Introduction
Sepsis is defined as a systemic inflammatory
response syndrome that is caused by infection. Despite advances in
the management of septic shock, it remains the primary cause of
mortality in intensive care units (1). Lipopolysaccharide (LPS) from
gram-negative bacteria is the primary cause of severe sepsis
(2). LPS enhances the inflammatory
reaction by activating cells associated with inflammation, and
causing the synthesis and release of associated pro-inflammatory
cytokines, and ultimately the inflammatory response of sepsis may
lead to irreversible cardiac dysfunction (2). Cardiac dysfunction is a frequently
occurring, serious complication of sepsis, which is correlated with
the mortality of septic patients (3). Compared with septic patients without
cardiac dysfunction, patients with cardiac dysfunction exhibit an
evidently increased mortality rate (3). Therefore, it is urgent to identify an
effective treatment for this dysfunction in septic patients.
Toll-like receptor (TLR)-4 is a member of the TLR
family, which serves a vital role in the innate immune system and
may be activated by LPS (4). TLR4
and its associated mitogen-activated protein kinase (MAPK) and
nuclear factor-κB (NF-κB) signaling pathways have been demonstrated
to be correlated with LPS-induced inflammation (5,6). An
increased level of cytokines, including tumor necrosis factor α
(TNF-α), interleukin (IL)-1β and IL-6, aggravates the development
and progression of the septic myocardial dysfunction (7). The downregulation of TLR4-mediated MAPK
and NF-κB signaling pathways inhibits the LPS-induced inflammatory
response, and provides a protective effect in various organs
against sepsis (5,6). Previous studies have reported that
glutamine (GLN), as the most abundant free amino acid in the human
body, is able to regulate the immune response induced by LPS,
attenuate the release of cytokines and alleviate LPS-induced acute
liver, renal, intestinal mucosal and lung injuries (8–10).
However, the effect and underlying mechanisms of GLN in LPS-induced
cardiac dysfunction remains unclear.
In order to investigate whether GLN has a potential
protective effect against cardiac dysfunction, the present study
established an LPS-induced sepsis model in Sprague-Dawley rats and
attempted to identify the possible underlying mechanisms. It was
hypothesized that GLN may be used against LPS-induced cardiac
dysfunction by blocking the activation of the TLR4/MAPK and NF-κB
signaling pathways.
Materials and methods
Animals and cardiac dysfunction
model
All animal experiments were performed in accordance
with the Guide for the Care and Use of Laboratory Animals (11), and approved by the Animal Care and
Use Committee of Renmin Hospital of Wuhan University (Wuhan,
China). A total of 45 specific pathogen-free male Sprague-Dawley
rats (8–10 weeks-old; 180–220 g) were purchased from the Hunan SJA
Laboratory Animal Co., Ltd. (Changsha, China). All animals were
housed in a light-controlled room with a 12 h light/dark cycle, at
a temperature of 25°C and a humidity of 45–50%. All animals had
free access to food and water for 1 week prior to the commencement
of the study to allow them to acclimatize to the laboratory
environment. Rats were randomly divided into three groups (15 rats
per group), including the control, LPS and LPS+GLN groups. Rats in
the LPS+GLN group were intragastrically administered with GLN (cat.
no. V900419; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a
dose of 1 g/kg once per day for 5 days, while rats in the control
and LPS groups received the same volume of normal saline. On day 6,
cardiac dysfunction was induced by a single intraperitoneal
injection of 10 mg/kg LPS (cat. no. L2880; Sigma-Aldrich; Merck
KGaA), as described previously (12). The rats in the control group were
injected with the same volume of saline. At 24 h following LPS
injection the echocardiography and catheter-based analyses were
performed on the surviving rats (control group, n=15; LPS group,
n=9; and LPS+GLN group, n=13) under 1.5% inhaled isoflurane
anesthesia (13).
Observation of the mortality rate
The time of LPS injection was assigned as the
baseline (time point 0). The survival of rats was observed at 6,
12, 18 or 24 h, respectively, following LPS injection and the
mortality rates of the three groups were analyzed.
Echocardiography and hemodynamics
analysis
Transthoracic echocardiography was performed at 24 h
after the animals were injected with LPS or normal saline.
Echocardiography was performed on anesthetized (inhaled 1.5%
isoflurane) rats using a MyLab 30CV (Esaote SpA, Genoa, Italy)
equipped with a 10-MHz linear array ultrasound transducer. The left
ventricular (LV) dimensions were assessed in parasternal short-axis
view during systole or diastole, with a sweep speed of 50 mm/s at
the mid-papillary muscle level from the LV M-mode tracing. The LV
end-systolic diameter (LVESD), LV end-diastolic dimension L (LVEDD)
and fractional shortening (FS) were also measured. Hemodynamic
variables were analyzed using a microtip transducer catheter
(SPR-839; Millar, Inc., Houston, TX, USA) and a Millar
Pressure-Volume system (MPVS-400; Millar, Inc.). The maximal rate
of pressure development (dP/dt max), and minimal rate of pressure
decay (dP/dt min) were then processed with the PVAN data analysis
software LabChart version 7.3.7 (Millar, Inc.; ADInstruments,
Dunedin, New Zealand).
Analysis of blood samples by
ELISA
Following the collection of echocardiography and
catheter-based measurements, blood was collected from the retro
orbital plexus in the rats eyes. All blood samples were centrifuged
at 4,200 × g for 10 min at room temperature, and the supernatants
were subsequently collected and stored at −20°C. The levels of
TNF-α (cat. no. KRC3011), IL-1β (cat. no. BMS630) and IL-6 (cat.
no. KRC0061) in the blood samples were measured using ELISA kits
(eBioscience; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocols.
Histological examination
The animals were sacrificed following the collection
of the blood samples and the hearts were excised, washed with 10%
KCl and fixed with 10% formalin. Next, the heart tissues were
embedded in paraffin and then cut transversely close to the apex to
visualize the left and right ventricles. Subsequently, 4–5-µm
cardiac tissue sections were stained with hematoxylin and eosin
(HE) for histopathological examination, and the pathological
changes were evaluated under a light microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In order to investigate the relative mRNA expression
of B-type natriuretic peptide (BNP), TLR4, TNF-α, IL-1β and IL-6,
pulverized heart tissues were homogenized and total RNA was
isolated using TRIzol reagent (cat. no. 15596-026; Invitrogen;
Thermo Fisher Scientific, Inc.). A DU 730 Series UV/Vis
spectrophotometer (Beckman Coulter, Inc., Brea, CA, USA) was used
to determine the quality of RNA using the A260/A280 ratio. Total
mRNA (2 µg of each sample) was reverse transcribed using a
PrimeScript™ RT reagent kit (cat. no. RR047A; Takara Bio, Inc.,
Otsu, Japan). According to the manufacturer's protocol, PCR
amplification was conducted using a SYBR® Green Master
Mix kit (cat. no. RR820A; Takara Bio, Inc.) and a 7500 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The thermocycling conditions for PCR were as
follows: Initial denaturation step at 95°C for 30 sec; followed by
40 cycles of denaturation at 95°C for 5 sec, annealing at 60°C for
34 sec; and a dissociation stage at 95°C for 15 sec, followed by
60°C for 1 min and 95°C for 15 sec. The primers used for
amplification of the respective genes are listed in Table I. All results were normalized against
GAPDH gene expression by using the 2−ΔΔCq method
(14).
 | Table I.List of primer sequences used in
quantitative polymerase chain reaction. |
Table I.
List of primer sequences used in
quantitative polymerase chain reaction.
|
| Primer sequence
(5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| GAPDH |
GACATGCCGCCTGGAGAAAC |
AGCCCAGGATGCCCTTTAGT |
| TLR4 |
TTATCCAGAGCCGTTGGTGT |
CCCACTCGAGGTAGGTGTTT |
| BNP |
CTCAAAGGACCAAGGCCCTA |
TAAAACAACCTCAGCCCGTC |
| TNF-α |
AGCATGATCCGAGATGTGGA |
ATCTGAGTGTGAGGGTCTGG |
| IL-1β |
CCTGTGTGATGAAAGACGGC |
TATGTCCCGACCATTGCTGT |
| IL-6 |
GTTGCCTTCTTGGGACTGATG |
TACTGGTCTGTTGTGGGTGGT |
Western blotting
In order to determine the activation state of the
TLR4/MAPK/NF-κB signaling pathway, the nuclear and cytoplasmic
proteins were extracted from the cardiac tissues. Cardiac tissues
were harvested and homogenized on ice for 15 min using a
radioimmunoprecipitation assay lysis buffer (cat. no. P0013B;
Beyotime Institute of Biotechnology, Haimen, China), a cOmplete
Protease Inhibitor Cocktail (cat. no. 4693159001; Roche
Diagnostics, Basel, Switzerland) and PhosStop Phosphatase Inhibitor
Cocktail (cat. no. 4906837001; Roche Diagnostics). The homogenates
were subsequently collected and transferred to microcentrifugation
tubes for centrifugation at 12,000 × g for 30 min at 4°C. The
supernatant was collected and the protein concentration of samples
was measured using a BCA kit (cat. no. 23227; Thermo Fisher
Scientific, Inc.). The nuclear proteins were extracted using
Nuclear and Cytoplasmic Extraction Reagents (cat. no. P0027;
Beyotime Institute of Biotechnology), according to the
manufacturer's protocol. A total of 50 µg of protein was loaded per
lane, subjected to 10% SDS-PAGE and subsequently transferred onto
polyvinylidene difluoride membranes (IPFL00010; EMD Millipore,
Billerica, MA, USA). The membranes were subsequently blocked with
5% non-fat milk for 1 h at room temperature and washed with PBS 3
times for 5–10 min each time. The membranes were incubated
overnight at 4°C with the following primary antibodies directed
against TLR4 (dilution, 1:1,000; cat. no. 14358), total
extracellular signal-regulated kinase 1/2 (dilution, 1:1,000;
ERK1/2; cat. no. 4695), phosphorylated (p-)ERK1/2 (dilution,
1:2,000; cat. no. 4370), total p38 (dilution, 1:1,000; cat. no.
8690), p-p38 (dilution, 1:1,000; cat. no. 4511), total c-Jun
N-terminal kinase (dilution, 1:1,000; JNK; cat. no. 9592), p-JNK
(dilution, 1:1,000; cat. no. 4668), p65 (dilution, 1:1,000; cat.
no. 8242), IκBα (dilution, 1:1,000; cat. no. 4814) and GAPDH
(dilution, 1:1,000; cat. no. 97166; all purchased from Cell
Signaling Technology, Inc., Danvers, MA, USA), as well as Lamin B1
(dilution, 1:300; cat. no. BA1228; Wuhan Boster Biological
Technology, Ltd., Wuhan, China). This was followed by incubation
with the following secondary antibodies for 1 h at room temperature
in the dark: IRDye 800CW goat anti-mouse 926-32210 (dilution,
1:10,000; cat. no. C50316-03) and IRDye 800CW goat anti-rabbit
926-32211 (dilution 1:10,000; cat. no. C50602-08) (both LI-COR
Biosciences, Lincoln, NE, USA). The blots were scanned by a
two-color infrared imaging system (Odyssey; LI-COR Biosciences,
Lincoln, NE, USA). Specific protein expression levels were
normalized to the GAPDH protein expression for total tissues
lysates and the membranes were also probed for Lamin B1 as
additional loading controls for nuclear protein expression levels.
Densitometry analysis was performed using Odyssey Software, version
3.0.29 (LI-COR Biosciences).
Statistical analysis
Data are expressed as the means ± standard
deviation. Differences among groups were determined by two-way
analysis of variance followed by a post hoc Tukey's test.
Comparisons between two groups were performed by an unpaired
Student's t-test. Kaplan-Meier curves were used to analyze the
mortality rate of the three groups and the log-rank test was used
to compare the survival distributions of two groups and determine
the efficacy of GLN treatment. All data were analyzed by SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA), and P<0.05 was
considered to be an indicator of a statistically significant
difference.
Results
GLN improves the impaired cardiac
function induced by LPS
To evaluate the protective effect of GLN
pretreatment on the progression of LPS-induced cardiac dysfunction,
the rats were assessed by echocardiography and hemodynamic analysis
at 24 h after LPS administration. The results revealed that LVEDD,
and LVESD were significantly increased in the LPS group compared
with the control group, whereas the dP/dt max, dP/dt min and FS
were significantly decreased (Fig. 1A
and B). These results suggest that LPS aggravated cardiac
dysfunction. Pretreatment of the rats with GLN resulted in a
significant decrease in the level of LVEDD and LVESD compared with
the LPS group, and also caused a significant increase in the dP/dt
max, dP/dt min and FS. These results indicate that treatment with
GLN has a beneficial effect on the impaired cardiac function in
LPS-induced sepsis in rats. As an indicator of cardiac function,
the mRNA level of BNP was also investigated (Fig. 1C). The results demonstrated that the
mRNA level of BNP in the LPS+GLN group was significantly decreased
compared with the LPS only group, but still significantly increased
compared with the control group.
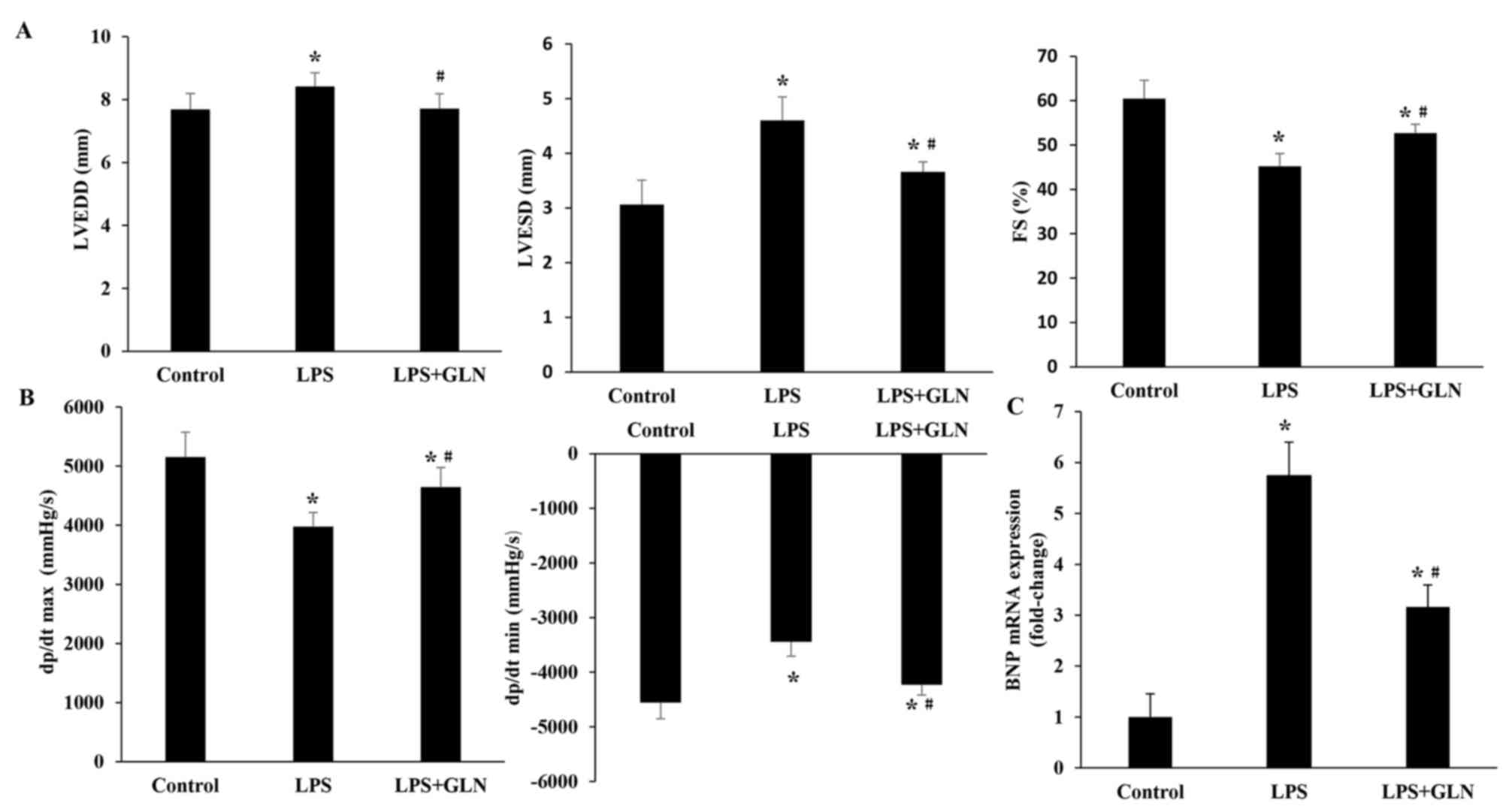 | Figure 1.GLN treatment improved cardiac
function as observed by echocardiography and pressure-volume
analysis. (A) GLN improved the LPS-induced cardiac dysfunction by
normalizing the LVEDD, LVESD and FS. (B) Cardiac function data of
hemodynamic parameters, including dP/dt max and dP/dt min, were
normalized by GLN pretreatment. (C) BNP mRNA expression was
examined using reverse transcription-quantitative polymerase chain
reaction analysis. *P<0.05 vs. the control group;
#P<0.05 vs. the LPS group. GLN, glutamine; LPS,
lipopolysaccharide; LVESD, left ventricular end-systolic diameter;
LVEDD, left ventricular end-diastolic dimension; FS, fractional
shortening; dP/dt max, maximal rate of pressure development; dP/dt
min, minimal rate of pressure decay; BNP, B-type natriuretic
peptide. |
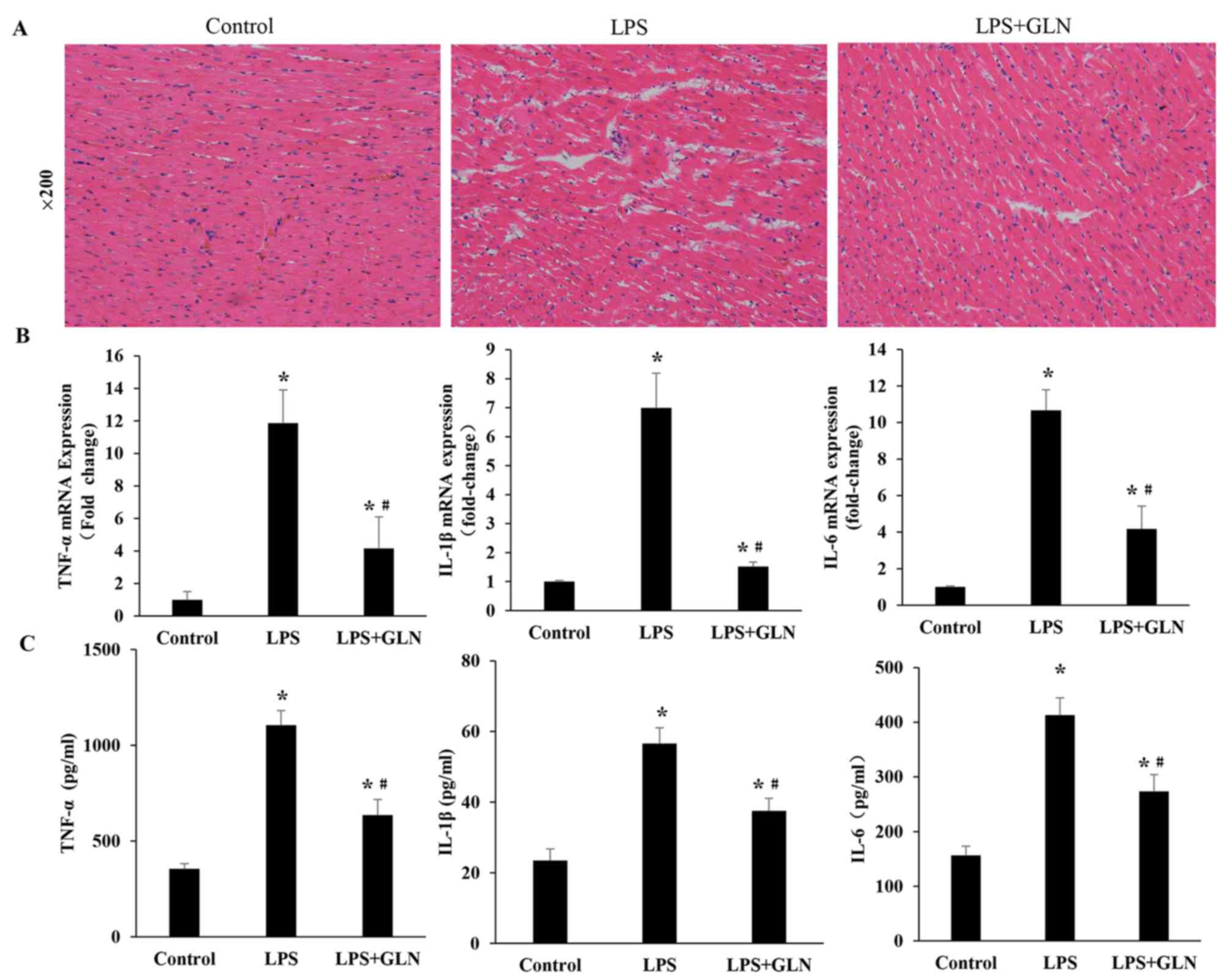
GLN alleviates the inflammation
infiltration and the expression levels of inflammatory
cytokines
Compared with the control group, the LPS rats
demonstrated numerous necrotic alterations in the myofibril and
neutrophil granulocyte infiltration, which were confirmed by HE
staining. The necrotic alterations in the myofibrils and neutrophil
granulocyte infiltration were attenuated in the LPS+GLN group
compared with the LPS only group (Fig.
2A). RT-qPCR revealed that the mRNA expression levels of
inflammatory cytokines, including TNF-α, IL-1β and IL-6, in the
LPS+GLN group were significantly decreased compared with the LPS
only group, however, they were still significantly increased
compared with the control group (Fig.
2B). Similarly, the levels of inflammatory cytokines in the
serum were also markedly reduced in the LPS+GLN group compared with
the LPS only group and significantly higher compared with the
control group (Fig. 2C). These
results suggest that GLN may alleviate inflammation and the
expression levels of inflammatory cytokines induced by LPS.
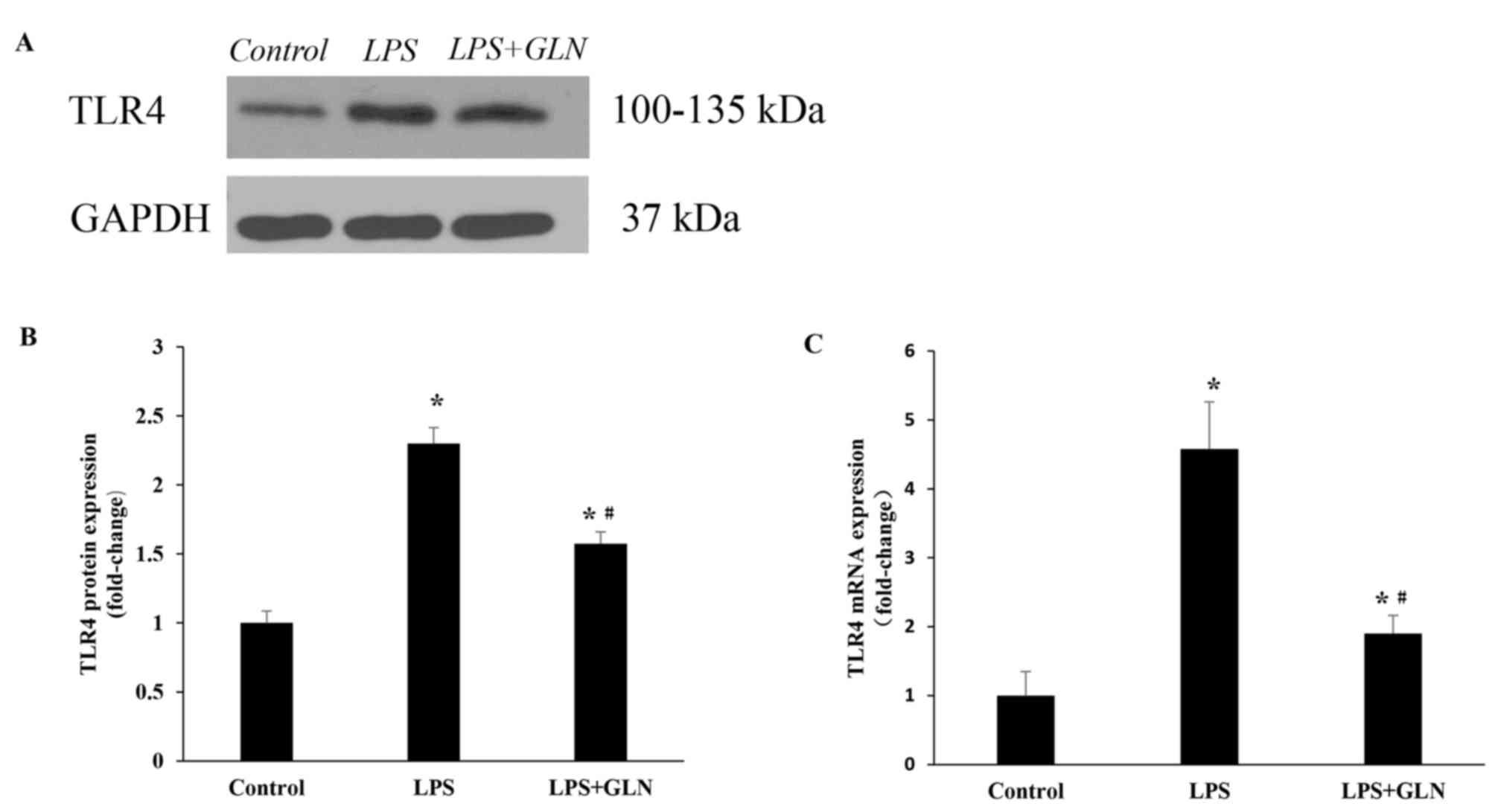
GLN downregulates the expression of
TLR4 protein and mRNA
Subsequent to stimulation for 24 h with LPS, the
level of TLR4 protein expression was evidently higher compared with
that in the control group. By contrast, upon pretreatment with GLN,
TLR4 protein expression was significantly decreased compared with
the LPS only group, however, TLR4 protein expression was still
significantly higher in the LPS+GLN group compared with the control
group (Fig. 3A and B). This trend
was also detected in the level of TLR4 mRNA expression (Fig. 3C).
GLN inhibits the activation of the
MAPK and NF-κB signaling pathways
In order to elucidate the molecular mechanisms
underlying the anti-inflammatory effect of GLN, the activation
state of the TLR4 downstream signaling pathways, including MAPK and
NF-κB pathways, were examined. As revealed in Fig. 4A and B, following the activation of
TLR4 induced by LPS, the protein expression levels of p-ERK, p-P38
and p-JNK were significantly increased in the LPS group compared
with the control group. GLN pretreatment regulated the MAPK
signaling pathway by significantly decreasing the LPS-induced
expression of p-ERK, p-P38 and p-JNK compared with the LPS only
group. The protein expression levels of p-ERK, p-P38 and JNK in the
LPS+GLN group were also significantly increased compared with the
control group. Similarly, the protein expression of nuclear p65
(normalized to Lamin B1 protein expression) was significantly
increased in the LPS group compared with the control group, but
significantly decreased in the LPS+GLN group compared with the LPS
only group (Fig. 4C and D). The
expression of nuclear p65 in the LPS+GLN group was still
significantly increased compared with the control group.
Conversely, the protein expression of IκBα was significantly
decreased in the LPS group compared with the control group, but was
significantly increased in the LPS+GLN group compared with the LPS
group. The expression of IκBα in the LPS+GLN group was still
significantly decreased compared with the control group. These
results suggest that the activation of the MAPK and NF-κB signaling
pathways may be partially inhibited by GLN.
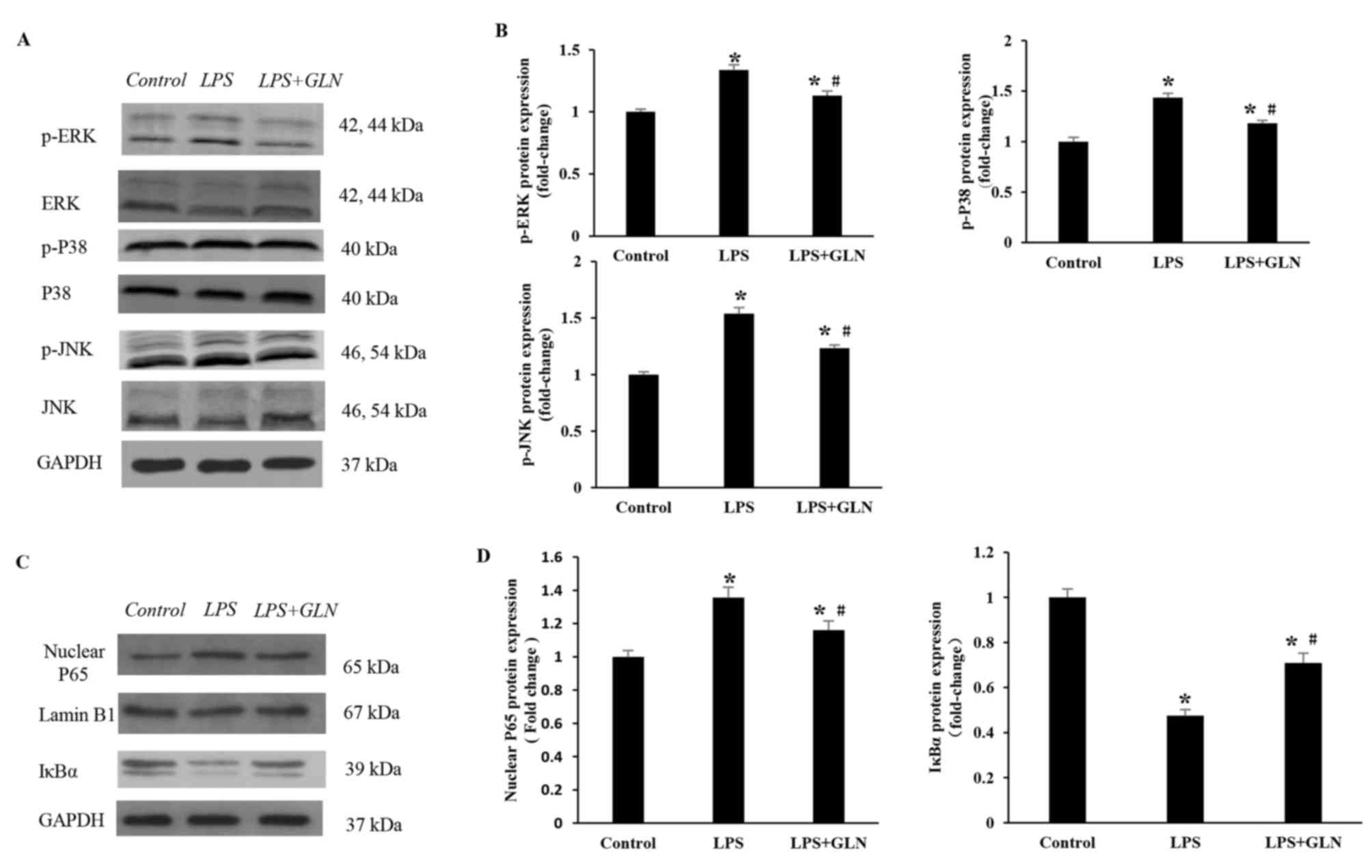 | Figure 4.Effects of GLN on MAPK and NF-κB
signaling pathways in response to LPS stimulation. (A) Western
blots and (B) quantified protein expression levels of
phosphorylated and total ERK, P38 and JNK in heart tissues of rats
in each group. (C) Western blots and (D) quantified protein
expression levels of P65, Lamin B1 and IκBα in the rat tissues.
*P<0.05 vs. the control group; #P<0.05 vs. the LPS
group. GLN, glutamine; LPS, lipopolysaccharide; MAPK,
mitogen-activated protein kinase; NF-κB, nuclear factor-κB; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; p-, phosphorylated; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
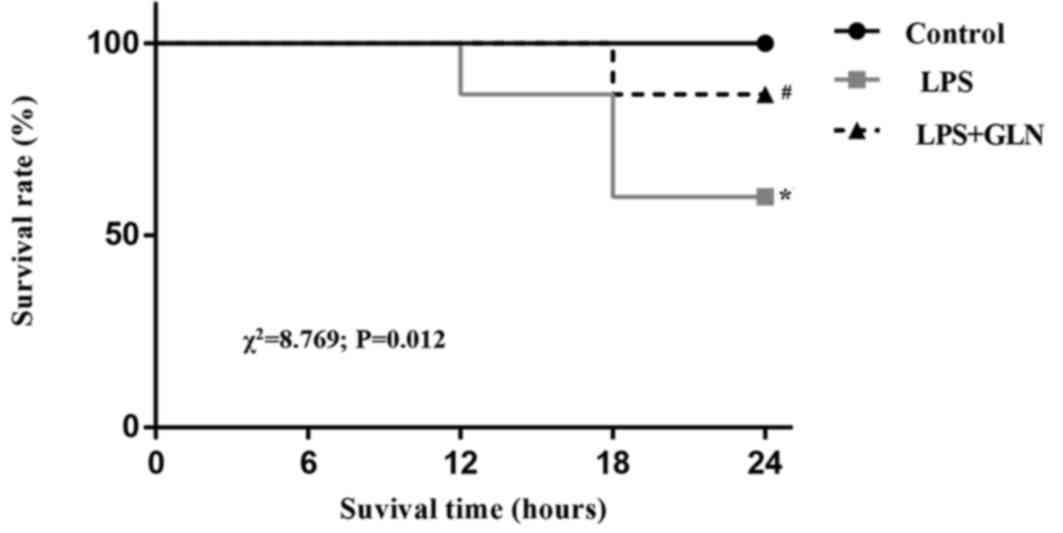
GLN pretreatment decreases the
mortality of rats with sepsis induced by LPS
Kaplan-Meier survival curves (Fig. 5) demonstrated that the rats with
LPS-induced sepsis without GLN pretreatment presented a 24 h
mortality rate of up to 40%. However, GLN pretreatment reduced the
24 h mortality rate to 13.3% of the sepsis group rats. The result
of the log-rank test revealed a statistically significant
difference between the LPS group and the LPS+GLN group
(χ2=8.769; P=0.012). After 24 h, the rats were examined
by echocardiography and hemodynamic analysis; therefore, further
observation of the mortality rate was not possible.
Discussion
Sepsis is a common condition with a high mortality
rate; however, no effective anti-sepsis treatments are currently
available for this condition (15,16). The
cardiovascular system is frequently affected by sepsis and cardiac
dysfunction, as it has been widely observed in patients with severe
sepsis (17). As a primary innate
immune receptor, TLR4 can be stimulated by LPS and participated in
the sepsis-induced acute myocardial dysfunction (18). GLN has been proven to be beneficial
against LPS-induced acute liver, renal, intestinal mucosal and lung
injury. Therefore, the present study investigated whether GLN
exerted a beneficial effect on LPS-induced myocardial dysfunction.
It was observed that, compared with the LPS group, GLN pretreatment
normalized the echocardiography and hemodynamic indices, and had a
positive effect on reducing the mortality rate of rats. These data
suggest that GLN is an effective cardioprotective agent in septic
rats.
Numerous studies have demonstrated that macrophages
served a key role in the progression of LPS-induced sepsis, since
macrophages release various pro-inflammatory cytokines, including
TNF-α, IL-1β and IL-6 (19–21). Among these, TNF-α leads to
inflammation and tissue damage, and the level of TNF-α is
positively correlated with mortality in patients with septic shock
(22). IL-1β is a type of
pro-inflammatory cytokine that enhances the activation of
inflammatory cells and amplifies the systemic inflammatory response
(23). As an important pleiotropic
cytokine in the initial stage of inflammation, IL-6 has the effect
of mediating a variety of inflammatory responses, and can reflect
the degree of inflammation and disease (24,25).
Upon stimulation with LPS, macrophages generate these inflammatory
cytokines, and persistent release of TNF-α, IL-1β and IL-6 can
aggravate sepsis and even lead to multiple organ failure (26). After 24 h of LPS stimulation, the
mRNA expression and serum levels of inflammatory cytokines were
markedly increased in the present study. However, GLN was able to
reduce the expression and release of TNF-α, IL-1β and IL-6, thus
protecting against LPS-induced cardiac dysfunction by inhibiting
the production of inflammatory cytokines.
TLR4 is one of the most studied members of the TLR
family, it is a major receptor and signal transduction molecule for
the identification of LPS, and serves an important role in
mediating the pathophysiological effects of LPS (27). In addition, TLR4 is responsible for
the recognition of LPS and for the initiation of sepsis (28). Subsequent to stimulation by LPS, the
mRNA and protein expression levels of TLR4 were significantly
increased in the current study. The results also revealed that GLN
was able to normalize these expression levels of TLR4, suggesting
that GLN may be a potential target for the treatment of sepsis.
MAPK is induced by the activation of TLR4, and
several studies have reported that the MAPK signaling pathway was
involved in the pathogenesis of septic shock (29–31).
MAPKs constitute one of the major kinase families associated with
immune defense and inflammatory reaction. Mitogen-activated protein
kinase phosphatase-1 is a natural negative regulator of MAPKs, and
subsequent to its knockdown, mice presented an increased level of
pro-inflammatory cytokines, such as TNF-α and IL-6, and exhibited a
marked increase in mortality (32).
Furthermore, ERK1/2, JNK and p38 are three major subfamilies of
MAPK signaling. Several studies of systemic inflammation have
demonstrated that MAPKs are key mediators promoting the production
of inflammatory cytokines during sepsis (33,34). It
has also been observed that the inhibition of the p38
MAPK-dependent mechanism is able to significantly reduce the
mortality associated with sepsis in rats with endotoxic shock
(35). Additionally, the activation
of MAPK signaling increases the synthesis of TNF-α, IL-1β and IL-6.
These pro-inflammatory cytokines, in turn, promote further
activation of MAPK (26).
The synthesis of inflammatory cytokines also mainly
depends on the activation of NF-κB (26). Under normal conditions, NF-κB and
IκBα form a complex, which is present in the cytoplasm in an
inactive form, the degradation of IκBα can be induced by the
activation of MAPK signaling pathway, which then leads to
activation of NF-κB and nuclear translocation (36,37).
However, the activation of TLR4 also leads to the activation of
NF-κB, increasing the expression of inflammatory cytokines TNF-α,
IL-1β and IL-6 in the serum and heart tissue samples (38,39).
Furthermore, the activation of NF-κB led to an increase in
oxidative stress levels and aggravation of cardiac dysfunction in
type II diabetes (40).
Previous studies reported that NF-κB serves a key
role in the development of sepsis and septic shock, while
inhibition of TLR4-mediated signaling reduced the mortality rate of
septic mice (41,42). In LPS-treated rats, the activity of
NF-κB was increased, while suppression of the NF-κB activity had a
beneficial effect on LPS-induced myocardial dysfunction (43). In the present study, it was
identified that the NF-κB and MAPK signaling pathways were involved
in regulating inflammatory processes following stimulation by LPS.
However, treatment with GLN significantly decreased the protein
expression levels of p-ERK, p-P38, p-JNK and nuclear NF-κB p65,
whereas the expression of IκBα was significantly increased.
Therefore, it is suggested that GLN may alleviate cardiac
dysfunction by partially preventing the activation of MAPK/NF-κB
signaling pathway.
In conclusion, GLN pretreatment effectively improved
cardiac dysfunction and reduced the mortality rate induced by LPS
in rats. The underlying mechanism of its action may be associated
with the inhibition of the TLR4/MAPK/NF-κB signaling pathway. GLN
may provide a potentially effective therapy for cardiac dysfunction
in patients with sepsis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81371789).
Glossary
Abbreviations
Abbreviations:
|
GLN
|
glutamine
|
|
LPS
|
lipopolysaccharide
|
|
LVEDD
|
left ventricular end-diastolic
dimension
|
|
LVESD
|
left ventricular end-systolic
diameter
|
|
FS
|
fractional shortening
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
HE
|
hematoxylin and eosin
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
TLR4
|
Toll-like receptor 4
|
|
TNF-α
|
tumor necrosis factor α
|
|
IL-1β
|
interleukin-1β
|
|
BNP
|
B-type natriuretic peptide
|
References
|
1
|
Kimmoun A, Ducrocq N and Levy B:
Mechanisms of vascular hyporesponsiveness in septic shock. Curr
Vasc Pharmacol. 11:139–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao H, Zhang M, Zhou F, Cao W, Bi L, Xie
Y, Yang Q and Wang S: Cinnamaldehyde ameliorates LPS-induced
cardiac dysfunction via TLR4-NOX4 pathway: The regulation of
autophagy and ROS production. J Mol Cell Cardiol. 101:11–24. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Merx MW and Weber C: Sepsis and the heart.
Circulation. 116:793–802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teo JD, Macary PA and Tan KS: Pleiotropic
effects of Blastocystis spp. Subtypes 4 and 7 on ligand-specific
toll-like receptor signaling and NF-κB activation in a human
monocyte cell line. PLoS One. 9:e890362014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li H, Yoon JH, Won HJ, Ji HS, Yuk HJ, Park
KH, Park HY and Jeong TS: Isotrifoliol inhibits pro-inflammatory
mediators by suppression of TLR/NF-κB and TLR/MAPK signaling in
LPS-induced RAW264.7 cells. Int Immunopharmacol. 45:110–119. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, Zheng Q, Hu X, Shen H and Li F:
Betulin attenuates kidney injury in septic rats through inhibiting
TLR4/NF-κB signaling pathway. Life Sci. 144:185–193. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rudiger A and Singer M: Mechanisms of
sepsis-induced cardiac dysfunction. Crit Care Med. 35:1599–1608.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang F, Wang X, Pan L, Wang W, Li N and
Li J: Glutamine attenuates lipopolysaccharide-induced acute lung
injury. Nutrition. 25:692–698. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia CJ, Dai CL, Zhang X, Cui K, Xu F and
Xu YQ: Alanyl-glutamine dipeptide inhibits hepatic
ischemia-reperfusion injury in rats. World J Gastroenterol.
12:1373–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou X, Wu X, Yin Y, Zhang C and He L:
Preventive oral supplementation with glutamine and arginine has
beneficial effects on the intestinal mucosa and inflammatory
cytokines in endotoxemic rats. Amino Acids. 43:813–821. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bayne K: Revised guide for the care and
use of laboratory animals available. American physiological
society. Physiologist. 39(199): 208–211. 1996.
|
|
12
|
Masson GS, Nair AR, Dange RB, Silva-Soares
PP, Michelini LC and Francis J: Toll-like receptor 4 promotes
autonomic dysfunction, inflammation and microglia activation in the
hypothalamic paraventricular nucleus: Role of endoplasmic reticulum
stress. PLoS One. 10:e01228502015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Plante E, Lachance D, Beaudoin J,
Champetier S, Roussel E, Arsenault M and Couet J: Comparative study
of vasodilators in an animal model of chronic volume overload
caused by severe aortic regurgitation. Circ Heart Fail. 2:25–32.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plunkett A and Tong J: Sepsis in children.
BMJ. 350:h30172015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen J, Vincent JL, Adhikari NK, Machado
FR, Angus DC, Calandra T, Jaton K, Giulieri S, Delaloye J, Opal S,
et al: Sepsis: A roadmap for future research. Lancet Infect Dis.
15:581–614. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
De Geer L, Engvall J and Oscarsson A:
Strain echocardiography in septic shock-a comparison with systolic
and diastolic function parameters, cardiac biomarkers and outcome.
Crit Care. 19:1222015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Avlas O, Fallach R, Shainberg A, Porat E
and Hochhauser E: Toll-like receptor 4 stimulation initiates an
inflammatory response that decreases cardiomyocyte contractility.
Antioxid Redox Signal. 15:1895–1909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He G, Zhang X, Chen Y, Chen J, Li L and
Xie Y: Isoalantolactone inhibits LPS-induced inflammation via NF-κB
inactivation in peritoneal macrophages and improves survival in
sepsis. Biomed Pharmacother. 90:598–607. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma F, Liu F, Ding L, You M, Yue H, Zhou Y
and Hou Y: Anti-inflammatory effects of curcumin are associated
with down regulating microRNA-155 in LPS-treated macrophages and
mice. Pharm Biol. 55:1263–1273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen ZB, Tang H, Liang YB, Yang W, Wu JG,
Hu XC, Li ZY, Zeng LJ and Ma ZF: Recombinant Trichinella spiralis
53-kDa protein activates M2 macrophages and attenuates the
LPS-induced damage of endotoxemia. Innate Immun. 22:419–432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian Y, Tao T, Zhu J, Zou Y, Wang J, Li J,
Bo L and Deng X: Soluble tumor necrosis factor related apoptosis
inducing ligand level as a predictor of severity of sepsis and the
risk of mortality in septic patients. PLoS One. 8:e822042013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Copray JC, Mantingh I, Brouwer N, Biber K,
Küst BM, Liem RS, Huitinga I, Tilders FJ, Van Dam AM and Boddeke
HW: Expression of interleukin-1 beta in rat dorsal root ganglia. J
Neuroimmunol. 118:203–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Gonzalo-Calvo D, Neitzert K, Fernández
M, Vega-Naredo I, Caballero B, García-Macía M, Suárez FM,
Rodríguez-Colunga MJ, Solano JJ and Coto-Montes A: Differential
inflammatory responses in aging and disease: TNF-alpha and IL-6 as
possible biomarkers. Free Radic Biol Med. 49:733–737. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waller Persson K, Colditz IG, Lun S and
Ostensson K: Cytokines in mammary lymph and milk during
endotoxin-induced bovine mastitis. Res Vet Sci. 74:31–36. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou XL, Tong Q, Wang WQ, Shi CY, Xiong W,
Chen J, Liu X and Fang JG: Suppression of inflammatory responses by
dihydromyricetin, a flavonoid from ampelopsis grossedentata, via
inhibiting the activation of NF-κB and MAPK signaling pathways. J
Nat Prod. 78:1689–1696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pellegrino P, Falvella FS, Cheli S,
Perrotta C, Clementi E and Radice S: The role of Toll-like receptor
4 polymorphisms in vaccine immune response. Pharmacogenomics J.
16:96–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li HR, Liu J, Zhang SL, Luo T, Wu F, Dong
JH, Guo YJ and Zhao L: Corilagin ameliorates the extreme
inflammatory status in sepsis through TLR4 signaling pathways. BMC
Complement Altern Med. 17:182017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Frazier WJ, Xue J, Luce WA and Liu Y: MAPK
signaling drives inflammation in LPS-stimulated cardiomyocytes: The
route of crosstalk to G-protein-coupled receptors. PLoS One.
7:e500712012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng S, Pan Y, Wang C, Liu Y, Shi M and
Ding G: HMGB1 turns renal tubular epithelial cells into
inflammatory promoters by interacting with TLR4 during sepsis. J
Interferon Cytokine Res. 36:9–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feng H, Guo W, Han J and Li XA: Role of
caveolin-1 and caveolae signaling in endotoxemia and sepsis. Life
Sci. 93:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Nelin LD, Kuhlman JR, Meng X,
Welty SE and Liu Y: The role of MAP kinase phosphatase-1 in the
protective mechanism of dexamethasone against endotoxemia. Life
Sci. 83:671–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim SJ, Baek KS, Park HJ, Jung YH and Lee
SM: Compound 9a, a novel synthetic histone deacetylase inhibitor,
protects against septic injury in mice by suppressing MAPK
signalling. Br J Pharmacol. 173:1045–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang M, Wang X, Bai B, Zhang R, Li Y and
Wang Y: Oxymatrine protects against sepsis-induced myocardial
injury via inhibition of the TNF-α/p38-MAPK/caspase-3 signaling
pathway. Mol Med Rep. 14:551–559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morales MG, Olguín H, Di Capua G, Brandan
E, Simon F and Cabello-Verrugio C: Endotoxin-induced skeletal
muscle wasting is prevented by angiotensin-(1–7) through a p38
MAPK-dependent mechanism. Clin Sci (Lond). 129:461–476. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee S, Choi SY, Choo YY, Kim O, Tran PT,
Dao CT, Min BS and Lee JH: Sappanone A exhibits anti-inflammatory
effects via modulation of Nrf2 and NF-κB. Int Immunopharmacol.
28:328–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lawrence T, Bebien M, Liu GY, Nizet V and
Karin M: IKKalpha limits macrophage NF-kappaB activation and
contributes to the resolution of inflammation. Nature.
434:1138–1143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang W, Hu X, Shen P, Zhang N and Fu Y:
Sodium houttuyfonate inhibits LPS-induced inflammatory response via
suppressing TLR4/NF-kB signaling pathway in bovine mammary
epithelial cells. Microb Pathog. 107:12–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu XL, Zhao CH, Zhang H and Yao XL: iRhom2
is involved in lipopolysaccharide-induced cardiac injury in vivo
and in vitro through regulating inflammation response. Biomed
Pharmacother. 86:645–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mariappan N, Elks CM, Sriramula S,
Guggilam A, Liu Z, Borkhsenious O and Francis J: NF-kappaB-induced
oxidative stress contributes to mitochondrial and cardiac
dysfunction in type II diabetes. Cardiovasc Res. 85:473–483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ha T, Xia Y, Liu X, Lu C, Liu L, Kelley J,
Kalbfleisch J, Kao RL, Williams DL and Li C: Glucan phosphate
attenuates myocardial HMGB1 translocation in severe sepsis through
inhibiting NF-κB activation. Am J Physiol Heart Circ Physiol.
301:H848–H855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Y, Lu Y, Ma L, Cao X, Xiao J, Chen
J, Jiao S, Gao Y, Liu C, Duan Z, et al: Activation of vascular
endothelial growth factor receptor-3 in macrophages restrains
TLR4-NF-κB signaling and protects against endotoxin shock.
Immunity. 40:501–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vaez H, Najafi M, Rameshrad M, Toutounchi
NS, Garjani M, Barar J and Garjani A: AMPK activation by metformin
inhibits local innate immune responses in the isolated rat heart by
suppression of TLR 4-related pathway. Int Immunopharmacol.
40:501–507. 2016. View Article : Google Scholar : PubMed/NCBI
|