Introduction
Vitamin E is a generic term for all naturally
occurring tocopherols and tocotrienol as well as their derivatives
(1). This fat-soluble vitamin was
discovered and named by Evans in the 1920s (2,3). Since
its hydroxyl moiety on carbon 6 can be easily oxidized, vitamin E
has a strong reducibility, which protects important substances from
oxidation in vivo and has an important role in the
maintenance of normal metabolic processes and physiological
function in the body (4). Vitamin E
is required to protect the cell membrane from peroxide damage,
maintain immunity and enhance resistance to disease, whilst it is
tightly associated with embryonic development, nucleic acid
metabolism, ascorbic acid biosynthesis as well as maintenance of
tissue quality (5–9). Vitamin E has become one of the most
important vitamins in aquatic animal breeding. A large number of
studies have reported that vitamin E deficiency impairs aquatic
animal performance, including a reduced weight gain, protein
efficiency ratio as well as feed coefficient (10–15).
The channel catfish (Ictalurus punctatus,
Rafinesque 1818) is well known for its meat that is tasty and high
in amino acids. It is naturally distributed within the USA and
Canada and has been widely introduced to other countries worldwide
due to its value (16). This fish
has become one of the most economically important fish in China.
Channel catfish require relatively high levels of vitamin E and its
deficiency significantly inhibits fish growth (17). The intestine is a major organ for
energy and nutrition absorption to maintain survival, growth and
reproduction of fish. Although several studies have investigated
the effects of vitamin E on weight gain, tissue ascorbate
concentration as well as stress responses (18–20), few
studies have investigated the effects of vitamin E on the
intestinal structure and function.
The first purpose of the present study was to
investigate the effect of vitamin E supplementation on growth
performance and to determine the optimum dietary vitamin E
supplementation level for channel catfish. The second purpose of
the study was to explore the effects of vitamin E supplementation
on the intestinal structure and function of channel catfish. In
addition, the present study assessed the effects of vitamin E
supplementation on the expression of somatostatin, which is
distributed in the gastrointestinal tract and acts as a hormone as
well as a neurotransmitter to regulate a variety of physiological
processes, such as the body's growth, development and metabolism
(21–23). The present study provided a
scientific reference for the breeding of channel catfish.
Materials and methods
Experimental diets
All experimental protocols were approved by the
University of Sichuan Agricultural Animal Care Advisory Committee
(Ya'an, China). The experimental diets were varied with regard to
the level of vitamin E. The basal diet (Table I) was formulated according to the
Nutrient Requirements of Fish from the NRC National Research
Council (24) and was supplemented
with 0, 50, 100 or 1,000 mg/kg Dl-all-rac-α-tocopherol acetate
(Sanyou Institute of Special Additives, Chengdu, China). A study
utilizing the dose of 1,000 mg/kg has been described previously
(25). For diet preparation, the
ingredients were ground into fine powder and screened through a
mesh (0.38 mm). All ingredients were then thoroughly mixed and made
into a 4 mm-diameter soft pellet diet using a pellet feed mill.
After natural air drying in the dark, the pellets were preserved in
a freezer at −20°C. In addition, to assess the proximate
composition of the diets, crude protein, crude fat and energy were
determined using a Kjeldahl apparatus (nitrogen ×6.25; Xi'an HEB
Biotechnology Co., Ltd., Xi'an, China), extraction with petroleum
ether by a Soxhlet apparatus (Xi'an HEB Biotechnology Co., Ltd.)
followed by ash incineration at 600°C for 3 h and bomb calorimetry,
respectively. The composition of the catfish's diet is shown in
Table I.
 | Table I.Composition of the basal diet of the
catfish. |
Table I.
Composition of the basal diet of the
catfish.
| Ingredients and
proximate composition | Amount |
|---|
| Ingredients (g/kg
dry weight) |
|
|
Gelatin | 6.9 |
| Fish
meal | 12.4 |
| Rice
protein concentrate | 19.5 |
| Soybean
meal | 23.68 |
|
α-Starch | 32.77 |
|
CaH2O4 | 0.8 |
|
Chlorocholine | 0.4 |
|
Ethoxyquin | 0.05 |
| vitamin mixture
(vitamin E-free)a | 0.1 |
| Mineral
mixtureb | 1 |
| Soybean
oil | 1 |
| Fish
oil | 1.4 |
| Proximate
composition (%) |
|
| Crude
protein | 37.47 |
| Crude
fat | 5.24 |
|
Ash | 12.18 |
|
Available phosphorus | 0.45 |
| Vitamin
E (mg/kg) | 2.09 |
| Gross
energy (MJ/kg)c | 17.38 |
Experimental animals
A total of 900 healthy channel catfish (Ictalurus
punctatus, Rafinesque 1818; weight, 5.20±0.15 g; age, 2 years
old) were purchased from a fish farm (Sichuan Fisheries Science and
Technology Development Co., Ltd., Xinjin, China), where they were
originally hatched and reared. They were randomly divided into four
groups (225 fish per group) according to the vitamin E content in
the experimental diet. For each group, fish were equally divided
among three circular tanks with a volume of 20 l (total number of
tanks, 12), leading to a density of 75 fish per tank.
Prior to starting the feeding trial, fish were
acclimatized for two weeks on a basal diet that was not
supplemented with vitamin E and then fed one of the four
experimental diets by hand three times daily (07:30, 13:30 and
19:30 h) to approximate satiation for 15 weeks based on percentage
body weight. Feed consumption was monitored at each feeding. The
feeding rate was adjusted once or twice a week based on the feeding
activity of fish. The tanks were configured as a flow-through
culture system and the incoming water was filtered with aeration.
The average water temperature was 21±2°C with a pH of 6.8–7.5, and
the amount of dissolved oxygen was 8–10 mg/l. Fish were reared in a
12 h light/dark cycle.
Growth performance
All of the fish in each tank were counted and
weighed to monitor growth rates at the end of the feeding trial.
The parameters indicating the growth performance of catfish in each
group, including average weight gain, average daily growth, feed
coefficient and protein efficiency ratio, were calculated following
standard protocols. The formulas were as follows: i) Average weight
gain (g/fish) = average final weight (g) - average initial weight
(g); ii) Average daily growth (g/fish/day) = average weight gain
(g/fish)/duration of experiment (days); iii) Protein efficiency
ratio = wet weight gain (g)/protein intake (g); iv) Feed
coefficient (%) = dry feed fed (g)/wet weight gain (g); and v)
Survival (%) = (final number of fish/initial number of fish) ×
100%.
Analysis of digestive enzyme in
hepatopancreas and gut
After recording of body measurements, 45 fish were
collected from each group. The hepatopancreas and gut of fish were
removed, frozen in liquid nitrogen and then preserved at −80°C for
further analysis. For digestive enzyme analyses, tissue samples
were homogenized in ice-cold physiological saline and the
supernatant was retained after centrifugation at 3,200 × g for 20
min. The activities of lipase were evaluated using phenolphthalein
indicator and the activities of protease were determined by the
Folin-phenol reagent method. In addition, the alkaline phosphatase
(AKP) assay kit (Nanjing Jiancheng, Nanjing, Jiangsu, China) were
used to evaluate the AKP activities, and
Na+/K+-ATPase activities were assessed using
a Na+/K+-ATPase assay kit (Nanjing Jiancheng,
Nanjing, China).
Gut performance
Another 45 fish collected from each group at the end
of the feeding trial were dissected and the guts were removed. The
gut weight and length of the fish from each group was recorded.
Moreover, the gut weight index and gut length index were calculated
as follows: i) Gut weight index (%) = [average gut weight
(g)/average final body weight (g)] × 100%; and ii) Gut length index
(%) = [average gut length (cm)/average final body length (cm)] ×
100%.
Histological analysis
After recording of body measurements, another 20
fish were collected from each group and 0.5 cm of each segment of
intestine was sampled on a chilled cutting board according to a
previous description (26). Samples
were fixed in Bouin solution for 12 h, embedded in paraffin
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and cut into 5-µm
sections using a Leica RM2128 microtome (Leica Microsystems,
Wetzlar, Germany). A proportion of the sections from each segment
of the intestine was stained with hematoxylin and eosin (H&E)
and observed under an Olympus BH-2 microscope (Olympus, Tokyo,
Japan). The height of intestinal folds and the thickness of the
mucous membrane were evaluated by analyzing more than ten different
views of each sample of intestine segment using Spot software
(Version 4.0.6; Diagnostic Instruments, Sterling Heights, MI,
USA).
The other part of the tissue sections was used for
immunohistochemical analysis for detecting the distribution and
expression of somatostatin. After washing with PBS three times,
sections were rehydrated and incubated in 3%
H2O2 for 10 min. Subsequently, sections were
incubated with rabbit anti-somatostatin primary antibody (BA0124;
Wuhan Boster Biological Technology, Ltd, Wuhan, Hubei, China) at a
dilution of 1:100 at 4°C for 17 h, followed by incubation with the
biotin-conjugated goat anti-rabbit secondary antibody (BA1003;
Wuhan Boster Biological Technology, Ltd, Wuhan, Hubei, China).
Finally, sections were stained using a Streptavidin-Biotin
Peroxidase Complex kit (Wuhan Boster Biological Technology, Ltd,
Wuhan, Hubei, China) and visualized using diaminobenzidine as a
chromogen. Sections incubated with PBS instead of primary antibody
were considered as a negative control. The slices were observed and
images were captured using a digital camera (Moticam 2500, Motic,
Hong Kong, China) attached to a Nikon microscope (Nikon Eclipse
50i, Nikon, Tokyo, Japan). The expression of somatostatin among
groups was evaluated by determining the number of
somatostatin-positive cells. Ten different views per section of
each segment of intestine (three sections for each segment
intestine from each group) were obtained to analyze the expression
and distribution of somatostatin.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Significant differences among groups were determined by
one-way analysis of variance followed by Duncan's method. P<0.05
was considered to indicate a statistically significant difference
between groups. All statistical analyses were performed with SPSS
19.0 software (International Business Machines, Inc., Armonk, NY,
USA).
Results
Effect of vitamin E supplementation on
the growth performance of channel catfish
Average weight gain, protein efficiency ratio, feed
coefficient as well as survival were calculated and presented in
Table II. Dietary supplementation
with vitamin E affected catfish survival and fish with vitamin E
supplementation at different concentrations showed a higher
survival rate than those without. In addition, the fish receiving
diets supplemented with vitamin E had significantly higher growth
parameters, including average weight gain, average daily growth as
well as protein efficiency ratio than those fed with the basal diet
(P<0.05) and the highest values occurred in fish receiving a
diet supplemented with vitamin E at 100 mg/kg. The feed coefficient
is used to evaluate the feed quality with a lower the feed
coefficient indicating a higher feed quality. As shown in Table II, dietary supplementation with 50
and 100 mg/kg vitamin E had an obviously lower feed coefficient
than that with 1,000 mg/kg vitamin E or without vitamin E
(P<0.05).
 | Table II.Effects of dietary supplementation of
vitamin E on growth performance of channel catfish (Ictalurus
punctatus, Rafinesque 1818). |
Table II.
Effects of dietary supplementation of
vitamin E on growth performance of channel catfish (Ictalurus
punctatus, Rafinesque 1818).
|
| Vitamin E
supplementation (mg/kg) |
|---|
|
|
|
|---|
| Variable | 0 | 50 | 100 | 1,000 |
|---|
| Initial weight
(g) |
5.21±0.08 |
5.22±0.09 |
5.20±0.06 |
5.19±0.11 |
| Final weight
(g) |
62.93±2.36 |
76.10±1.43a |
77.20±2.38a |
73.93±0.37a |
| Average weight gain
(g/fish) |
57.73±2.55 |
70.41±2.93a |
72.00±2.74a |
69.75±0.64a |
| Average daily
growth (g/fish/day) |
2.61±0.03 |
2.96±0.06a,b |
2.99±0.50a,b |
2.85±0.02a |
| Protein efficiency
ratio |
1.39±0.08 |
1.63±0.03a |
1.63±0.06a |
1.53±0.02a |
| Feed coefficient
(%) |
2.06±0.12 |
1.75±0.03a,b |
1.74±0.06a,b |
1.95±0.02 |
| Survival (%) |
70.56±4.19 |
97.22±2.55 |
97.78±0.96 |
96.34±3.85 |
Effects of vitamin E supplementation
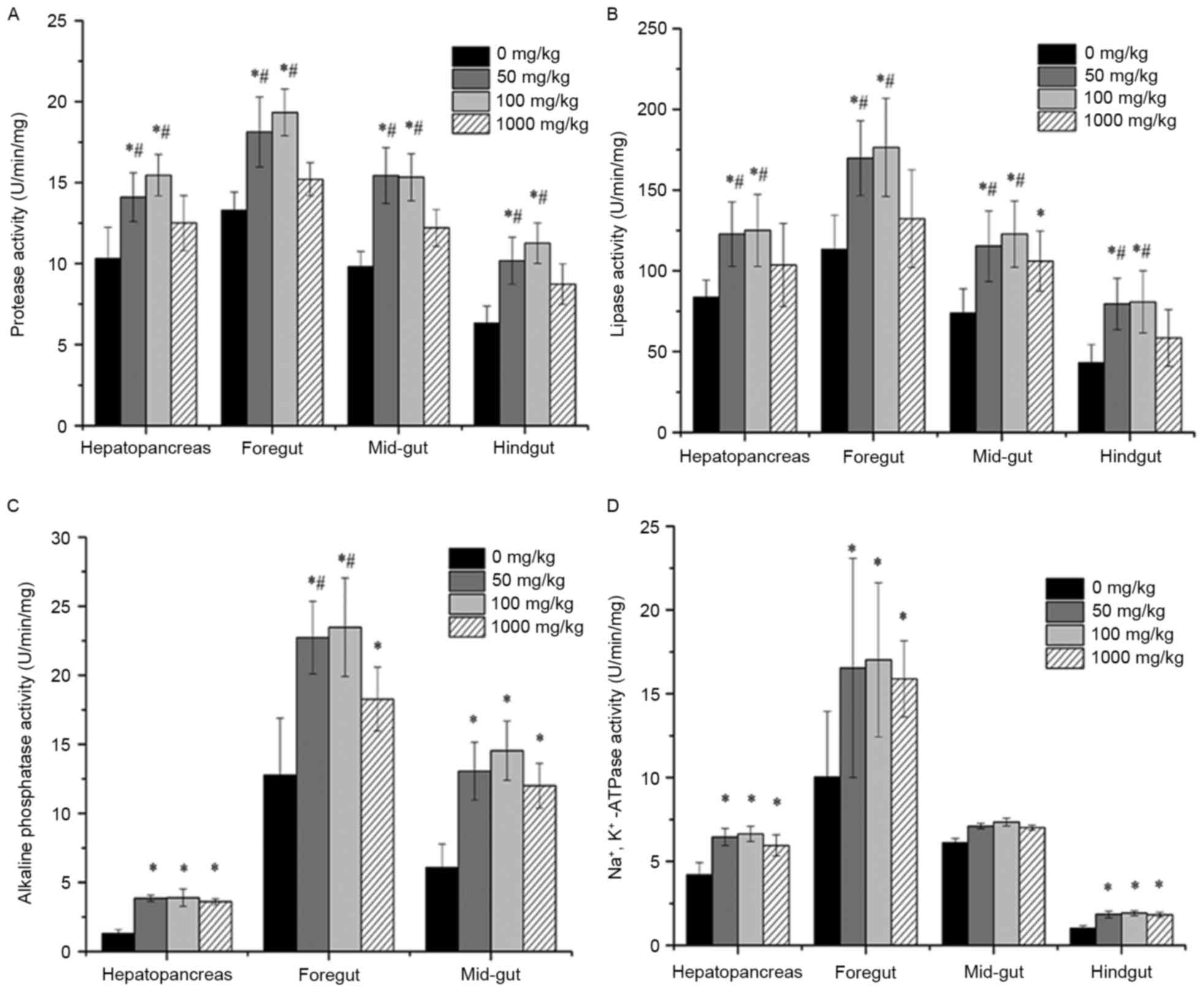
on digestive enzyme activity in channel catfish
Digestive enzyme activity in the hepatopancreas and
each gut segment of fish supplemented with different concentrations
of vitamin E was shown in Fig. 1. In
the hepatopancreas and all segments of the fish gut for 50 and 100
mg/kg vitamin E groups, protease activity was significantly
increased compared to that without vitamin E supplementation
(P<0.05) and that in 1,000 mg/kg group (P<0.05). However,
there was no significant difference in the 1,000 mg/kg group
compared with that of the 0 mg/kg group. Similarly, in the
hepatopancreas and all segments of the fish gut for 50 and 100
mg/kg groups, lipase activity was significantly increased compared
to that without vitamin E supplementation (P<0.05) and that in
1,000 mg/kg group (P<0.05). Additionally, there was no
significant difference in the hepatopancreas, foregut and hindgut
of fish in 1,000 mg/kg group compared with the 0 mg/kg group,
except in the mid-gut (P<0.05). AKP activity was significantly
increased in the hepatopancreas, foregut and mid-gut of fish in 50,
100 and 1,000 mg/kg groups compared to that without vitamin E
supplementation (P<0.05). Na+/K+-ATPase
activity was significantly increased in the hepatopancreas, foregut
and hindgut of fish in 50, 100 and 1,000 mg/kg groups compared to
that without vitamin E supplementation (P<0.05). However, there
was no significant difference in the mid-gut of fish in 50, 100 and
1,000 mg/kg groups compared with the 0 mg/kg group (P>0.05). In
addition, the concentration of supplemented vitamin E influenced
the digestive enzymes activity, since fish supplemented with
vitamin E at 50 and 100 mg/kg showed obviously higher activities of
protease, lipase and AKP than that without vitamin E (P<0.05).
Although the fish in 1,000 mg/kg group also exhibited increased
activities, the values were lower than those in the 50 and 100
mg/kg groups.
Effects of vitamin E supplementation
on gut performance of channel catfish
The results of gut weight, gut weight index, gut
length as well as gut length index of fish supplemented with
different concentrations of vitamin E are shown in Table III. Fish fed diets supplemented
with vitamin E had a greater gut length and gut weight compared to
those without (P<0.05). The gut weight index and gut length
index of fish with vitamin E supplementation were also markedly
higher than those of fish without vitamin E supplementation
(P<0.05).
 | Table III.Effects of dietary supplementation of
vitamin E on gut performance of channel catfish (Ictalurus
punctatus, Rafinesque 1818). |
Table III.
Effects of dietary supplementation of
vitamin E on gut performance of channel catfish (Ictalurus
punctatus, Rafinesque 1818).
|
| Vitamin E
supplementation (mg/kg) |
|---|
|
|
|
|---|
| Variable | 0 | 50 | 100 | 1,000 |
|---|
| Gut weight (g) |
0.62±0.03 |
0.96±0.02a |
0.97±0.03a |
0.88±0.05a |
| Gut weight index
(%) |
0.91±0.20 |
1.36±0.15a |
1.39±0.17a |
1.24±0.14a |
| Gut length
(cm) |
15.38±0.52 |
20.03±0.45a |
20.49±0.64a |
19.85±0.72a |
| Gut length index
(%) |
82.10±4.58 |
97.73±2.47a |
98.99±3.92a |
93.85±4.88a |
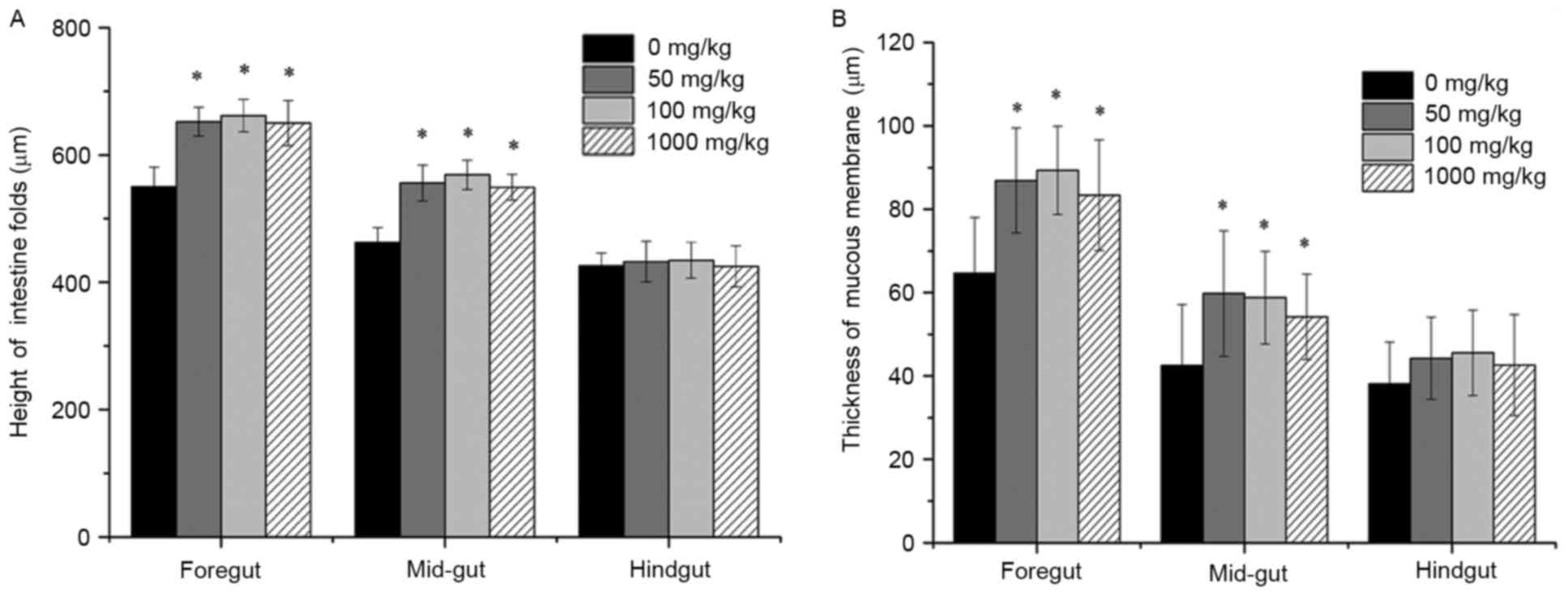
Effects of vitamin E supplementation
on the height of intestinal folds and the thickness of the mucous
membrane
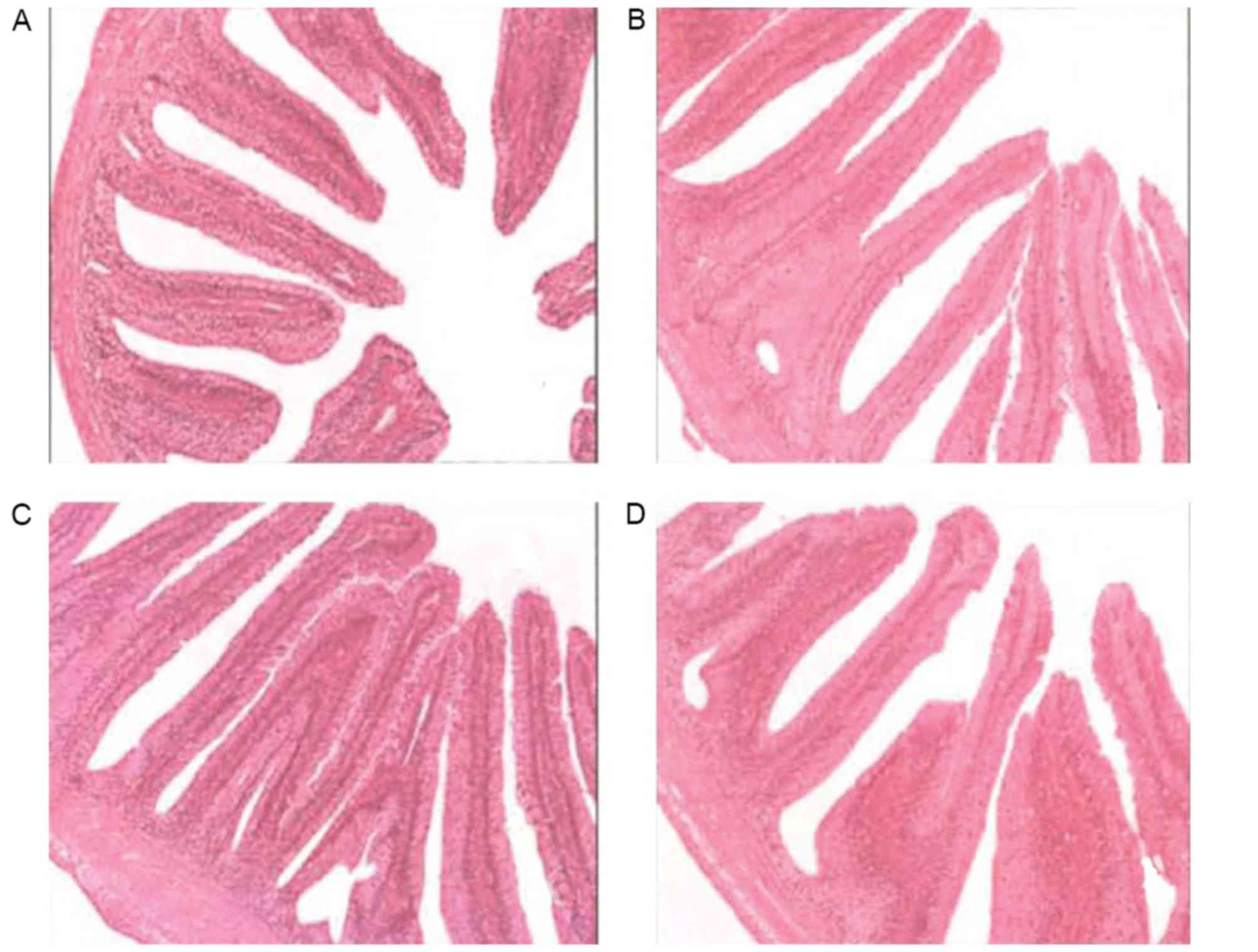
The intestinal fold height and of mucous membrane
thickness were observed under a microscope after H&E staining
and the statistical results are shown in Figs. 2 and 3. The intestinal folds in fish fed a diet
without vitamin E showed an irregular morphology and they were
small, thin and loosely arranged throughout, while they were much
larger and more tightly arranged in fish fed a diet supplemented
with vitamin E. Statistical analysis indicated that fished fed
diets containing vitamin E had significantly larger intestinal fold
height as well as a thicker mucous membrane (P<0.05).
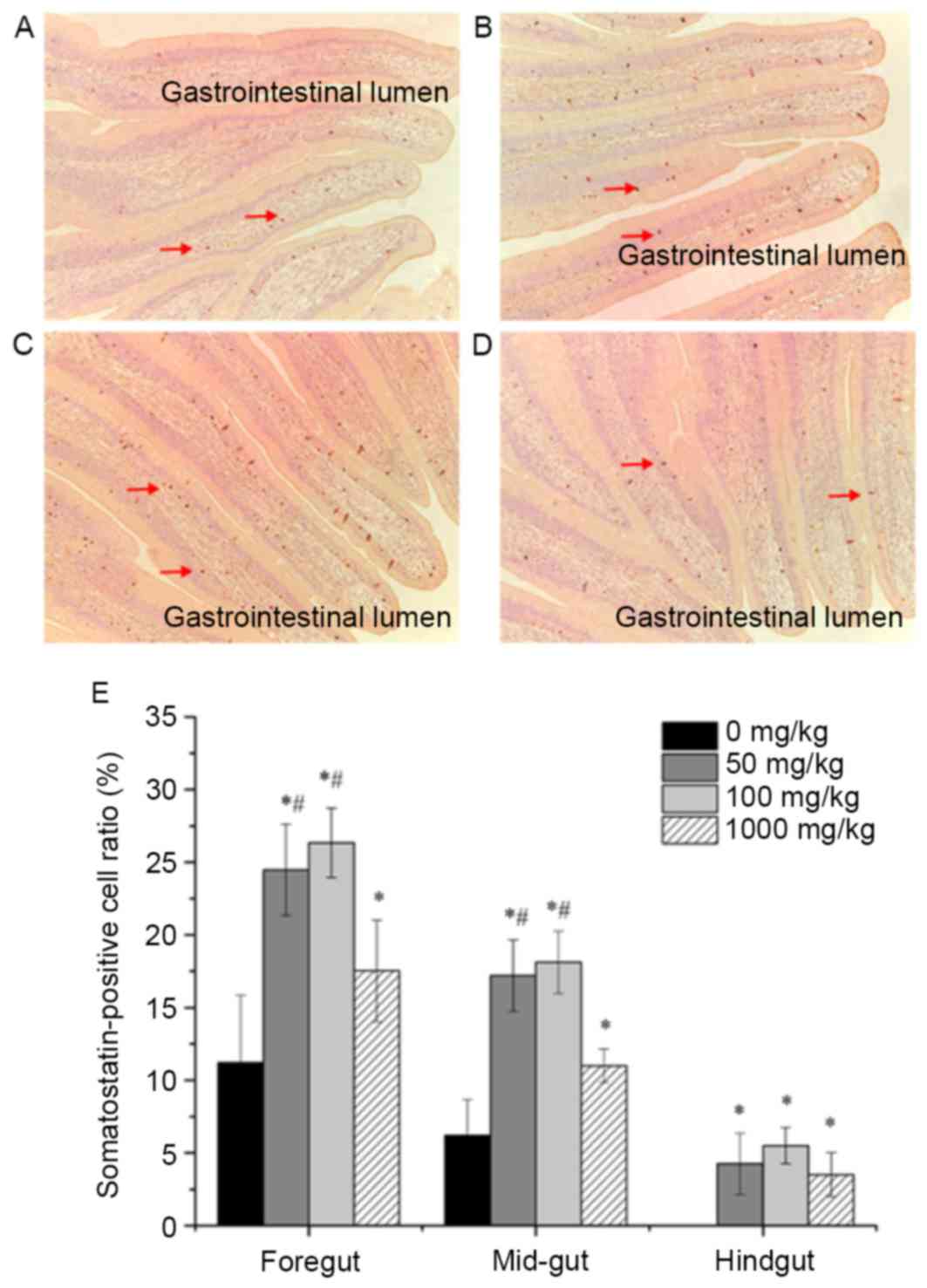
Effects of vitamin E supplementation
on the distribution and expression of somatostatin
Somatostatin-positive cells were observed in the
foregut, mid-gut as well as hindgut. The distribution of
somatostatin-positive cells in the foregut of fish fed a diet
supplemented with different amounts of vitamin E is shown in
Fig. 4A-D. Somatostatin-positive
cells (brown-yellow granules) with various cellular morphologies
were mainly located at the bottom of the intestinal epithelial cell
layer and some of them were found between cells of the mucous
epithelium. The distribution of somatostatin-positive cells among
the groups in the mid- and hindgut was similar to that in the
foregut (data not shown). In addition, the expression of
somatostatin was evaluated by quantifying the number of
somatostatin-positive cells in ten different views per section and
the results for each segment of the intestine are shown in Fig. 4E. The number of somatostatin-positive
cells was significantly higher in the foregut of fish with vitamin
E supplementation at different concentrations compared with that in
the foregut of fish without supplementation (P<0.05). Moreover,
dietary supplementation of vitamin E at 100 and 50 mg/kg resulted
in an obviously larger number of somatostatin-positive cells in the
foregut and mid-gut of fish than that at 1,000 mg/kg
(P<0.05).
Discussion
The present study was performed based on a dose
response design. The results indicated that the weight gain,
survival rate and protein efficiency ratio of fish increased with
dietary vitamin E supplementation at increasing doses of up to 100
mg/kg, after which a further increase did not achieve any
improvements or had a less pronounced effect. This trend was
similar to that previously described in other animals (27). These results suggested that dietary
supplementation with vitamin E is beneficial for the growth of
catfish. A large body of evidence has indicated that vitamin E at a
certain dose range promoted the growth of aquatic animals (28). Vitamin E deficiency induces excessive
production of toxic lipid peroxides, leading to a decrease of
weight gain and feed efficiency in animals. The addition of vitamin
E to their diet prevents variable unsaturated fatty acids in diets
and tissues of aquatic animals from oxidative rancidity and
maintains a normal metabolism, thus increasing the survival rate,
weight gain as well as feed efficiency of animals (29). In addition, dietary vitamin E
supplementation decreased the feed coefficient in the present
study, which has been an important and visual index to evaluate the
feed quality. As previously described, adding an appropriate dose
of vitamin E reduced the feed coefficient and increased the feed
quality and feed efficiency (13,14), and
the results of the present study were consistent with this.
It is well accepted that the intestine has an
important role as the site of nutrient digestion and absorption,
and digestive function correlates with the development of the
intestinal tract. In the present study, intestine weight and
length, which reflect the development of the intestine, were
significantly increased in response to high dietary vitamin E. In
addition, differences in gut length and weight among fish species
appear to be associated with their feeding habits as described
previously (30). In the present
study, dietary vitamin E at a certain dose range promoted gut
development. Aquatic animals have a relatively simple mucosal
structure. Instead of the intestinal villus as in terrestrial
animals, the intestinal fold directly reflects the digestive and
absorptive ability of fish (31,32).
Intestinal fold height has been considered as an indicator of the
absorptive ability of aquatic animals. Moreover, the thickness of
the mucous membrane correlates to the functional area of the
intestine, directly influencing the absorption and transportation
of nutrition. According to the results of the present study,
dietary vitamin E at a certain dose range significantly increased
the intestinal height and the thickness of the mucous membrane,
thus improving the intestine function in catfish.
The ability of digestion is associated with the
activity of digestive enzymes, which are affected by dietary intake
(33,34). In the present study, dietary vitamin
E at a certain dose range increased the activities of protease and
lipase. Intestinal AKP is a marker enzyme in the intestinal mucosa,
which reflects epithelial cell proliferation and absorbency ability
of the intestine (35). In addition,
this enzyme also correlates with the degree of differentiation of
enterocytes and its content reflects their maturation status
(36). In the present study, the
activity of AKP was observed only in the foregut and mid-gut and
was significantly higher in fish with vitamin E dietary
supplementation (at a certain range dose) than in those without. In
addition, the absorption of certain nutrients, including amino
acids and glucose, was associated with the absorption of
Na+ by Na+/K+-ATPase. The activity
of Na+/K+-ATPase indirectly reflects the cell
energy metabolism and function of the gut (37). As shown in the present study, vitamin
E dietary supplementation at a certain dose range also increased
the activity of this enzyme. Considering all of the above results,
it is concluded that vitamin E dietary supplementation at a certain
dose range significantly improved intestinal function. Furthermore,
vitamin E had a stimulatory effect on digestion enzyme activity in
the hepatopancreas of catfish. As previously reported, enzyme
secretion is closely associated with the development of the
hepatopancreas (38). Dietary
supplementation of vitamin E also promoted the development of the
hepatopancreas.
Somatostatin is a typical brain-gut peptide of dual
distribution (21,39). Somatostatin protects epithelial cells
in the digestive tract from damage and necrosis caused by a variety
of harmful substances (40). The
distribution of somatostatin-positive cells in the gastrointestinal
tract varied in different fish. For instance, in Korean aucha perch
(Coreoperca herzi), Monopterus albus and Perca
fluriatilis, somatostatin-expressing cells were only found in
the stomach (41,42). In Tilapia nilotca, oriental
sheatfish (Silurus asotus) and mandarin fish (Siniperca
chuatsi), somatostatin-expressing cells were found in the
stomach and intestines (42). In the
present study, somatostatin-expressing cells existed only in the
intestines of channel catfish rather than in the stomach. However,
channel catfish have more than two genes encoding different forms
of somatostatin (43,44). Different endogenous somatostatin
isoforms are already known to function differently not only between
tissues but also within the same target cell/tissue in the same
teleost species (45,46). Accordingly, further investigation of
the distribution of somatostatin isoforms should also be performed.
Due to the important role of somatostatin in the digestive tract,
the expression of this protein may be regarded as another index to
express the effects of vitamin E on gut function in channel
catfish. According to the results of the present study, dietary
supplementation of vitamin E at a certain dose range significantly
increased the expression of somatostatin.
In conclusion, dietary vitamin E supplementation at
doses within a certain range promote the growth of channel catfish
and improve their intestinal structure and function. The optimal
dietary vitamin E supplementation should be limited to the range of
50–100 mg/kg. Due to the feasibility and efficacy of oral vitamin E
supplementation for channel catfish, the present study provided an
experimental foundation for the use of vitamin E for breeding of
channel catfish in the aquaculture industry.
Acknowledgements
This study was supported by the Program for
Changjiang Scholars, the Innovative Research Team in University
(no. IRTO848), the Sichuan Provincial Office of Education
Scientific Research Project (no. 10ZB035) and the Scientific
Research Fund of Sichuan Provincial Education Department (no.
13SZA0249).
References
|
1
|
Yu W, Simmons-Menchaca M, Gapor A, Sanders
BG and Kline K: Induction of apoptosis in human breast cancer cells
by tocopherols and tocotrienols. Nutr Cancer. 33:26–32. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blaner WS: Vitamin E: The enigmatic one! J
Lipid Res. 54:2293–2294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niki E: Role of vitamin E as a
lipid-soluble peroxyl radical scavenger: in vitro and in vivo
evidence. Free Radic Biol Med. 66:3–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sen CK, Khanna S and Roy S: Tocotrienols:
Vitamin E beyond tocopherols. Life Sci. 78:2088–2098. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McDowell LR: The vitaminsFish nutrition
Academic Press, San Diego, California. Vitamin E Academic Press;
London: pp. 93–131. 1989
|
|
6
|
Halver JE: The vitamins. Fish nutrition
Academic Press; San Diego, California: pp. 61–141. 2002
|
|
7
|
Aggarwal BB, Sundaram C, Prasad S and
Kannappan R: Tocotrienols, the vitamin E of the 21st century: Its
potential against cancer and other chronic diseases. Biochem
Pharmacol. 80:1613–1631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salinthone S, Kerns AR, Tsang V and Carr
DW: α-Tocopherol (vitamin E) stimulates cyclic AMP production in
human peripheral mononuclear cells and alters immune function. Mol
Immunol. 53:173–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fang Y, Zhou Y, Zhong Y, Gao X and Tan T:
Effect of vitamin E on reproductive functions and anti-oxidant
activity of adolescent male mice exposed to bisphenol A. Wei Sheng
Yan Jiu. 42:18–22. 2013.(In Chinese). PubMed/NCBI
|
|
10
|
Sau Sk, Paul BN, Mohanta KN and Mohanty
SN: Dietary vitamin E requirement, fish performance and carcass
composition of rohu (Labeo rohita) fry. Aquaculture.
240:359–368. 2004. View Article : Google Scholar
|
|
11
|
Thorarinsson R, Landolt ML, Elliott DG,
Pascho RJ and Hardy RW: Effect of dietary vitamin E and selenium on
growth, survival and the prevalence of Renibacterium
salmoninarum infection in chinook salmon (Oncorhynchus
tshawytscha). Aquaculture. 121:343–358. 1994. View Article : Google Scholar
|
|
12
|
Lee KJ and Dabrowski K: Interaction
between vitamins C and E affects their tissue concentrations,
growth, lipid oxidation, and deficiency symptoms in yellow perch
(Perca flavescens). Br J Nutr. 89:589–596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai SC and Lee KJ: Different levels of
dietary DL-α-tocopheryl acetate affect the vitamin E status of
juvenile Korean rockfish, Sebastes schlegeli. Aquaculture.
161:405–414. 1998. View Article : Google Scholar
|
|
14
|
Huang CH and Lin WY: Effects of dietary
vitamin E level on growth and tissue lipid peroxidation of
soft-shelled turtle, Pelodiscus sinensis (Wiegmann). Aquac
Res. 35:948–954. 2004. View Article : Google Scholar
|
|
15
|
Paul B, Sarkar S and Mohanty SN: Dietary
vitamin E requirement of mrigal, Cirrhinus mrigala fry.
Aquaculture. 242:529–536. 2004. View Article : Google Scholar
|
|
16
|
Keenan E, Warner S, Crowe A and Courtney
M: Length, Weight and Yield in Channel Catfish. Lake Diane, MI:
arXiv preprint arXiv:1102.4623. 2011
|
|
17
|
Jobling M: National Research Council
(NRC): Nutrient requirements of fish and shrimp. Aquac Int.
20:601–602. 2012. View Article : Google Scholar
|
|
18
|
Gatlin DM, Bai SC and Erickson MC: Effects
of dietary vitamin E and synthetic antioxidants on composition and
storage quality of channel catfish, Ictalurus punctatus.
Aquaculture. 106:323–332. 1992. View Article : Google Scholar
|
|
19
|
Bai SC and Gatlin DM: Dietary vitamin E
concentration and duration of feeding affect tissue α-tocopherol
concentrations of channel catfish (Ictalurus punctatus).
Aquaculture. 113:129–135. 1993. View Article : Google Scholar
|
|
20
|
Li MH, Wise DJ and Robinson EH: Effect of
dietary vitamin C on weight gain, tissue ascorbate concentration,
stress response and disease resistance of channel catfish
ictalurus punctatus1. J World Aquac Soc. 29:1–8.
1998. View Article : Google Scholar
|
|
21
|
Patel YC: Somatostatin and its receptor
family. Front Neuroendocrinol. 20:157–198. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diakatou E, Kaltsas G, Tzivras M, Kanakis
G, Papaliodi E and Kontogeorgos G: Somatostatin and dopamine
receptor profile of gastroenteropancreatic neuroendocrine tumors:
An immunohistochemical study. Endocr Pathol. 22:24–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheridan MA and Hagemeister AL:
Somatostatin and somatostatin receptors in fish growth. Gen Comp
Endocrinol. 167:360–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Council NR: Nutrient requirements of fish.
Washington: National Academy; 1993
|
|
25
|
Wen J, Morrissey PA, Buckley DJ and Pja S:
Supranutritional vitamin E supplementation in pigs: Influence on
subcellular deposition of α-tocopherol and on oxidative stability
by conventional and derivative spectrophotometry. Meat Science.
47:301–310. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kühlwein H, Merrifield DL, Rawling MD,
Foey AD and Davies SJ: Effects of dietary β-(1,3)(1,6)-D-glucan
supplementation on growth performance, intestinal morphology and
haemato-immunological profile of mirror carp (Cyprinus
carpio L.). J Anim Physiol Anim Nutr. 98:279–289. 2014.
View Article : Google Scholar
|
|
27
|
Falahatkar B, Amlashi AS and Conte F:
Effect of dietary vitamin E on cortisol and glucose responses to
handling stress in juvenile beluga Huso huso. J Aquat Anim
Health. 24:11–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taveekijakarn P, Miyazaki T, Matsumoto M
and Arai S: Study on vitamin E deficiency in amago salmon. Bull Fac
Biores Mie Univ. 16:17–24. 1996.
|
|
29
|
Wassef E, El Masry MH and Mikhail FR:
Growth enhancement and muscle structure of striped mullet, Mugil
cephalus L., fingerlings by feeding algal meal-based diets. Aquac
Res. 32:315–322. 2001. View Article : Google Scholar
|
|
30
|
De Silva SS and Anderson TA: Fish
nutrition in aquaculture. Springer Science Business Media; 1994
|
|
31
|
Olli JJ and Krogdahi Å: Nutritive value of
four soybean products as protein sources in diets for rainbow trout
(Oncorhynchus mykiss, Walbaum) reared in fresh water. Acta
Agric Scand A Anim Sci. 44:185–192. 1994.
|
|
32
|
Farhangi M and Carter CG: Growth,
physiological and immunological responses of rainbow trout
(Oncorhynchus mykiss) to different dietary inclusion levels
of dehulled lupin (Lupinus angustifolius). Aquac Res.
32:329–340. 2001. View Article : Google Scholar
|
|
33
|
Galgani F and Ceccaldi HJ: Effet de
l'incorporation de farines de soja et de poisson dans l'aliment sur
la croissance et les enzymes digestives de Penaeus vannamei. Aquat
Living Resour. 1:181–187. 1988.(In French). View Article : Google Scholar
|
|
34
|
Kumlu M and Jones D: The effect of live
and artificial diets on growth, survival and trypsin activity in
larvae of Penaeus indicus. J World Aquac Soc. 26:406–415.
1995. View Article : Google Scholar
|
|
35
|
Cuvier-Péres A and Kestemont P:
Development of some digestive enzymes in Eurasian perch larvae
Perca fluviatilis. Fish Physiol Biochem. 24:279–285. 2001.
View Article : Google Scholar
|
|
36
|
Wood SR, Zhao Q, Smith LH and Daniels CK:
Altered morphology in cultured rat intestinal epithelial IEC-6
cells is associated with alkaline phosphatase expression. Tissue
Cell. 35:47–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rhoads JM, Chen W, Chu P, Berschneider HM,
Argenzio RA and Paradiso AM: L-glutamine and L-asparagine stimulate
Na+-H+ exchange in porcine jejunal
enterocytes. Am J Physiol. 266:G828–G838. 1994.PubMed/NCBI
|
|
38
|
Yan L and Qiu-Zhou X: Dietary glutamine
supplementation improves structure and function of intestine of
juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture.
256:389–394. 2006. View Article : Google Scholar
|
|
39
|
Lin X and Peter RE: Somatostatins and
their receptors in fish. Comp Biochem Physiol B Biochem Mol Biol.
129:543–550. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reichlin S: Somatostatin. N Engl J Med.
309:1495–1501. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JH, Ku SK, Park KD and Lee HS:
Immunohistochemical study of the gastrointestinal endocrine cells
in the Korean aucha perch. J Fish Biol. 65:170–181. 2004.
View Article : Google Scholar
|
|
42
|
Pan QS, Fang ZP and Huang FJ:
Identification, localization and morphology of APUD cells in
gastroenteropancreatic system of stomach-containing teleosts. World
J Gastroenterol. 6:842–847. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hobart P, Crawford R, Shen L, Pictet R and
Rutter WJ: Cloning and sequence analysis of cDNAs encoding two
distinct somatostatin precursors found in the endocrine pancreas of
anglerfish. Nature. 288:137–141. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moore CA, Kittilson JD, Ehrman MM and
Sheridan MA: Rainbow trout (Oncorhynchus mykiss) possess two
somatostatin mRNAs that are differentially expressed. Am J Physiol.
277:R1553–R1561. 1999.PubMed/NCBI
|
|
45
|
Hanssen L, Hanssen KF and Myren J:
Inhibition of secretin release and pancreatic bicarbonate secretion
by somatostatin infusion in man. Scand J Gastroenterol. 12:391–394.
1977. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dollinger HC, Raptis S and Pfeiffer EF:
Effects of somatostatin on exocrine and endocrine pancreatic
function stimulated by intestinal hormones in man. Horm Metab Res.
8:74–78. 1976. View Article : Google Scholar : PubMed/NCBI
|