Introduction
Purulent meningitis is a serious central nervous
system infection caused by pyogenic bacteria (1,2), and
neonatal purulent meningitis refers to its occurrence within the
first 4 weeks after birth. Common etiological agents include Group
B streptococci, Escherichia coli, Staphylococcus aureus and
Listeria monocytogenes (3,4). The
morbidity rate in live-born infants for neonatal purulent
meningitis is approximately 0.06–1%, and in premature infants is up
to 3% (5,6). The disability and mortality rates in
those affected are both high: In developed countries, the
disability rate reaches 20–50%, and the mortality rate is
approximately 10–15% (7–10). While in developing countries, the
mortality rate is as high as 40–58% (11). Recent medical advancements are likely
to reduce the morbidity and mortality rates for neonatal purulent
meningitis, but the disability rate in neonates is still going to
remain high. Hearing impairment, which is the most common type of
disability caused by the infection (9), can seriously affect a child's
development. CD64+ neutrophils and procalcitonin (PCT)
are specific markers for bacterial infection that are widely used
in the diagnosis of infectious diseases such as neonatal pneumonia,
sepsis and others. In this study, we retrospectively recorded the
levels of CD64 and PCT in cerebrospinal fluid samples of 156 cases
of neonatal purulent meningitis, also auditory tests and clinical
follow-ups were performed to detect degrees of hearing impairment
left as sequelae. Statistical analyses were performed to explore
the correlation of CD64 and PCT levels with hearing impairment and
their prognostic values.
Patients and methods
Clinical data
Clinical data of 156 cases of neonatal purulent
meningitis were recorded during a period from April 2012 to April
2016 in Zhongnan Hospital. There were 91 males and 65 females. All
the patients received brainstem response audiometry tests. During
the cases review each case was assigned either to a normal hearing
or an impaired hearing group. The patients in the impaired hearing
group were followed up for 1 year. During follow-up, 1 patient died
and 7 patients failed to be present. The family members of all the
patients signed informed consents. The study was approved by the
Ethics Committee of Zhongnan Hospital of Wuhan University (Protocol
no. 2015019).
Experimental methods
Diagnostic and exclusion criteria for all the
children in the study were taken from the fourth edition of
‘Practical Pediatrics’. All children received lumbar puncture for
cerebrospinal fluid (CSF) examination and the results of the test
included at least one of the following eight possibilities for the
CSF routine test: The CSF appeared turbid after smear or culture.
The CSF pressure was higher than 2.94–7.84 kPa. The CSF white blood
cell count was higher than 29×106/l in premature
infants, higher than 32×106/l in term infants and higher
than 10×106/l in patients at 1 week after birth. More
than 57–61% of the cells were multinucleated cells. The protein
content was higher than 0.65–1.5 g/l in premature infants and
higher than 0.1–1.7 g/l in term infants. The Pan test gave ++ or
+++. The lactate dehydrogenase was often higher than 1000 U/l. In
addition, the glucose levels were often lower than 1.1–2.2 mmol/l.
For CSF cell counts, the proportions of white blood cells and
erythrocytes were significantly higher than those in blood. CSF
smear and cultures were negative. For patients with abnormalities
in hearing screening, a family history of deafness, neonatal
hyperbilirubinemia, intrauterine infection history, perinatal
hypoxia asphyxia history, congenital brain development and other
abnormalities were excluded.
Audiological examination was performed using the
evoked potentials instrument (Chartr-EP) at the ENT Department of
Zhongnan Hospital of Wuhan University. The test was performed
either during sleep or after a phenobarbital injection. The test
was performed in a soundproof audiometric test room. The recording
electrode was affixed to the cranial CZ site, and the reference
electrode was attached to the ipsilateral mastoid bone prominence.
After clearing foreign bodies in the ear, appropriate earplugs were
used. One ear was stimulated with sparse polar burst sound, and the
other ear was treated with flat noise. The data were recorded at
the intensity of 70 dB. Each ear was tested at least twice. The
threshold for audiological examination was set with the sound
stimulation from an intensity of 30 dB, increasing gradually to a
maximum stimulation up to 97 dB. The high frequency hearing
threshold was defined as the sound intensity that can be recognized
up to the V wave. The normal high frequency hearing threshold of
this experiment was 30 dB. According to audiology standards, less
than 40 dB was classified as mild hearing impairment, 41–55 dB
indicated a moderate hearing impairment, 56–70 dB indicated a
moderate to severe hearing impairment, 71–90 dB indicated a severe
hearing impairment and higher than 91 dB indicated an extremely
severe hearing impairment.
Detection of CD64 and PCT
CSF was collected through lumbar puncture within the
first day after hospitalization for a CSF routine test, and
biochemical and virological tests. Additionally, a portion of the
fluid was used for CD64 and PCT detection. A flow cytometer
(Cytomics FC500, Berea, OH, USA) was used to detect the CD64
content. The detection of PCT was performed through enzyme-linked
fluorescence analysis using a ELISA kit (Biomerica, Irvine, CA,
USA), and a PCT ≥0.5 ng/ml was considered to be positive.
Statistical analysis
The statistical analysis was performed using SPSS
19.0 statistical software (SPSS Inc., Chicago, IL, USA).
Measurement data were expressed as mean ± SD, the comparisons
between two groups were performed using independent sample t-tests,
and the comparisons among three groups were performed by one-way
ANOVA. Graded data were expressed as counts, and the Kruskal-Wallis
H test was used for the comparisons among more than 2 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Hearing conditions of the patients on
the day of admission
This study includes the analysis of data from 156
cases of neonatal purulent meningitis. The data from patients were
divided into two groups, a normal hearing and a hearing impairment
group (68 patients accounting for 43.59% of all the patients). At
the same time, dta from children with hearing impairment were
subdivided into three groups according to mild (18 cases), moderate
(23 cases) or severe (27 cases) hearing impairment degree. All
children with abnormal hearing were followed up for a minimum of
six months and up to three years. During the follow-up, 1 patient
died and 7 failed to show up to complete the follow-ups. Among the
60 patients who finished the follow-ups, 16 showed mild hearing
impairment, 21 moderate hearing impairment, and 23 severe hearing
impairment on the day of admission (Table I).
 | Table I.Hearing conditions of the patients on
the day of admission. |
Table I.
Hearing conditions of the patients on
the day of admission.
| Patient groups | Mild hearing
impairment | Moderate hearing
impairment | Severe hearing
impairment |
|---|
| Normal hearing group
(n=88) | – | – | – |
| Hearing impairment
group (n=68) | 18 | 23 | 27 |
| Patients who finished
follow-up (n=60) | 16 | 21 | 23 |
Comparison of hearing conditions of
patients during follow-up
Among the 16 patients who presented mild hearing
impairment at the time of admission, 13 completely recovered, 2
showed partially recovery, and 1 did not recover at all. Among the
21 patients with moderate hearing impairment at the time of
admission, 10 recovered completely to normal hearing conditions, 8
recovered partially, and 3 did not recover. Finally, among the 23
patients showing severe hearing impairment at the time of
admission, 4 recovered completely, 6 recovered partially, and 13
did not recover (2 had even worse conditions at the time of the
follow-ups). The Kruskal-Wallis H test was used for comparison
among the three groups with different degrees of hearing
impairment, and the results showed significant differences among
the three groups (χ2=18.315, P<0.01) (Table II).
 | Table II.Comparison of hearing conditions of
patients during follow-up. |
Table II.
Comparison of hearing conditions of
patients during follow-up.
| Patient groups | Complete
recovery | Partial recovery | No recovery |
|---|
| Mild hearing
impairment (n=16) | 13 | 2 | 1 |
| Moderate hearing
impairment (n=21) | 10 | 8 | 3 |
| Severe mild hearing
impairment (n=23) | 4 | 6 | 13 |
Comparison of PCT levels in CSF
between patients
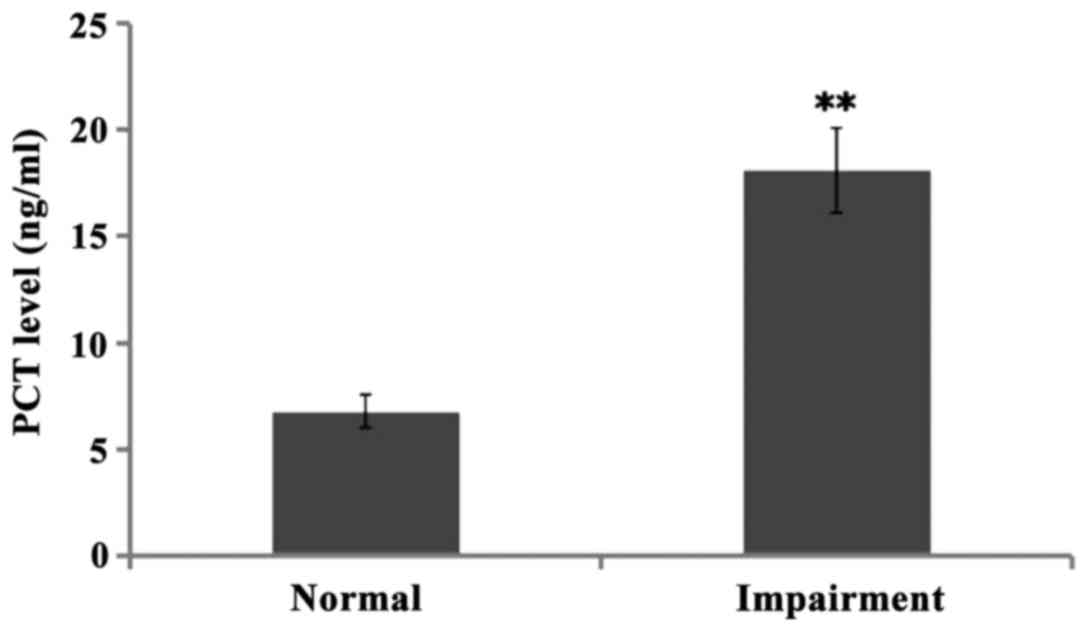
The levels of PCT in CSF of children with normal
hearing and hearing impairment were both high when compared to the
standard normal level, namely 6.78±0.770.5 ng/ml and 18.12±1.980.5
ng/ml, respectively. There was significant difference between the
two groups (P<0.01). Further comparison of PCT levels in
cerebrospinal fluid of patients with varying degrees of hearing
impairment showed that the levels of PCT increased gradually with
the severity of the impairment, and the differences between groups
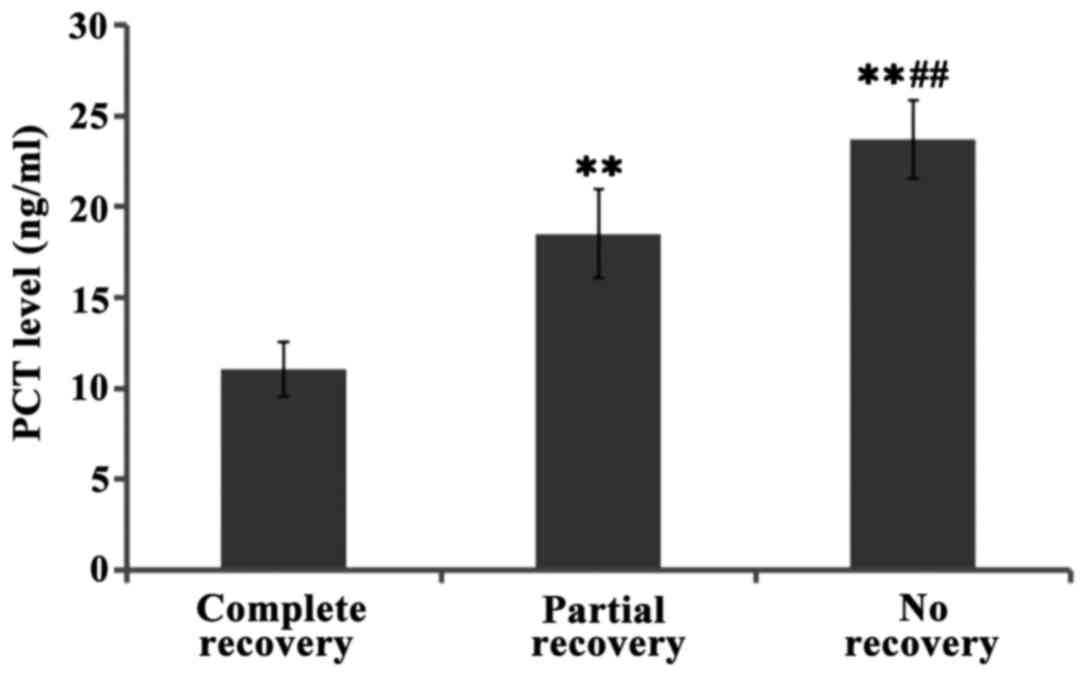
were significant (P<0.01). Further comparisons showed the levels
of PCT levels in cerebrospinal fluid of patients with full recovery
were lower than those of patients with partial recovery, and even
lower than those of patients who did not recover at all, and the
differences between groups were significant (P<0.01) (Figs. 1–3).
Comparison of CD64 levels CSF between
patients
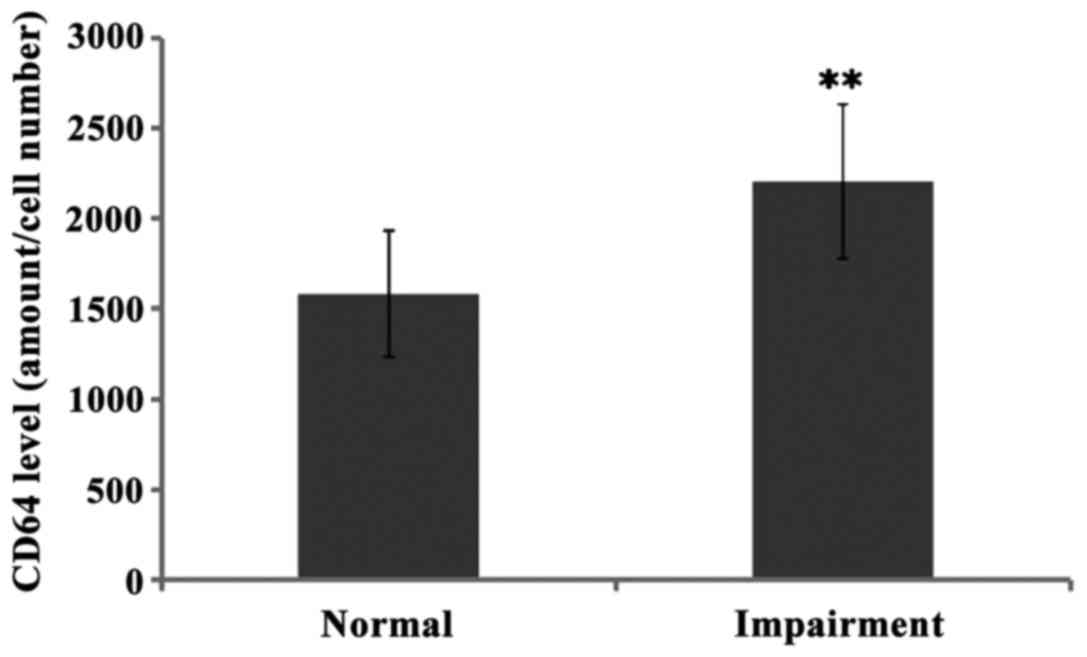
The levels of CD64 in cerebrospinal fluid of normal
hearing and hearing impairment group patients were 1584.05±351.12
and 2208.79±427.30, respectively, and there was significant
difference between the two groups (P<0.01). Further comparison
of CD64 levels showed the levels increased gradually from mild, to
moderate and to severe hearing impairment groups, and the
differences between groups were significant (P<0.01).
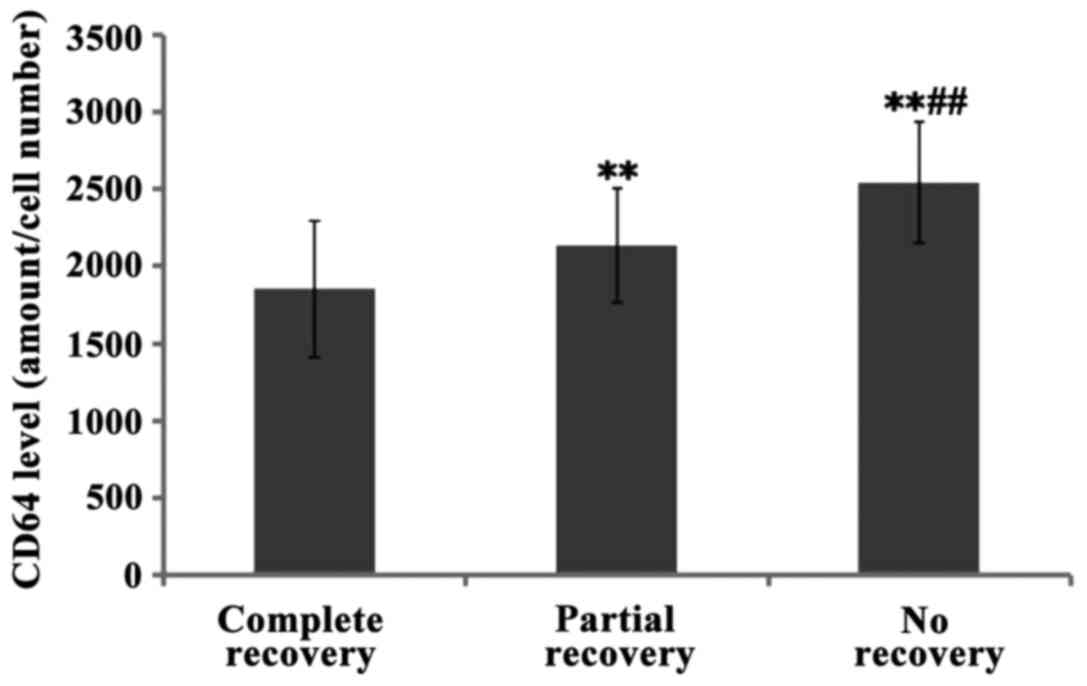
Furthermore, the levels of CD64 in children with complete hearing
recovery were lower than the levels in those who recovered
partially and even lower than those who did not recover at all
(with significant differences P<0.01) (Figs. 4–6).
Discussion
Neonatal purulent meningitis is a common central
nervous system pediatric infection. It can leave serious sequelae,
that negatively impact the development and life quality of
patients. Even though medical advances have lowered the mortality
due to purulent meningitis, its sequelae are still frequent
(12). These include hemiparesia,
epilepsy, hearing impairment, small head deformity, blindness and
cerebral palsy (13). A follow-up
study carried out in Wales and England found that the most common
sequelae of purulent meningitis is hearing impairment, and the
second is language disorder, followed by vision disorder, and then
behavioral and motor dysfunction (9). Another study also confirmed that
hearing impairment is the most common sequelae, followed by mental
retardation, dyskinesia and epilepsy (14). In this study, 43.59% of the patients
(60) showed different degrees of hearing impairment, and 55% of the
patients with hearing impairment failed to recover completely.
Therefore, we speculate that approximately 1/4 of the patients who
develop neonatal purulent meningitis will be left with long-term
hearing impairment. We also found that the hearing recovery is
related to the degree of the hearing impairment detected on the day
of admission, whereas a higher degree of impairment increases the
chances of incomplete recovery. It is possible that the inner ear
hairy cells in patients with severe hearing impairment were
damaged, resulting in irreversible changes in the microenvironment
of the cochlea; supporting this notion is the fact that the
patients with severe hearing impairment usually had congenital
inner ear malformations. Mild hearing impairment, on the other
hand, may possibly have been caused by otitis media tympanitis,
which can easily be controlled. Further studies are still needed in
order to understand the mechanisms that lead to life-long hearing
impairment.
CD64, which is a member of the immunoglobulin
superfamily, is a receptor of the Fc region of immunoglobulin IgG.
Genes encoding CD64 are located on chromosome 1p13 and 1q21, and
the molecular weight of the cognate protein is 72 kD (15). There are three types of IgG Fc region
receptors, namely CD64, CD32, and CD16, these receptors link cell
and humoral immunities (16). CD64
is the only high affinity receptor for IgG. The expression of CD64
in the body is normally kept to low levels. After a bacterial
infection, however, the body produces interferon, granulocyte
colony stimulating factor, bacterial cell wall lipopolysaccharide
and a series of neutrophil stimulating factors, causing increased
expression of CD64 on the surface of neutrophils (17). A study found that the expression
levels of CD64 differ in children and adults in different age
groups (18), while the expression
level does not significantly changed after viral infection, making
it a useful marker for bacterial infection prediction. Yet another
study showed that in children with septic shock, the expression
levels of CD64 were positively correlated with the severity of
infection, and higher expression levels indicated a more severe
infection, suggesting that the expression level of CD64 can be used
as an indicator of the severity of infection (19). In this study, the expression levels
of CD64 in CSF in neonatal purulent meningitis patients were
significantly increased; suggesting they can be used to assist in
the diagnosis of neonatal purulent meningitis. Moreover, our study
found that the level in CSF was related with the degree of hearing
impairment of neonatal purulent meningitis patients, and higher
expression levels of CD64 always came along with a higher degree of
hearing impairment and lower degree of hearing recovery. This
suggests that the expression level of CD64 can be used as a
predictor of the degree of hearing impairment and prognosis for
children with purulent meningitis.
PCT, a hormone activity-free glycoprotein, is a
calcitonin pro-peptide. The PCT protein is composed of 16 amino
acids with a total molecular weight of 13000 (20). Under physiological conditions, PCT is
mainly produced and secreted by thyroid C cells. The normal content
of PCT is very low in blood and undetectable in cerebrospinal fluid
(20). After a bacterial infection,
toxins secreted by bacteria can stimulate glandular cells to
release a large amount of PCT, resulting in significantly increased
PCT levels in blood and cerebrospinal fluid. Clinical studies have
shown that PCT, WBC and CRP levels in blood and cerebrospinal fluid
of patients with purulent meningitis get significantly increased
(21,22). Another study showed that the PCT
level was significantly increased in the body 6 h after a bacterial
infection in the body, and the increase of PCT appeared much
earlier than the increase of CRP, indicating that PCT can be used
as an early sensitive indicator of bacterial infection (23). This study found that the PCT levels
in CSF of neonates with purulent meningitis were significantly
increased. Furthermore, our study found that the PCT levels in CSF
of the neonates with purulent meningitis correlate with the degree
of hearing impairment, suggesting that the expression level of PCT
can be used as a predictor of the degree of hearing impairment and
prognosis for these patients.
In conclusion, this study found that approximately
1/4 of children with purulent meningitis showed long-term hearing
impairment. CD64 and PCT were highly expressed in the CSF of the
patients. In addition, the expression levels were positively
correlated with the degree of hearing impairment, whereas higher
PCT levels were found in those cases with higher levels of hearing
impairment and lower levels of recovery. Further clinical studies
are still needed to understand the underlying mechanisms involved
in the pathogenesis of neonatal purulent meningitis.
References
|
1
|
Kim KS: Pathogenesis of bacterial
meningitis: From bacteraemia to neuronal injury. Nat Rev Neurosci.
4:376–385. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mook-Kanamori BB, Geldhoff M, van der Poll
T and van de Beek D: Pathogenesis and pathophysiology of
pneumococcal meningitis. Clin Microbiol Rev. 24:557–591. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stoll BJ, Hansen NI, Sánchez PJ, Faix RG,
Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID
III, Hale EC, et al: Eunice Kennedy Shriver National Institute of
Child Health and Human Development Neonatal Research Network: Early
onset neonatal sepsis: The burden of group B Streptococcal and
E. coli disease continues. Pediatrics. 127:817–826. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ku LC, Boggess KA and Cohen-Wolkowiez M:
Bacterial meningitis in infants. Clin Perinatol. 42:29–45. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KS: Neonatal bacterial meningitis.
Neoreviews. 16:e535–e543. 2015. View Article : Google Scholar
|
|
6
|
May M, Daley AJ, Donath S and Isaacs D:
Australasian Study Group for Neonatal Infections: Early onset
neonatal meningitis in Australia and New Zealand, 1992–2002. Arch
Dis Child Fetal Neonatal Ed. 90:F324–F327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaufman D, Zanelli S, Cantey JB and
Sánchez PJ: Neonatal meningitis: current treatment
optionsNeurology: Neonatology Questions and Controversies. Perlman
JMD: 2nd edition. Elsevier; Amsterdam: pp. 181–201. 2012
|
|
8
|
Holt DE, Halket S, de Louvois J and Harvey
D: Neonatal meningitis in England and Wales: 10 years on. Arch Dis
Child Fetal Neonatal Ed. 84:F85–F89. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reading R: Meningitis in infancy in
England and Wales: Follow up at age 5 years. Child Care Health Dev.
28:533–536. 2002.
|
|
10
|
Stevens JP, Eames M, Kent A, Halket S,
Holt D and Harvey D: Long term outcome of neonatal meningitis. Arch
Dis Child Fetal Neonatal Ed. 88:F179–F184. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furyk JS, Swann O and Molyneux E:
Systematic review: Neonatal meningitis in the developing world.
Trop Med Int Health. 16:672–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grimwood K: Legacy of bacterial meningitis
in infancy. Many children continue to suffer functionally important
deficits. BMJ. 323:523–524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Klinger G, Chin CN, Beyene J and Perlman
M: Predicting the outcome of neonatal bacterial meningitis.
Pediatrics. 106:477–482. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bargui F, D'Agostino I, Mariani-Kurkdjian
P, Alberti C, Doit C, Bellier N, Morin L, Galli Gibertini G, Smail
A, Zanin A, et al: Factors influencing neurological outcome of
children with bacterial meningitis at the emergency department. Eur
J Pediatr. 171:1365–1371. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ravetch JV: Fc receptors: Rubor redux.
Cell. 78:553–560. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fossati G, Bucknall RC and Edwards SW:
Fcgamma receptors in autoimmune diseases. Eur J Clin Invest.
31:821–831. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du J, Li L, Dou Y, Li P, Chen R and Liu H:
Diagnostic utility of neutrophil CD64 as a marker for early-onset
sepsis in preterm neonates. PLoS One. 9:e1026472014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung JW, Park CJ, Cha CH, Cho YU, Jang S,
Chi HS, Seo EJ, Lee JH, Lee JH, Lee KH, et al: A combination of
CD15/CD10, CD64/CD33, CD16/CD13 or CD11b flow cytometric
granulocyte panels is sensitive and specific for diagnosis of
myelodysplastic syndrome. Ann Clin Lab Sci. 42:271–280.
2012.PubMed/NCBI
|
|
19
|
Jeune Simonin-Le K, Le Jeune A, Jouneau S,
Belleguic C, Roux PF, Jaguin M, Dimanche-Boitre MT, Lecureur V,
Leclercq C, Desrues B, et al: Impaired functions of macrophage from
cystic fibrosis patients: CD11b, TLR-5 decrease and sCD14,
inflammatory cytokines increase. PLoS One. 8:e756672013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konstantinidis T, Cassimos D, Gioka T,
Tsigalou C, Parasidis T, Alexandropoulou I, Nikolaidis C,
Kampouromiti G, Constantinidis T, Chatzimichael A, et al: Can
procalcitonin in cerebrospinal fluid be a diagnostic tool for
meningitis? J Clin Lab Anal. 29:169–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdelkader NA, Mahmoud WA and Saber SM:
Serum procalcitonin in Egyptian patients with acute meningitis and
a negative direct cerebrospinal fluid examination. J Infect Public
Health. 7:106–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choi HJ: Procalcitonin in diagnosis of
post-operative bacterial meningitis: A promising but limited role.
Infect Chemother. 45:346–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Viallon A, Desseigne N, Marjollet O,
Birynczyk A, Belin M, Guyomarch S, Borg J, Pozetto B, Bertrand JC
and Zeni F: Meningitis in adult patients with a negative direct
cerebrospinal fluid examination: value of cytochemical markers for
differential diagnosis. Crit Care. 15:R1362011. View Article : Google Scholar : PubMed/NCBI
|