Introduction
Inflammation is a complex event involving the
activation of physiological and pathological immune system
processes (1,2). In the normal state, proinflammatory
mediators, such as nitric oxide (NO) and prostaglandin
(PG)E2, and proinflammatory cytokines generated from
macrophage cells serve an essential role in host survival and
tissue repair (1,2). However, when macrophages are activated
by specific stimuli, these proinflammatory mediators and cytokines
are overproduced, leading to various inflammatory diseases, such as
arthritis, inflammatory bowel disease and asthma (3,4).
Therefore, inhibition of macrophage activation has been suggested
as a potential therapeutic mechanism for mitigating the progression
of inflammatory diseases.
Lipopolysaccharide (LPS)-induced activation of RAW
264.7 macrophages is a well-established model of inflammation, with
previous studies demonstrating that LPS, a cell wall component of
gram-negative bacteria, activates macrophages to produce
proinflammatory mediators and cytokines, thereby mimicking an
inflammatory reaction (5,6). Studies have also shown that the
LPS-induced stimulation of macrophages initiates the toll-like
receptor (TLR)-4/myeloid differentiation primary response gene 88
signaling pathway, which involves the activation of nuclear factor
(NF)-κB, phosphatidylinositol 3-kinase (PI3K)/RAC-α
serine/threonine-protein kinase (Akt) and mitogen-activated protein
kinase (MAPK) signaling pathways (7,8). This,
in turn, has been indicated to upregulate proinflammatory cytokines
and inducible enzymes, such as inducible NO synthase (iNOS) and
cyclooxygenase (COX)-2 (9,10).
The transcription factor NF-κB plays a major role in
the regulation of genes associated with inflammation (11,12). The
activation of NF-κB occurs via phosphorylation and degradation of
the suppressor protein inhibitor of NF-κB α (IκBα) bound to NF-κB,
resulting in the translocation of NF-κB into the nucleus and the
promotion and induction of the expression of various inflammatory
genes (11,12). TLR4-mediated signaling leads to rapid
activation of the PI3K/Akt pathway, which has an important role in
the regulation of LPS-induced acute inflammatory responses
(5,6). Previous studies have indicated that the
PI3K/Akt signaling pathway contributes to TLR4-mediated NF-κB
activation and cytokine release (7,8). Studies
have also demonstrated that MAPKs, such as extracellular
signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and
p38 MAPK, function as a group of signaling molecules that are
critical in relaying inflammatory information (13,14). The
TLR4-induced activation of MAPKs has been shown to activate the
nuclear translocation of NF-κB and to culminate in the initiation
of proinflammatory responses (7,8). Thus,
inhibition of the NF-κB, PI3K/Akt and MAPK signaling pathways is
critical in combating the actions of anti-inflammatory
molecules.
Daehwangmokdantang (DHMDT) is a traditional
polyherbal formulation that has been used since ancient times in
Korea. As described in the Donguibogam, an ancient Korean medical
book published in the early 17th century (15), DHMDT is composed of five medicinal
herbs: Paeonia suffruticosa Andr., Prunus persica
(L.) Batsch, Trichosanthes kirilowii Maxim, Rheum
plamatum L. and mirabilite. DHMDT has been used to treat
patients with digestive tract cancers and to prevent diarrhea and
inflammation (15). Despite the
valuable clinical effects of DHMDT on patients, the molecular
mechanism of its pharmacological effects has yet to be elucidated.
As part of the present research group's search for novel
biologically active substances from traditional medicinal resources
that are able to prevent and treat inflammation, the inhibitory
effect of DHMDT on LPS-induced inflammatory responses induced in
RAW 264.7 macrophages was evaluated in the present study. For the
first time, to the best of our knowledge, whether DHMDT inhibits
inflammatory responses via suppression of the NF-κB, PI3K/Akt, and
MAPK signaling pathways was also examined.
Materials and methods
Materials and reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were purchased from WelGENE Inc. (Daegu, South
Korea). LPS (Escherichia coli Serotype 055:B5), MTT and
Griess reagent were obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). PGE2 (cat. no. KGE004B) tumor
necrosis factor (TNF)-α (cat. no. MTA00B) and interleukin (IL)-1β
(cat. no. MLB00C) ELISA detection kits were purchased from R&D
Systems, Inc. (Minneapolis, MN, USA). Antibodies directed against
iNOS (1:1,000; cat. no. sc-509), COX-2 (1:500; cat. no. sc-19999),
IL-1β (1:1,000; cat. no. sc-32294), p65 (1:500; cat. no. sc-109),
ERK (1:1,000; cat. no. sc-154), p38 (1:1,000; cat. no. sc-535),
IKK-α (1:1,000; cat. no. sc-1643), IKKβ (1:1,000; cat. no.
sc-8014), Akt (1:1,000; cat. no. sc-8312), lamin B (1:1,000; cat.
no. sc-6216), β-actin (1:1,000; cat. no. sc-69879), goat anti-mouse
IgG-horseradish peroxidase (HRP) (1:1,500; cat. no. sc-2005), goat
anti-rabbit IgG-HRP (1:1,500; cat. no. sc-2004) and bovine
anti-goat IgG-HRP (1:1,500; cat. no. sc-2350) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Anti- TNF-α
(1:1,000; cat. no. 3707), phospho-JNK (1:500; cat. no. 9255), JNK
(1:1,000; cat. no. 9252S), phospho-ERK (1:500; cat. no. 9106S),
phospho-p38 (1:500; cat. no. 9211S), phospho-IKKα/β (1:50; cat. no.
2694) and p-Akt (1:500; cat. no. 9271) were purchased from Cell
Signaling Technology Inc. (Danvers, MA, USA). All other chemicals
were purchased from Sigma-Aldrich (Merck KGaA).
Preparation of the DHMDT extract
All herbs and mirabilite (natrii sulfas) (Table I) were obtained from Dongeui Oriental
Hospital, Dongeui University College of Korean Medicine (Busan,
South Korea). Each of the four herbal components of DHMDT and
mirabilite were cut into small pieces and the components were then
mixed together in the ratios shown in Table I to provide a total weight of 42 g.
The mixture was boiled with distilled water (42 g/500 ml) for 3 h.
The extract was filtered with a 0.45 µM filter to remove insoluble
materials. The filtrate was lyophilized and then crushed into a
thin powder. The extracts were dissolved in dimethyl sulfoxide
(DMSO) to a concentration of 100 mg/ml, and the stock solution was
then diluted with DMEM to the required concentration prior to use.
The final concentration of DMSO was always ≤0.1%.
 | Table I.Components and their quantities in the
Daehwangmok-dantang decoction. |
Table I.
Components and their quantities in the
Daehwangmok-dantang decoction.
| Component
(pharmacognostic nomenclature) | Quantity of raw
material, g (%) |
|---|
| Paeonia
suffruticosa | 10.0 (23.8) |
| Andr. (Moutan
Cortex) |
|
| Prunus persica
L. Batsch | 10.0 (23.8) |
| (Persicae Semen) |
|
| Trichosanthes
kirilowii Maxim | 10.0 (23.8) |
| (Trichosanthis
Fructus) |
|
| Rheum plamatum
L. | 6.0 (14.3) |
| (Rhei Radix) |
|
| Mirabilite (Natrii
Sulfas) | 6.0 (14.3) |
| Total | 42 (100) |
Cell culture and LPS stimulation
Murine macrophage-like RAW 264.7 cells were obtained
from the Korean Cell Line Bank (Seoul, South Korea) and grown in
DMEM, supplemented with 10% FBS, glucose (4.5 g/l), sodium pyruvate
(1 mM), L-glutamine (2 mM), penicillin (100 U/ml) and streptomycin
(100 U/ml) in an incubator at 37°C, with 5% CO2 and 95%
humidity. To stimulate the cells, the medium was exchanged for
fresh DMEM, and LPS (500 ng/ml) was added in the presence or
absence of DHMDT for various time periods, as described in the
following experiments.
Assessment of cell viability
The effects of DHMDT on cell viability were
evaluated using an MTT assay. In brief, RAW 264.7 cells were seeded
at a density of 1×104 cells/well in a 96-well plate and
incubated at 37°C for 24 h, followed by treatment with 800 µg/ml
DHMDT alone, 500 ng/ml LPS alone, or 500 ng/ml LPS plus 200, 400 or
800 µg/ml DHMDT for a further 24 h. Untreated RAW 264.7 cells
served as the control. Following this, MTT solution was added to
each well and the plate was further incubated for 4 h at 37°C. The
medium was then discarded, and DMSO was added to dissolve the
formazan dye. The optical density was then read at 450 nm using an
ELISA reader (Infinite M200; Tecan Group, Ltd., Männedorf,
Switzerland).
Measurement of NO production
The accumulation of NO was assayed using Griess
reagent (1% sulfanilamide, 0.1% naphthylethylenediamine
dihydrochloride and 2.5% phosphoric acid). The cells were
pretreated with 200, 400 or 800 µg/ml DHMDT for 1 h and then
stimulated with 500 ng/ml LPS for 24 h. Untreated RAW 264.7 cells
served as the control group. Subsequently, 100 µl Griess reagent
was mixed with an equal volume of cell supernatant and the mixture
was incubated at room temperature for 5 min. The optical density at
540 nm was measured, and the concentration of nitrite was
calculated according to the standard curve generated from known
concentrations of sodium nitrite.
PGE2, TNF-α, and IL-1β
immunoassay
The amounts of PGE2 and proinflammatory
cytokines released in the culture medium were measured using mouse
PGE2, TNF-α and IL-1β ELISA kits based on the
quantitative sandwich enzyme immunosorbent technique. Pretreatment
with DHMDT was conducted for 1 h prior to LPS stimulation for 24 h,
as described for the NO production assay. The levels of
PGE2, TNF-α and IL-1β in the culture media were
quantified using ELISA kits according to the manufacturer's
protocol. The absorbance was read at a wavelength of 450 nm using a
microplate reader.
Western blot analysis
Following treatment with the various concentrations
of DHMDT in the presence or absence of LPS, as described for the NO
production assay, the cells were washed, scraped into cold PBS and
centrifuged at 500 × g for 5 min at 4°C. The cell pellets were
suspended in lysis buffer [20 mM sucrose, 1 mM EDTA, 20 µM Tris-HCl
(pH 7.2), 1 mM dithiothreitol, 10 mM KCl, 1.5 mM MgCl2
and 5 µg/ml aprotinin]. Following removal of the cell debris by
centrifugation at 13,000 × g for 15 min at 4°C, the protein
concentration was determined using Bio-Rad Protein Assay Dye
Reagent Concentrate (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). In a parallel experiment, nuclear and cytosolic proteins were
prepared using nuclear extraction reagents (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) according to the manufacturer's
protocol. Equal amounts of protein (20–30 µg) from each sample were
separated by 10% SDS-PAGE and transferred onto nitrocellulose
membranes (Schleicher and Schuell; GE Healthcare Life Sciences,
Little Chalfont, UK). Nonspecific sites were blocked by incubation
of the membranes for 1 h at room temperature with 5% (w/v) non-fat
milk powder in Tris-buffered saline containing 0.05% (v/v)
Tween-20. Thereafter, the membranes were incubated overnight at 4°C
with the corresponding primary antibodies and subsequently
incubated with the appropriate HRP-conjugated secondary antibodies
for 1 h at room temperature. The specific proteins were detected
using an Amersham ECL Western Blotting Detection Reagent (Amersham;
GE Healthcare Life Sciences). Densitometric analyses were completed
using the ImageJ software (version 1.50i; National Institutes of
Health, Bethesda, MD, USA) to quantify protein expression levels.
All protein measurements were normalized to β-actin expression and
these normalized values were used for all statistical analyses.
Immunofluorescent staining of NF-κB
p65
RAW 264.7 cells were seeded on glass coverslips in
6-well plates for 24 h. The cells were then treated with DHMDT
(200–800 µg/ml) for 1 h, followed by stimulation with 500 ng/ml LPS
for 30 min. Untreated RAW 264.7 cells served as the control. The
cells were then rinsed twice with PBS and fixed with 3.7%
paraformaldehyde in PBS for 10 min at 4°C. Following fixation, the
cells were incubated with 0.4% Triton X-100 for 10 min and blocked
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 1 h at
room temperature, followed by probing with rabbit anti-p65 NF-κB
antibody (1:50; cat. no. sc-109; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. The cells were then incubated with fluorescein
isothiocyanate-conjugated donkey anti-rabbit IgG (1:200; cat. no.
711-095-152; Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) for 2 h at room temperature. After washing with PBS, the
nuclei were counterstained with DAPI solution (1 mg/ml) for 15 min
in the dark, and fluorescence was visualized using a fluorescence
microscope (Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
Data from at least three independent experiments are
expressed as the mean ± standard deviation (SD). Differences among
the groups were evaluated using one-way analysis of variance
followed by Duncan's multiple range tests. The statistical analysis
was conducted using SPSS 19.0 software (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
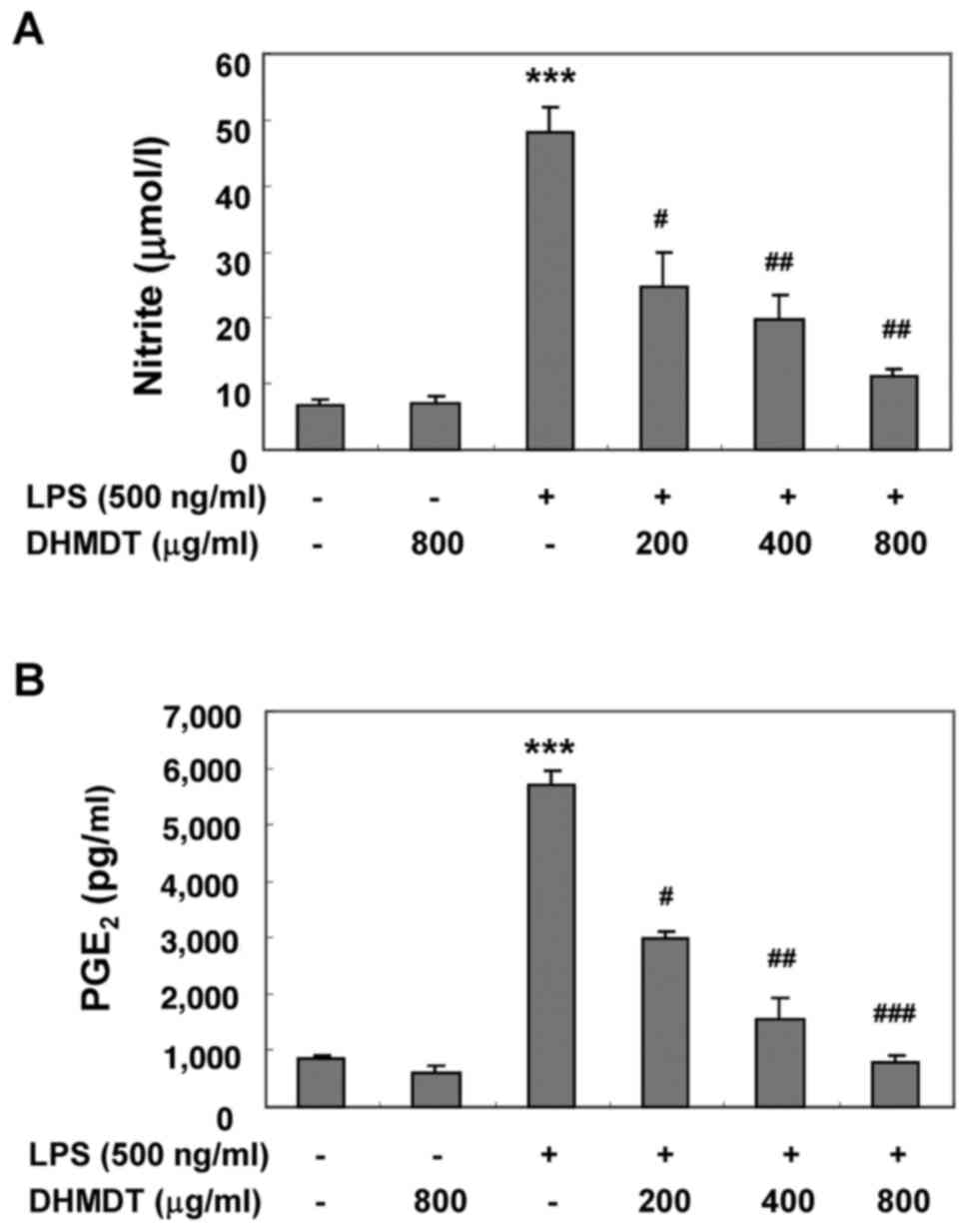
Inhibition of the LPS-induced
production of NO and PGE2 by DHMDT in RAW 264.7
macrophages
The potential inhibitory effects of DHMDT on
LPS-induced NO and PGE2 production in RAW 264.7
macrophages were first examined using Griess reagent and ELISA
analyses, respectively. The cells were pretreated with various
concentrations of DHMDT for 1 h prior to stimulation with 500 ng/ml
LPS for 24 h. As illustrated in Fig.
1, NO and PGE2 production was significantly induced
in the LPS-stimulated RAW 264.7 cells compared with the
unstimulated negative control, and pretreatment with DHMDT
significantly prevented this increase in a dose-dependent manner.
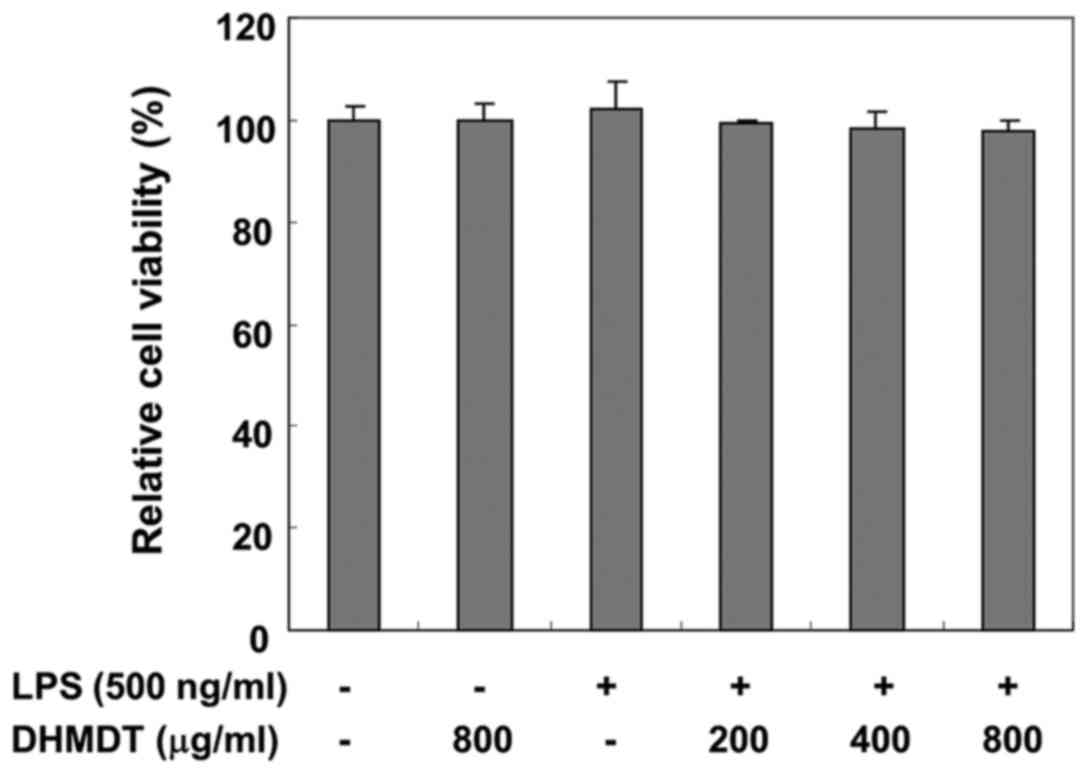
To exclude the possibility of DHMDT having a cytotoxic effect on
the RAW 264.7 cells, the viability of the cells was evaluated using
an MTT assay. The concentrations (200, 400 and 800 µg/ml) used for
the inhibition of NO and PGE2 production did not exhibit
any cytotoxicity (Fig. 2),
confirming that inhibition of NO and PGE2 production in
the LPS-stimulated RAW 264.7 cells was not due to a cytotoxic
action of DHMDT.
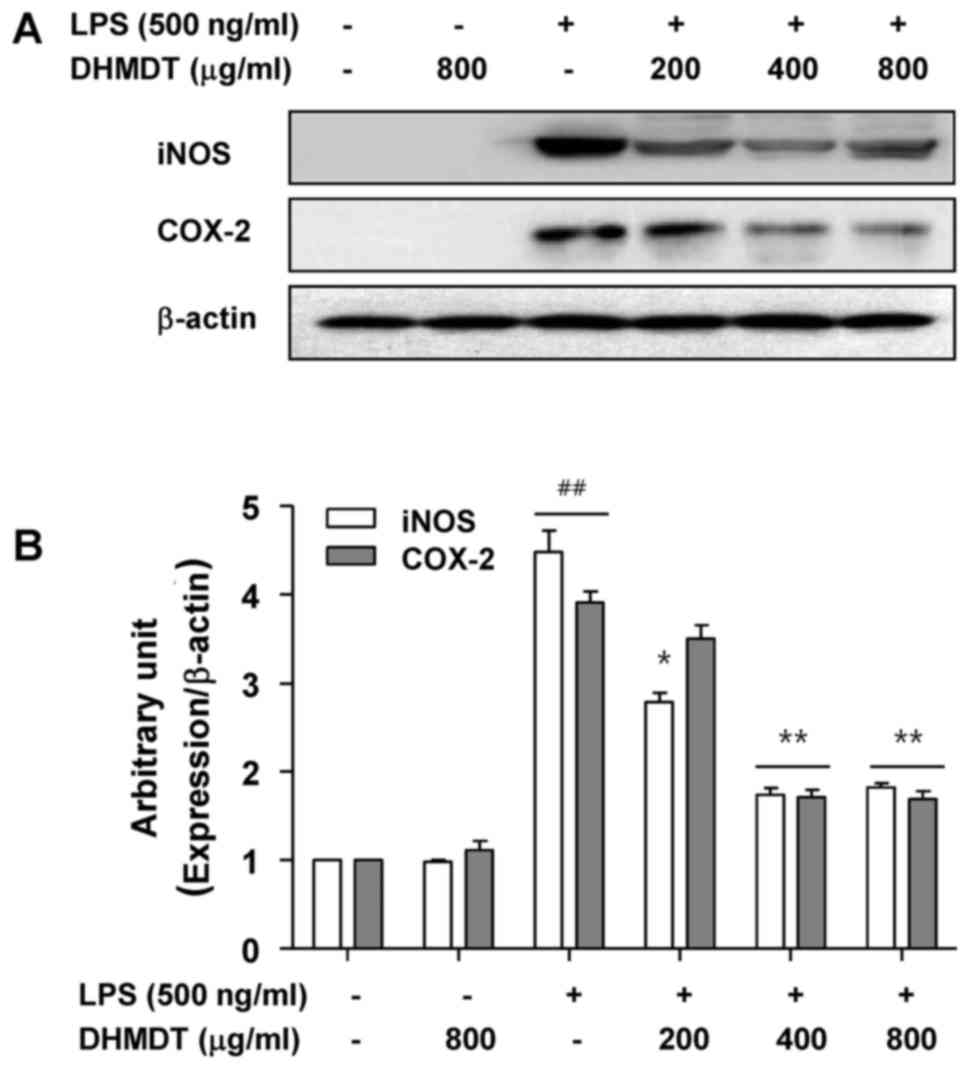
Inhibition of the LPS-induced
expression of iNOS and COX-2 by DHMDT in RAW 264.7 macrophages
To further investigate the mechanism of the
inhibitory effect of DHMDT on NO and PGE2 production,
the protein expression of iNOS and COX-2, the enzymes that mediate
the synthesis of NO and PGE2 (9,10), was
determined using western blot analysis. As shown in Fig. 3, the protein levels of iNOS and COX-2
were undetectable in the RAW 264.7 cells in the absence of LPS
stimulation, and treatment with LPS alone significantly increased
iNOS and COX-2 protein levels. However, the pretreatment with DHMDT
significantly suppressed the expression of iNOS and COX-2 proteins.
These results indicate that DHMDT was able to inhibit the
expression of iNOS and COX-2 enzymes, which, in turn, reduced the
production of NO and PGE2, two key mediators of
inflammation.
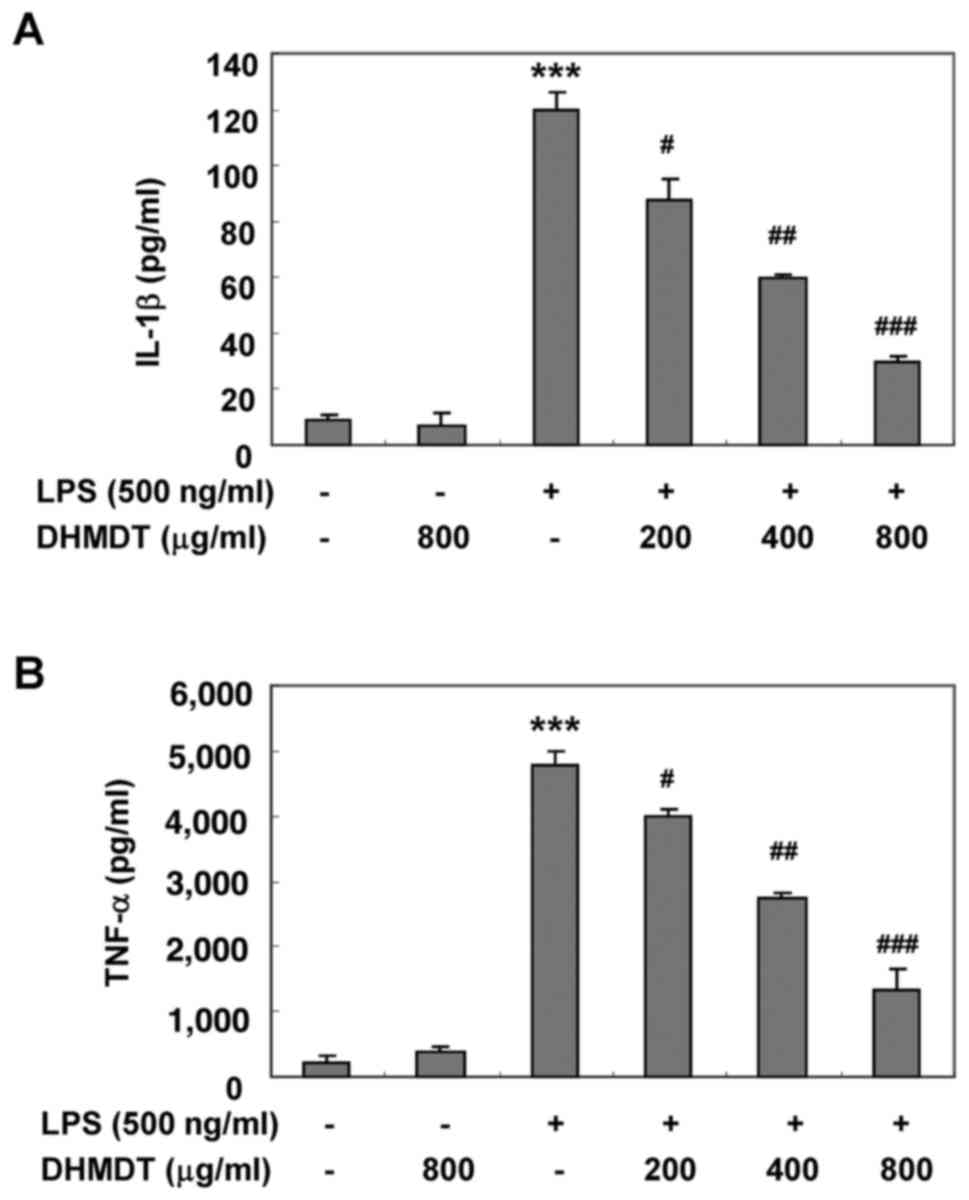
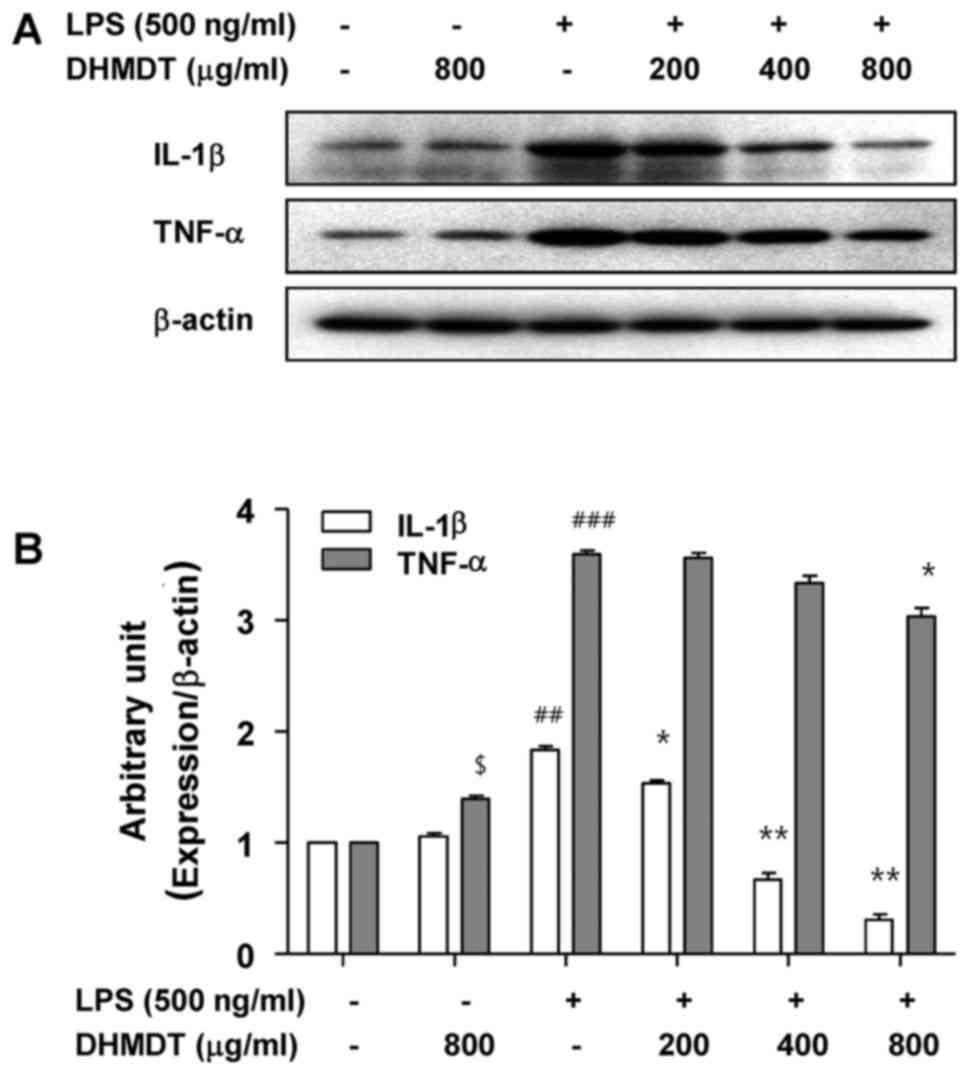
Inhibition of the LPS-induced
production and expression of TNF-α and IL-1β by DHMDT in RAW 264.7
macrophages
To examine the effects of DHMDT on the production of
proinflammatory cytokines following LPS treatment, the levels of
TNF-α and IL-1β in the culture media were measured using an ELISA.
As shown in Fig. 4, the challenge
with LPS alone induced significant increases in the levels of TNF-α
and IL-1β, whereas the pretreatment with DHMDT significantly
reduced the secretion of TNF-α and IL-1β in the cell media in a
concentration-dependent manner. Next, whether the inhibitory effect
of DHMDT on TNF-α and IL-1β release into the culture medium was
associated with modulation of the protein levels in the RAW 264.7
cells was investigated using western blotting. As shown in Fig. 5, DHMDT significantly suppressed the
protein expression of TNF-α and IL-1β compared with that in the LPS
only-treated group. The reduced expression of the TNF-α and IL-1β
proteins was consistent with the reduction in NO and
PGE2 in the culture media, suggesting that the
DHMDT-mediated inhibition of TNF-α and IL-1β production is
associated with the downregulation of TNF-α and IL-1β
expression.
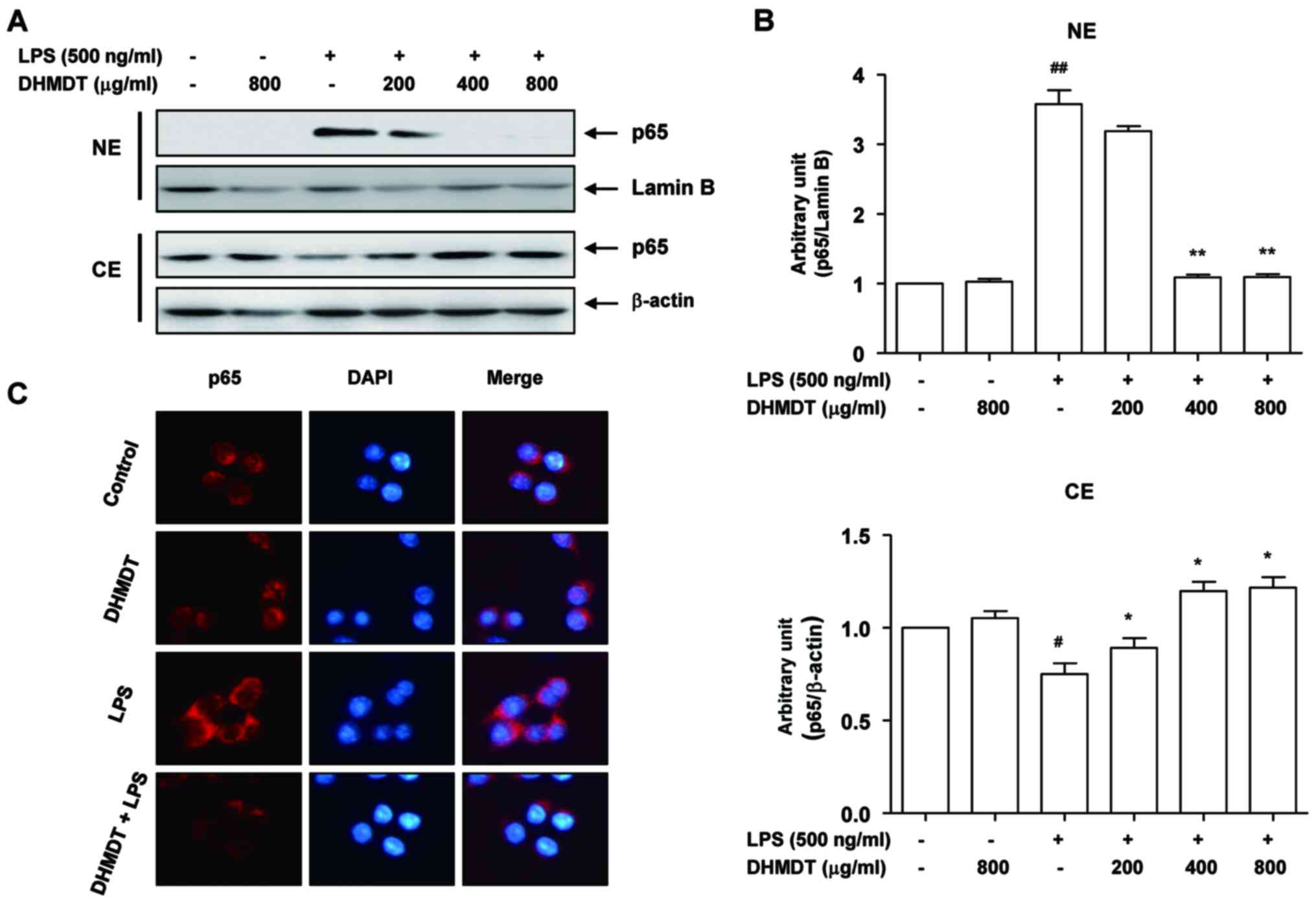
Attenuation of the LPS-induced nuclear
translocation of NF-κB by DHMDT in RAW 264.7 macrophages
As NF-κB plays a pivotal role in the regulation of
iNOS, COX-2 and proinflammatory cytokine expression (11,12), the
effects of DHMDT on the activation of NF-κB were examined. As
displayed in Fig. 6A, LPS
stimulation for 30 min caused the translocation of p65, a component
of the heterodimer of NF-κB, to the nucleus. However, DHMDT
pretreatment effectively blocked the LPS-induced nuclear
accumulation of p65 in the cells. These results were confirmed by
NF-κB and DAPI co-staining in the LPS-treated RAW 264.7 cells, with
DHMDT significantly preventing the nuclear translocation of p65
(Fig. 6B). As the phosphorylation of
IκB kinase (IKK) α/β allows IκBα to be phosphorylated and
ubiquitinated from the p50/p65 complex of NF-κB, the effects of
DHMDT on the inhibition of IKK activity in the RAW 264.7 cells were
explored. The western blotting data reveal that DHMDT inhibited the
LPS-induced phosphorylation of IKKα/β (Fig. 7A), suggesting that DHMDT effectively
inhibits LPS-induced NF-κB pathway activation by blocking the
nuclear translocation of NF-κB and the phosphorylation of
IKKα/β.
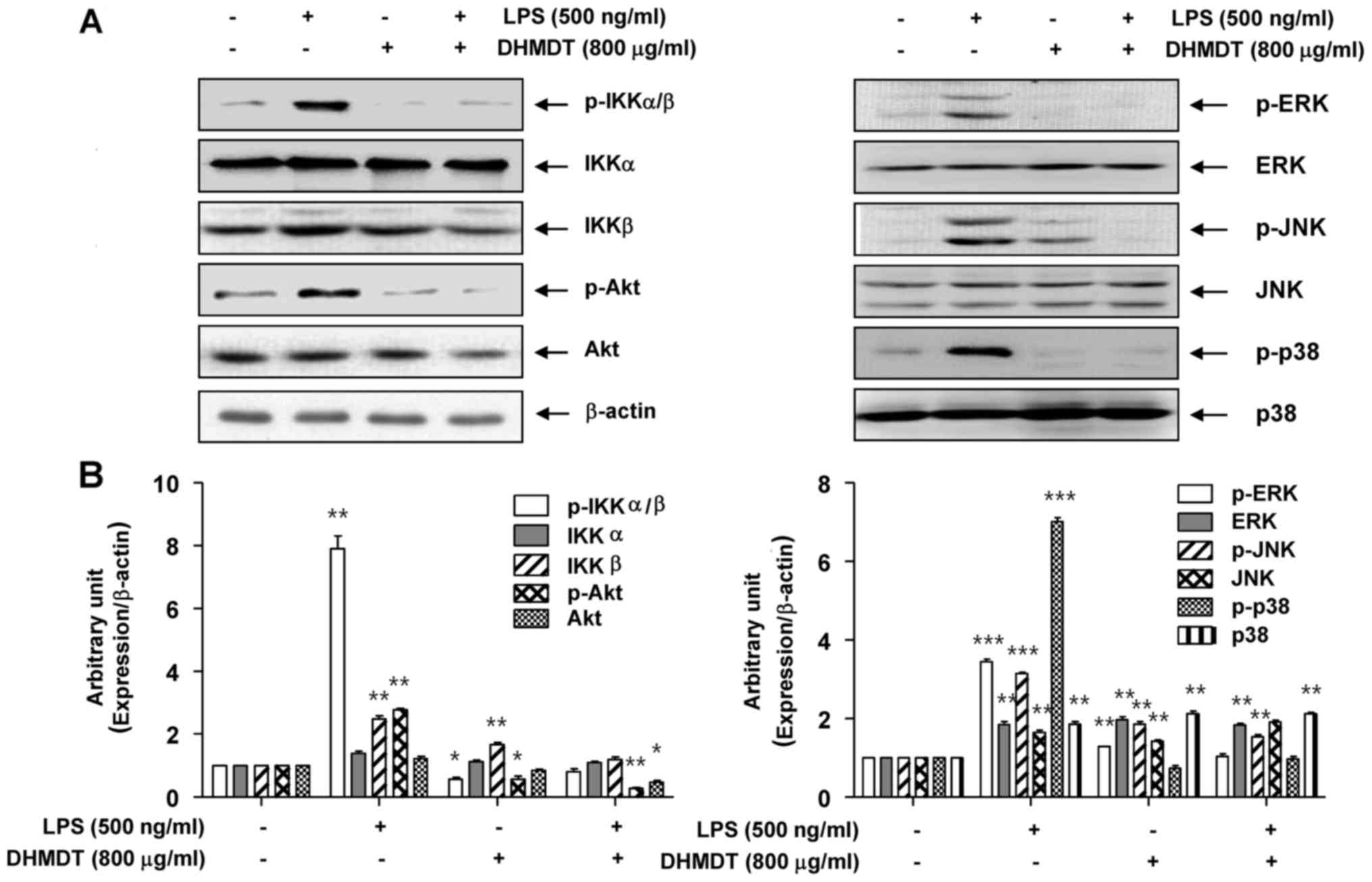 | Figure 7.Effect of DHMDT on the LPS-induced
phosphorylation of IKKα/β, Akt and MAPKs in RAW 264.7 macrophages.
The RAW 264.7 cells were pretreated with 800 µg/ml DHMDT for 1 h
prior to exposure to LPS for 30 min, and total proteins were
isolated. (A) The proteins were subjected to SDS-PAGE, followed by
western blot analysis. (B) ImageJ densitometric analysis of bands
expressed in relation to β-actin. Data are presented as mean ±
standard deviation of the mean. *P<0.05, **P<0.01 and
***P<0.005 vs. cells cultured with 500 ng/ml LPS only. DHMDT,
Daehwangmokdantang; LPS, lipopolysaccharide; p-, phosphorylated;
IKK, inhibitor of nuclear factor-κB kinase; Akt, protein kinase B;
ERK, extracellular signal-regulated kinase; JNK, c-Jun NH2-terminal
kinase. |
Suppression of the LPS-induced
phosphorylation of AKT and MAPKs by DHMDT in RAW 264.7
macrophages
To investigate whether the inhibition of
inflammatory reactions by DHMDT was mediated through the PI3K/Akt
and MAPK pathways, the effects of DHMDT on LPS-induced
phosphorylation of upstream kinases, including Akt, ERK, JNK, and
p38 MAPK in the RAW 264.7 cells were examined. As shown in Fig. 7, the phosphorylated levels of Akt and
the three MAPKs were significantly increased following treatment
for 30 min in the LPS-stimulated cell group, compared with the
control group without LPS treatment. DHMDT pretreatment clearly
attenuated the phosphorylation levels of these kinases compared
with those in the LPS only-treated group. These results suggest
that the anti-inflammatory effect of DHMDT may be due to modulation
of the PI3K/Akt and MAPK signaling pathways in RAW 264.7
macrophages.
Discussion
Macrophages are an important component in the immune
defense mechanism. Under normal circumstances,
inflammation-associated mediators, including NO and
PGE2, which are generated by iNOS and COX-2 enzymes,
respectively, and cytokines generated from macrophages have an
essential role in host survival and tissue repair (3,16).
However, these inflammatory mediators and cytokines are
overproduced in response to stimuli and may cause
inflammation-associated disorders (4,17).
Moreover, proinflammatory cytokines, including TNF-α and IL-1β,
evoke increases in the levels of iNOS and COX-2, followed by
significant increases in NO and PGE2 production
(1,2). Therefore, investigation of the
suppression of NO and PGE2 via the inhibition of iNOS
and COX-2 is important in the development of anti-inflammatory
agents. The results of the present study demonstrated that LPS
significantly upregulated the production of NO and PGE2,
and that pretreatment with DHMDT significantly inhibited the
LPS-induced release of NO and PGE2 by the RAW 264.7
macrophages, with no cytotoxicity at the concentrations employed.
In addition, the results from the western blot analysis revealed
that the application of DHMDT reduced the LPS-induced iNOS and
COX-2 levels, and ELISA results demonstrated that DHMDT
dose-dependently inhibited LPS-induced TNF-α and IL-1β production
by the RAW 264.7 macrophages. Consistent with these findings, it
was observed that DHMDT significantly suppressed the protein levels
of TNF-α and IL-1β. The results of the present study suggest that
DHMDT may elicit anti-inflammatory effects in RAW 264.7 macrophages
by reducing the LPS-stimulated production of proinflammatory
molecules.
NF-κB is a critical inducible transcription factor,
which serves a major role in the regulation of gene expression in
response to inflammation (11,12). The
activation of NF-κB involves phosphorylation and subsequent
proteolytic degradation of the inhibitory protein IκBα, which binds
to the NF-κB complex in the cytoplasm. Free NF-κB is then
translocated into the nucleus, where it binds to the NF-κB site in
the promoter regions of genes for inflammatory proteins, including
iNOS, COX-2, TNF-α, and IL-1β. The engagement of LPS activates IKK,
which initiates signal-induced degradation of IκB proteins. IKK
phosphorylates IκBα and then ubiquitinates p-IκBα from NF-κB, which
enables the translocation of NF-κB into the nucleus (8,16). To
investigate whether the inhibitory effect of DHMDT on the
production and expression of proinflammatory factors was associated
with NF-κB pathway activity, the effect of DHMDT on NF-κB nuclear
translocation was measured in the present study. The results
indicated that LPS alone reduced the levels of NF-κB p65 in the
cytoplasm of the RAW 264.7 macrophages and increased those in the
nucleus, and that this effect was reversible by pretreatment with
DHMDT. Furthermore, additional evidence that the inhibition of
NF-κB nuclear translocation by DHMDT resulted from the disturbance
of IKKα/β activation was obtained. The present study demonstrated
that DHMDT prevented the LPS-induced phosphorylation of IKKα/β in
RAW 264.7 macrophages. This suggests that inhibition of the NF-κB
signaling pathway by the suppression of IKK activation in RAW 264.7
macrophages may regulate the suppressive effects of DHMDT on
inflammatory mediators and cytokines.
Although the role of PI3K/Akt signaling in the
regulation of NF-κB transactivation is unclear, studies have shown
that phosphorylated Akt promotes the liberation of NF-κB upon the
activation of Akt by PI3K and that NF-κB subsequently translocates
into the cell nucleus to transcribe inflammatory mediators
(13,14). By inducing the nuclear translocation
of p65 in macrophages, the MAPK pathway activates pathways leading
to the production of proinflammatory mediators and cytokines
(18,19). Therefore, PI3K/Akt- and MAPK-targeted
therapeutics may be effective for the treatment of inflammatory
diseases, given that a wide variety of pharmacological agents
reportedly inhibit activation steps in the PI3K/Akt and MAPK
signaling pathways (8,20). To investigate whether the
anti-inflammatory effects of DHMDT were mediated through the
PI3K/Akt and MAPK pathways, the LPS-induced phosphorylation of Akt
and various MAPK family proteins, specifically ERK, JNK and p38
MAPK, were assessed in the present study. DHMDT pretreatment was
observed to markedly suppress the LPS-stimulated phosphorylation of
Akt, which is a critical step in PI3K activation (7,8).
Additionally, DHMDT significantly inhibited the LPS-induced
phosphorylation of three MAPKs. These results indicate that DHMDT
blocks LPS-induced NF-κB activation via changes in the
phosphorylation of Akt and MAPKs. In particular, inhibition of the
phosphorylation of Akt or MAPKs may lead to reductions in the
production and expression levels of inflammatory enzymes, including
iNOS and COX-2, and cytokines in RAW 264.7 macrophages.
In conclusion, the results of the present study
indicate that DHMDT has a strong inhibitory effect on the secretion
of NO, PGE2 and inflammatory cytokines, including TNF-α
and IL-1β. In the LPS-stimulated RAW 264.7 macrophages, this
inhibitory effect was exerted via suppression of iNOS, COX-2, TNF-a
and IL-1b protein expression. These inhibitory effects are possibly
mediated by the inhibition of NF-κB activation and blockade of the
PI3K/Akt and MAPK signaling pathways.
Although further studies are warranted to establish
the signaling pathways responsible for the observed effects, the
results of the present study suggest that DHMDT may be a useful
agent for the prevention or reversal of inflammatory responses.
Acknowledgements
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea
(NRF) grant funded by the Korea government (grant nos.
2013R1A1A2065537 and 2015R1A2A2A01004633).
References
|
1
|
Conti B, Tabarean I, Andrei C and Bartfai
T: Cytokines and fever. Front Biosci. 9:1433–1449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amin AR, Attur M and Abramson SB: Nitric
oxide synthase and cyclooxygenases: Distribution, regulation, and
intervention in arthritis. Curr Opin Rheumatol. 11:202–209. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X and Mosser DM: Macrophage
activation by endogenous danger signals. J Pathol. 214:161–178.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muralidharan S and Mandrekar P: Cellular
stress response and innate immune signaling: Integrating pathways
in host defense and inflammation. J Leukoc Biol. 94:1167–1184.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verstrepen L, Adib-Conquy M, Kreike M,
Carpentier I, Adrie C, Cavaillon JM and Beyaert R: Expression of
the NF-kappaB inhibitor ABIN-3 in response to TNF and toll-like
receptor 4 stimulation is itself regulated by NF-kappaB. J Cell Mol
Med. 12:316–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buchanan MM, Hutchinson M, Watkins LR and
Yin H: Toll-like receptor 4 in CNS pathologies. J Neurochem.
114:13–27. 2010.PubMed/NCBI
|
|
7
|
Li L, Jacinto R, Yoza B and McCall CE:
Distinct post-receptor alterations generate gene- and
signal-selective adaptation and cross-adaptation of TLR4 and TLR2
in human leukocytes. J Endotoxin Res. 9:39–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Broom OJ, Widjaya B, Troelsen J, Olsen J
and Nielsen OH: Mitogen activated protein kinases: A role in
inflammatory bowel disease? Clin Exp Immunol. 158:272–280. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
del Zoppo G, Ginis I, Hallenbeck JM,
Iadecola C, Wang X and Feuerstein GZ: Inflammation and stroke:
Putative role for cytokines, adhesion molecules and iNOS in brain
response to ischemia. Brain Pathol. 10:95–112. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McDaniel ML, Kwon G, Hill JR, Marshall CA
and Corbett JA: Cytokines and nitric oxide in islet inflammation
and diabetes. Proc Soc Exp Biol Med. 211:pp. 24–32. 1996,
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yenari MA and Han HS: Influence of
hypothermia on post-ischemic inflammation: Role of nuclear factor
kappa B (NFkappaB). Neurochem Int. 49:164–169. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Caivano M: Role of MAP kinase cascades in
inducing arginine transporters and nitric oxide synthetase in
RAW264 macrophages. FEBS Lett. 429:249–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Jiang S and Tapping RI: Toll-like
receptor signaling in cell proliferation and survival. Cytokine.
49:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heo J and Kim KJ: Dongui Bogam.
Beopinmunhwasa Publishing Corp.; Seoul: 1999, (In Korean).
|
|
16
|
Fujihara M, Muroi M, Tanamoto K, Suzuki T,
Azuma H and Ikeda H: Molecular mechanisms of macrophage activation
and deactivation by lipopolysaccharide: Roles of the receptor
complex. Pharmacol Ther. 100:171–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laveti D, Kumar M, Hemalatha R, Sistla R,
Naidu VG, Talla V, Verma V, Kaur N and Nagpal R: Anti-inflammatory
treatments for chronic diseases: A review. Inflamm Allergy Drug
Targets. 12:349–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haddad JJ: The role of inflammatory
cytokines and NF-kappaB/MAPK signaling pathways in the evolution of
familial Mediterranean fever: Current clinical perspectives and
potential therapeutic approaches. Cell Immunol. 260:6–13. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saklatvala J: Inflammatory signaling in
cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use
of inhibitors for research into pathogenesis and therapy of
osteoarthritis. Curr Drug Targets. 8:305–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei J and Feng J: Signaling pathways
associated with inflammatory bowel disease. Recent Pat Inflamm
Allergy Drug Discov. 4:105–117. 2010. View Article : Google Scholar : PubMed/NCBI
|