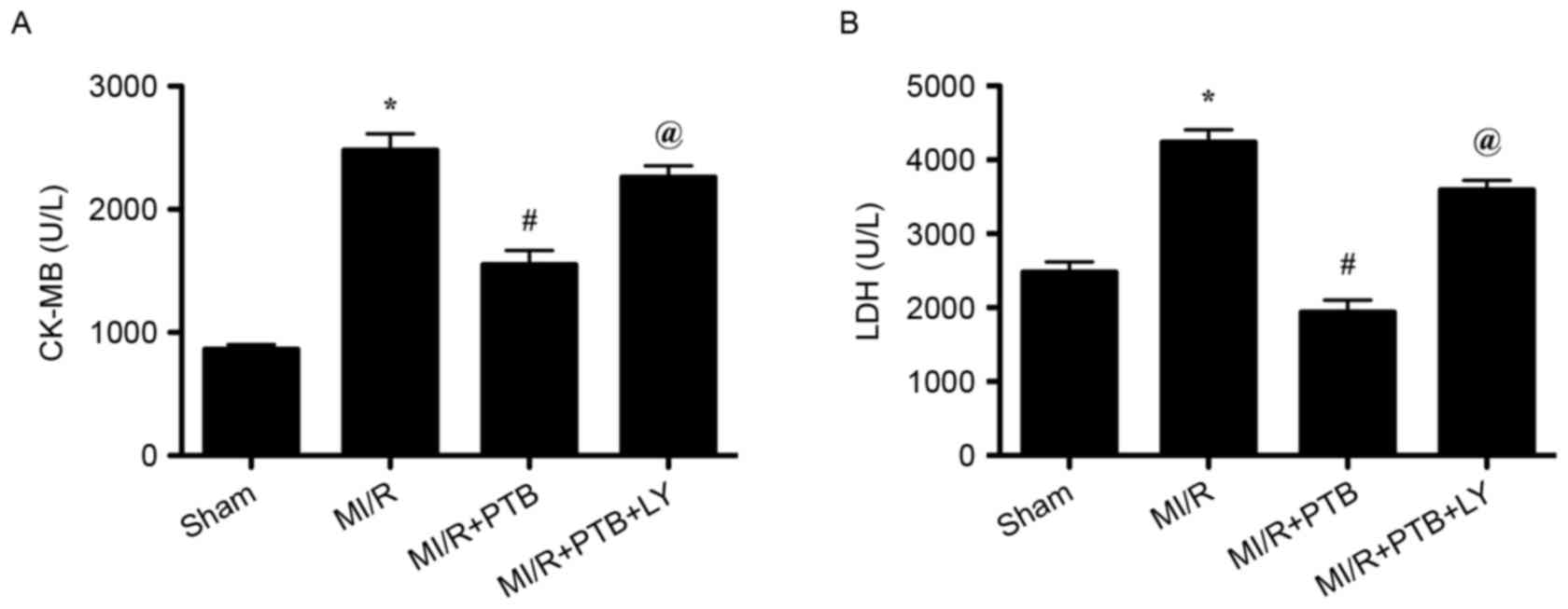
PTB decreases the release of
myocardial CK-MB and LDH following MI/R via PI3K/Akt signaling
pathway activation
The release of CK-MB and LDH is an indicator of
myocardial injury; therefore, serum levels of CK-MB and LDH were
measured. There was a significant increase in the release of serum
CK-MB and LDH in the MI/R group compared with the Sham group (both
P<0.05; Fig. 2). However, there
was a significant decrease in the release of CK-MB and LDH in the
MI/R+PTB group compared with the MI/R group (both P<0.05;
Fig. 2). The release of CK-MB and
LDH was significantly increased in the MI/R+PTB+LY group compared
with the MI/R+PTB group (P<0.05; Fig.
2) indicating that PI3K was involved in the protective effect
of PTB against MI/R injury.
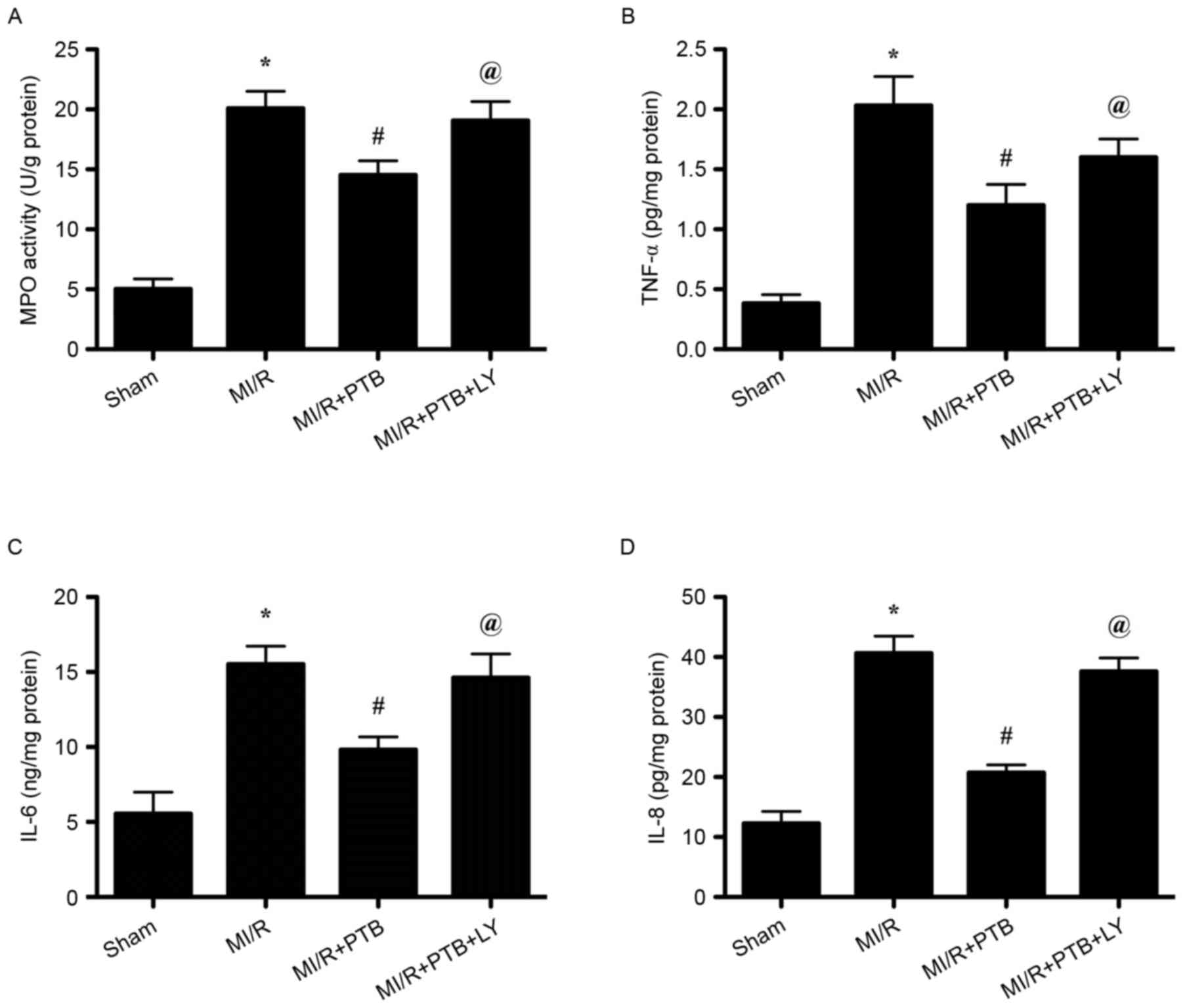
PTB decreases myocardial MPO activity
and the serum levels of TNF-α, IL-6 and IL-8 following MI/R via
activation of the PI3K/Akt signaling pathway
Increased MPO activity in the myocardium indicates
neutrophil infiltration. MPO activity in the MI/R group was
significantly increased compared with the Sham group (P<0.05;
Fig. 3A). However, PTB significantly
decreased myocardial MPO activity in the MI/R+PTB group compared
with the MI/R group and LY treatment reversed the effect of PTB
(both P<0.05; Fig. 3A). Levels of
TNF-α, IL-6, and IL-8 in the plasma were all significantly
increased following MI/R injury compared with the Sham group (all
P<0.05; Fig. 3B-D). Treatment
with PTB significantly decreased cytokine levels in the serum (all
P<0.05; Fig. 3B-D) and these
effects were reversed following LY treatment (all P<0.05;
Fig. 3B-D).
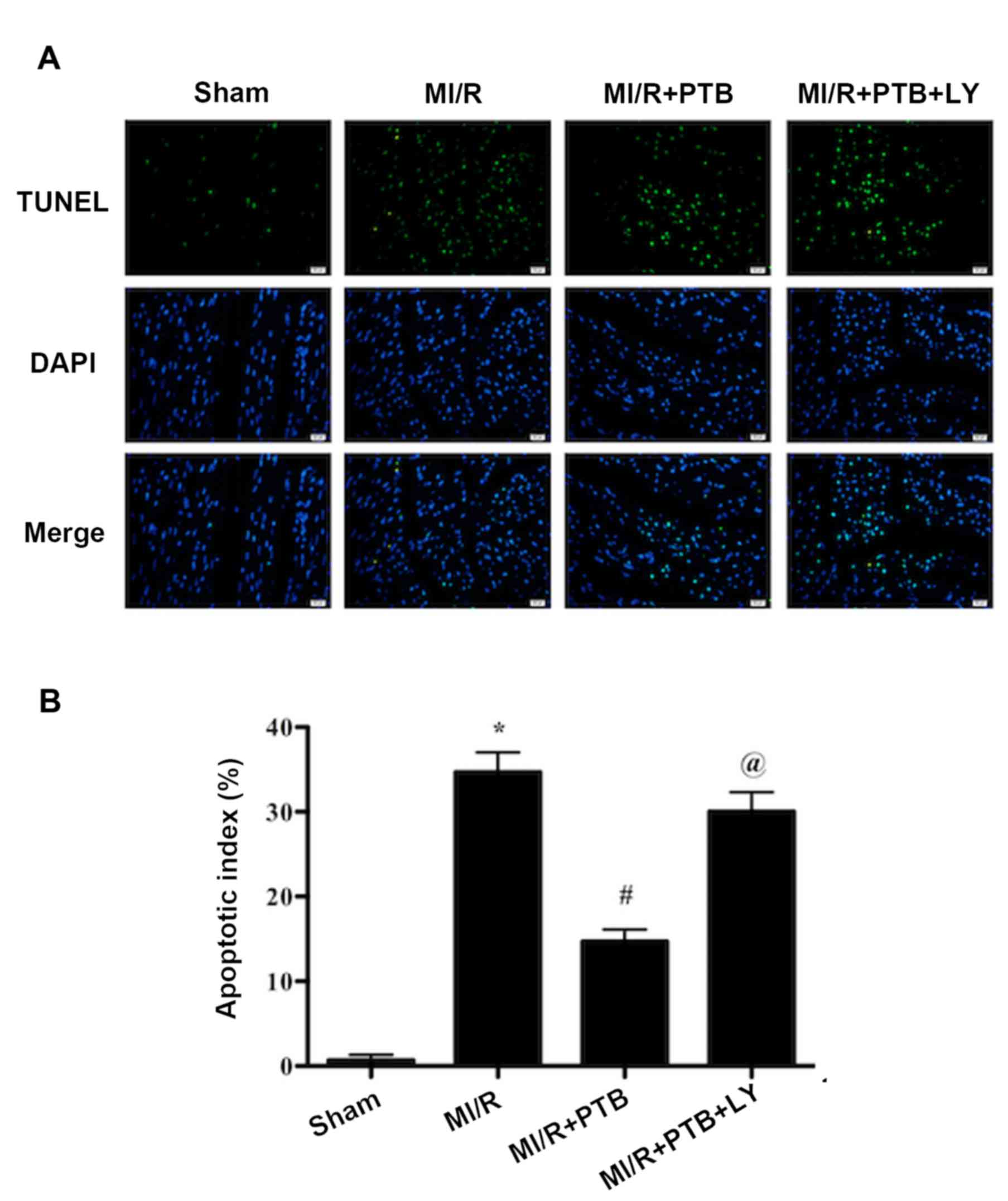
PTB reduces myocardial apoptosis
induced by MI/R via activation of the PI3K/Akt signaling
pathway
TUNEL staining was performed to evaluate apoptosis
in the rat hearts (Fig. 4). The
apoptotic index of the MI/R+PTB group was significantly decreased
compared with the MI/R group (P<0.05; Fig. 4). However, treatment with LY again
significantly reversed the protective effects of PTB (P<0.05;
Fig. 4), indicating that PI3K
activation is involved in the mechanism of action of PTB against
MI/R-induced myocardial apoptosis.
Effects of PTB on the expression of
p-Akt, Akt, Bcl-2 and Bax in hearts subjected to MI/R
MI/R injury in the MI/R group significantly
increased the phosphorylation of Akt compared with the Sham group
(P<0.05; Fig. 5A and B). The
phosphorylation of Akt was further increased following treatment
with PTB (P<0.05, Fig. 5A and B).
However, treatment with LY reversed the increase in Akt
phosphorylation that was induced by PTB (P<0.05; Fig. 5A and B). In addition, MI/R injury
significantly decreased Bcl-2 expression in the MI/R group compared
with the Sham group (P<0.05; Fig.
5C). Treatment with PTB significantly increased the expression
of Bcl-2 (P<0.05), which was subsequently decreased by
co-administration with LY (both P<0.05; Fig. 5C). By contrast, MI/R injury induced a
significant increase in Bax expression compared with the Sham group
(P<0.05; Fig. 5D). PTB
administration significantly decreased the expression of Bax in the
MI/R group compared with the MI/R+PTB group (P<0.05), which was
significantly reversed by co-administration with LY (both
P<0.05; Fig. 5D).
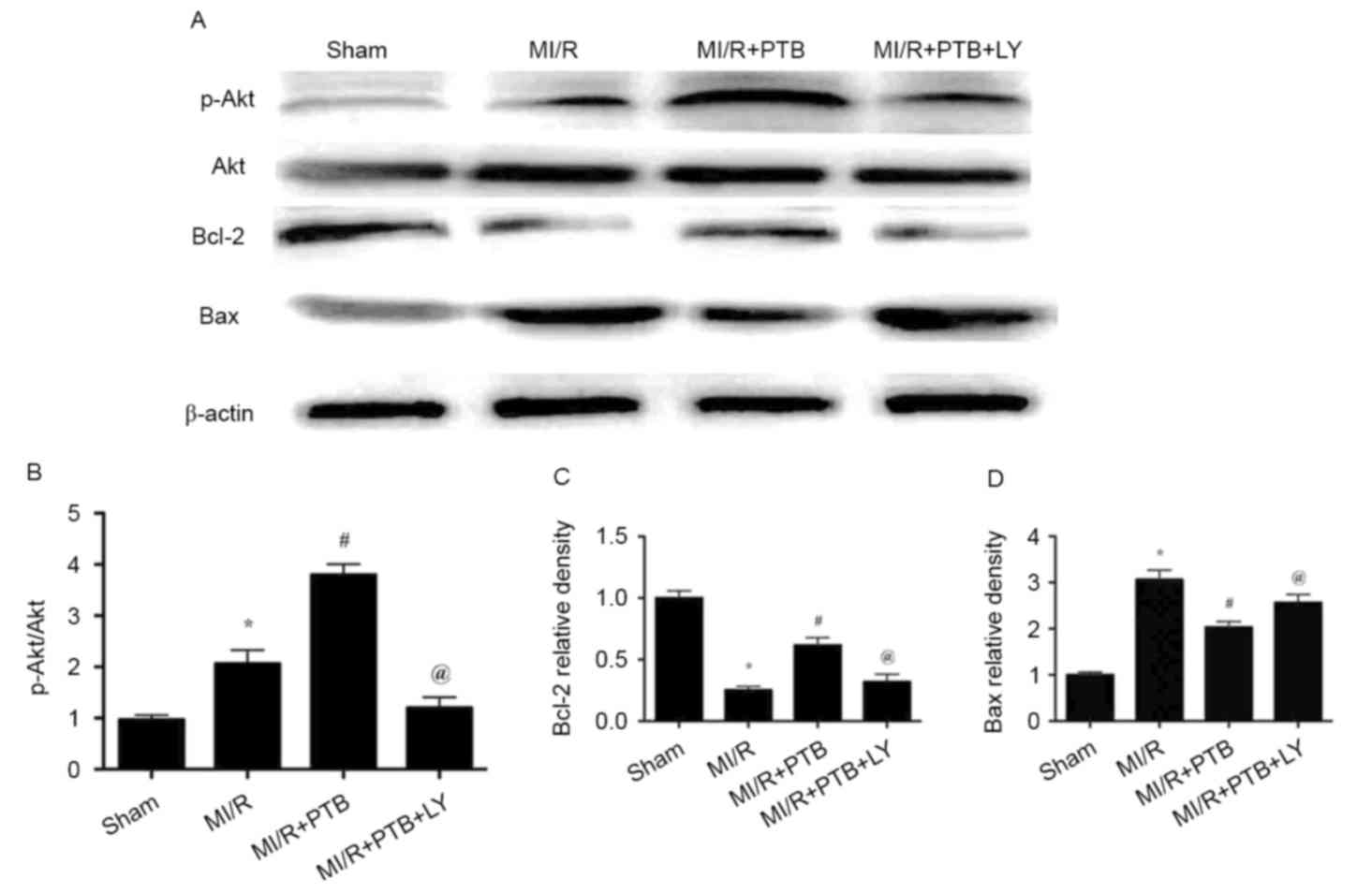 | Figure 5.PTB treatment activated PI3K/Akt
signaling and inhibited myocardial apoptosis signaling, which was
reversed by LY treatment. (A) Western blot analysis measuring the
expression of p-Akt, Akt, Bcl-2, Bax and β-actin in all groups. (B)
Expression of P-Akt/Akt. (C) Expression of Bcl-2. (D) Expression of
Bax. Data are expressed as the mean ± standard error of the mean
(n=6 for each group). *P<0.05 vs. the Sham group;
#P<0.05 vs. the MI/R group and @P<0.05
vs. the MI/R + PTB group. PTB, pterostilbene; MI/R, myocardial
ischemia/reperfusion; LY, LY294002; PI3K/Akt, phosphatidylinositol
3′-kinase-protein kinase B; Akt, protein kinase B; p,
phosphorylated. |
Discussion
The current study demonstrated that myocardial I/R
injury is attenuated by treatment with PTB. PTB decreased cardiac
injury, alleviated myocardial apoptosis and decreased the
inflammation induced by myocardial I/R injury. The protective
effect of PTB is strongly associated with activation of the
PI3K/Akt signaling pathway. Treatment with PTB significantly
decreased the release of CK-MB and LDH and the activity of MPO
following myocardial I/R injury. PTB administration also decreased
levels of TNF-α, IL-6, and IL-8 in the serum. Furthermore,
treatment with PTB reduced myocardial apoptosis. However, the
cardioprotective effects of PTB were reversed following treatment
with the PI3K signaling inhibitor LY, suggesting that activation of
the PI3K/Akt signaling is involved in the cardioprotective effects
of PTB.
CK is an enzyme expressed in various types of
tissues and its isoenzyme is CK-MB in the myocardium. LDH is an
enzyme that catalyzes the conversion of lactate to pyruvic acid.
The isoenzyme of LDH in the heart is LDH-1 and may be used to
identify damage in the heart (13).
Following cardiomyocyte damage, cellular CK-MB and LDH are released
into the blood, resulting in increased levels of CK-MB and LDH in
the serum. The results of the present study demonstrate that the
serum levels of CK-MB and LDH are decreased following PTB
treatment, suggesting that PTB treatment alleviates myocardial
injury following I/R.
MPO is a peroxidase enzyme, which is most abundantly
expressed in neutrophils. Therefore, MPO activity in the myocardium
is regarded as an indicator of neutrophil infiltration and
inflammation. The current study demonstrated that PTB treatment
decreased MPO activity in the myocardium, suggesting that PTB may
attenuate neutrophil infiltration and the inflammatory response
following MI/R injury. Inflammation serves an important role in
MI/R injury. Damaged cells stimulate inflammatory cells, which
subsequently release various inflammatory cytokines. The release of
cytokines causes further injury to endothelial cells, resulting in
increased vascular permeability (14). Therefore, in the current study, serum
levels of TNF-α, IL-6, and IL-8 were measured to determine the
anti-inflammatory effect of PTB. The results demonstrated that PTB
treatment deceases serum levels of TNF-α, IL-6, and IL-8. Overall,
these results demonstrate that PTB decreases neutrophil
infiltration and the inflammatory response during myocardial I/R
injury.
Myocardial apoptosis also serves an important role
in myocardial I/R injury. It is inhibited by Bcl-2 and induced by
Bax (15). The effect of PTB on
myocardial apoptosis was assessed using TUNEL staining in the
current study. The results indicate that PTB treatment reduces the
number of TUNEL-positive cells. Furthermore, levels of Bcl-2 and
Bax were measured using western blotting and the results
demonstrated that PTB treatment increases Bcl-2 expression and
decreases Bax expression. However, the protective effects of PTB
were inhibited by LY, a PI3K signaling inhibitor.
PI3K/Akt signaling is strongly associated with cell
survival (16,17). Previous studies have demonstrated
that PI3K/Akt signaling confers significant cardioprotective
effects (18,19). Akt phosphorylation suppresses
apoptosis and promotes cell survival in myocardial ischemia
(20–22). The results of the current study
demonstrate that PTB treatment upregulates Akt phosphorylation,
indicating that the protective effects of PTB are associated with
activation of the PI3K/Akt signaling pathway. Treatment with LY
reverses the increase in Akt phosphorylation, further confirming
this conclusion.
In conclusion, the present study demonstrates that
PTB, an antioxidant and anti-inflammatory agent, attenuates
myocardial I/R injury. The results also indicate that the
activation of the PI3K/Akt signaling pathway by PTB protects
against myocardial I/R injury. Thus, these findings may facilitate
the development of PTB as a therapeutic strategy to treat
myocardial I/R injury.
References
|
1
|
Ramzy D, Rao V and Weisel RD: Clinical
applicability of preconditioning and postconditioning: The
cardiothoracic surgeons's view. Cardiovasc Res. 70:174–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yue Y, Yang X, Feng K, Wang L, Hou J, Mei
B, Qin H, Liang M, Chen G and Wu Z: M2b macrophages reduce early
reperfusion injury after myocardial ischemia in mice: A predominant
role of inhibiting apoptosis via A20. Int J Cardiol. July
26–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Wang XL, Zhao J, Wang YJ, Lau WB,
Yuan YX, Gao EH, Koch WJ and Ma XL: Adiponectin inhibits
oxidative/nitrative stress during myocardial ischemia and
reperfusion via PKA signaling. Am J Physiol Endocrinol Metab.
305:E1436–E1443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimizu Y, Lambert JP, Nicholson CK, Kim
JJ, Wolfson DW, Cho HC, Husain A, Naqvi N, Chin LS, Li L and
Calvert JW: DJ-1 protects the heart against ischemia-reperfusion
injury by regulating mitochondrial fission. J Mol Cell Cardiol.
97:56–66. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCormack D and McFadden D: A review of
pterostilbene antioxidant activity and disease modification. Oxid
Med Cell Longev. 2013:5754822013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bhakkiyalakshmi E, Sireesh D,
Sakthivadivel M, Sivasubramanian S, Gunasekaran P and Ramkumar KM:
Anti-hyperlipidemic and anti-peroxidative role of pterostilbene via
Nrf2 signaling in experimental diabetes. Eur J Pharmacol. 777:9–16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Y, Zhang L, Li F, Hu CP and Zhang Z:
Restoration of sirt1 function by pterostilbene attenuates
hypoxia-reoxygenation injury in cardiomyocytes. Eur J Pharmacol.
776:26–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, Liu H, Yue L, Li X, Zhao L, Yang
X, Wang X, Yang Y and Qu Y: Neuroprotective effects of
pterostilbene against oxidative stress injury: Involvement of
nuclear factor erythroid 2-related factor 2 pathway. Brain Res.
1643:70–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fruman DA and Cantley LC: Phosphoinositide
3-kinase in immunological systems. Semin Immunol. 14:7–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujio Y, Nguyen T, Wencker D, Kitsis RN
and Walsh K: Akt promotes survival of cardiomyocytes in vitro and
protects against ischemia-reperfusion injury in mouse heart.
Circulation. 101:660–667. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Research Council, . Guide for the
Care and Use of Laboratory Animals. 8th edition. National Academies
Press (US); Washington, DC: 2011, PubMed/NCBI
|
|
13
|
Li GH, Luo B, Lv YX, Zheng F, Wang L, Wei
MX, Li XY, Zhang L, Wang JN, Chen SY, et al: Dual effects of VEGF-B
on activating cardiomyocytes and cardiac stem cells to protect the
heart against short- and long-term ischemia-reperfusion injury. J
Transl Med. 14:1162016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lefer AM, Ma XL, Weyrich A and Lefer DJ:
Endothelial dysfunction and neutrophil adherence as critical events
in the development of reperfusion injury. Agents Actions Suppl.
41:127–135. 1993.PubMed/NCBI
|
|
15
|
Niture SK and Jaiswal AK: INrf2 (Keap1)
targets Bcl-2 degradation and controls cellular apoptosis. Cell
Death Differ. 18:439–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Armstrong SC: Protein kinase activation
and myocardial ischemia/reperfusion injury. Cardiovasc Res.
61:427–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He F, Xu BL, Chen C, Jia HJ, Wu JX, Wang
XC, Sheng JL, Huang L and Cheng J: Methylophiopogonanone A
suppresses ischemia/reperfusion-induced myocardial apoptosis in
mice via activating PI3K/Akt/eNOS signaling pathway. Acta Pharmacol
Sin. 37:763–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Song F, Duan LR, Sheng JJ, Xie YH,
Yang Q, Chen Y, Dong QQ, Zhang BL and Wang SW: Paeonol and
danshensu combination attenuates apoptosis in myocardial infarcted
rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and
PI3K/Akt pathway. Sci Rep. 6:236932016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang J, Hu F, Ke D, Yan Y, Liao Z, Yuan X,
Wu L, Jiang Q and Chen L: N,N-dimethylsphingosine attenuates
myocardial ischemia-reperfusion injury by recruiting regulatory T
cells through PI3K/Akt pathway in mice. Basic Res Cardiol.
111:322016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mullonkal CJ and Toledo-Pereyra LH: Akt in
ischemia and reperfusion. J Invest Surg. 20:195–203. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pei YH, Chen J, Xie L, Cai XM, Yang RH,
Wang X and Gong JB: Hydroxytyrosol protects against myocardial
pschemia/reperfusion injury through a PI3K/Akt-dependent mechanism.
Mediators Inflamm. 2016:12321032016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Ye L, Jiang C, Jiang J, Hong H and
Qiu L: Over-expression of HSPA12B protects mice against myocardium
ischemic/reperfusion injury through a PPARγ-dependent PI3K/Akt/eNOS
pathway. Am J Transl Res. 7:2724–2737. 2015.PubMed/NCBI
|