Introduction
Normal tongue development has a crucial role in
craniofacial development, particularly for jaw and palate
development (1). Tongue primordium
is comprised of mesenchyme cells derived from cranial neural crest
cells (CNCCs), ectoderm-derived lingual epithelium in the distal
portion and the endoderm-derived proximal portion (2). The myoblasts migrated from the
occipital somites surround the CNCCs during the process of tongue
development (2). Core myogenic
regulators, such as MyoD, myogenic factor (Myf)5 and Myf6 (also
known as MRF4) have important roles in tongue muscle development
(3). However, the tongue muscle also
exhibits unique characteristics that are distinct from those of
other skeletal muscles, and different regulatory mechanisms may be
in place. Previous studies demonstrated that transforming growth
factor β1, Smad4 and fibroblast growth factor 6 signaling pathways
regulate myogenic differentiation and myoblast fusion during tongue
development (2,3). It should be noted that most previous
studies focused on embryonic development of mice tongues, whereas
the postnatal development of the tongue has remained to be fully
explored. However, the onset of numerous tongue diseases, such as
geographic tongue and fissured tongue, have a postnatal onset, and
numerous factors, including trauma and tumors, may lead to
postnatal tongue defects (4). Thus,
intensive research on tongue development may offer useful clues for
better understanding the possible mechanisms of tongue disease, and
provide novel approaches for tongue regeneration. The present study
performed a histological analysis of the postnatal development of
mouse tongues with the aim of identifying possible regulatory
mechanisms underlying postnatal tongue development.
Materials and methods
Animals
C57BL/6J genetic background mice were obtained from
Shanghai SIPPR-Bk Lab Animal Co., Ltd. (Shanghai, China). A total
of 3 maternal mice (12 weeks old; 22–24 g) were used to obtain
newborn mice in the present study. All experimental animal
protocols were reviewed and approved by the Ethics Committee of
Linyi People's Hospital (Linyi, China). Mice lived in a specific
pathogen-free animal room with a temperature of 22°C, and a 12-h
light/dark cycle. The development time of the mouse embryos was
calculated with the day of vaginal plug detection designated as
E0.5. At every time-point, the nodes of fifteen tongues were
harvested from immature mice, and five tongues from P90 mice were
obtained and fixed with 4% paraformaldehyde overnight at 4°C,
followed by washing with PBS. Subsequently, these tissues were
embedded in paraffin and sectioned at a thickness of 5 µm for the
subsequent experiments.
Hematoxylin and eosin (H&E)
staining
Tongue sections of P1, P3, P6, P10, P15 and P90 mice
were deparaffinized at 60°C for 2 h, followed by rehydration in a
graded series of ethanol and washing briefly in distilled water.
Sections were stained using the regular H&E staining method at
room temperature. Briefly, sections were stained with Harris
hematoxylin solution for 8 min and eosin-phloxine solution for 1
min. Slices were cleared in xylene and mounted with resinous
mounting medium to be observed and under a microscope, where images
were captured.
Immunohistochemical staining
Tongue slices of P1, P3, P6, P10, P15 and P90 mice
were dewaxed and immunohistochemical staining was performed
according to the following procedures: First, the sections were
blocked using 3% bovine serum albumin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in PBS for 40 min. Subsequently, the sections
were incubated with the respective primary antibody to Ki67
(ab15580; Abcam, Cambridge, MA, USA; 1:150) or C-kit (ab5506;
Abcam; 1:150) overnight at 4°C. The slices were then washed with
PBS and incubated with horseradish peroxidase-labeled secondary
antibody (KIT-5905; Maixin, Fuzhou, China; 1:250) at room
temperature for 60 min, followed by development using a
diaminobenzidine staining kit (KIT-5905; Maixin, Fuzhou, China) for
3–5 min at room temperature. Finally, the slices were
counterstained with hematoxylin at room temperature for 1 min, and
mounted with resinous mounting medium for observation and capturing
of images.
Immunofluorescence staining
Slices of tongue samples of P1, P3, P6, P10, P15 and
P90 mice were prepared as described above. Then sections were
incubated with primary antibody to msh homeobox 2 (Msx2; ab223692;
Abcam; 1:150 dilution) overnight at 4°C. Subsequently, the slices
were washed with PBS and incubated with secondary antibody (Alexa
Fluor 488-conjugated donkey anti-rabbit; A-21206; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at a dilution of
1:500 for 45 min at 37°C, and were counterstained with DAPI.
Finally, the slices were mounted with glycerinum mounting medium
for observation and image capturing under a fluorescence
microscope.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
For RT-qPCR analysis, the anterior mouse tongues
were dissected and the total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). The total RNA was
converted to complementary DNA according to the instructions of the
Prime Script-RT reagent kit (RR037A; Takara Bio, Inc., Otsu,
Japan). qPCR was performed using the SYBR-Green system (DRR041A;
Takara Bio, Inc.) in a total volume of 20 µl with the MyiQ
Single-Color Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The expression of c-kit relative to
β-actin and calculated using the 2−ΔΔCq method (5) with normalization to the expression
levels at P15. Each qPCR reaction was repeated three times. The
primers for c-kit and β-actin were identical to those used in a
previous study (6).
Statistical analysis
Values are presented as the mean ± standard
deviation. The statistical analysis of the qPCR results was
performed using SPSS v.16 software (SPSS, Inc., Chicago, IL, USA).
Analysis of variance followed by a post-hoc multiple comparisons
test (Duncan's test) was used to compare the results between
different groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Histological structures of postnatal
mouse tongues
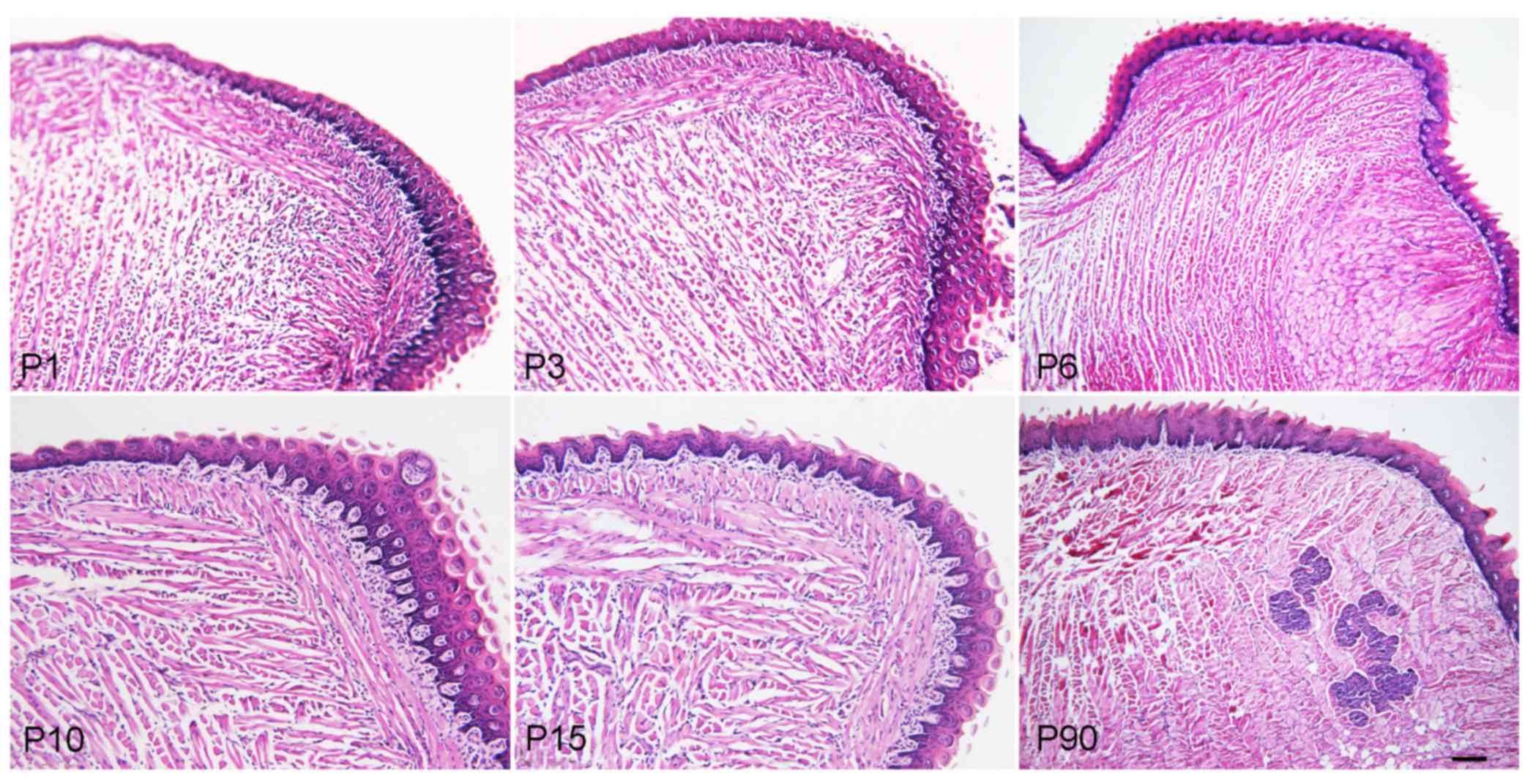
The H&E staining results indicated that the
tongue muscle fibers of the mice became larger, mature and stronger
during the development from newborn to adult, and an increased
amount of adipose tissues surrounded the tongue muscle in adult
mice. The foliate and fungiform papillae also became mature, but
the foliate papillae gradually decreased from newborn to adult mice
(Fig. 1).
Expression of C-kit and Ki-67 in
postnatal mouse tongues
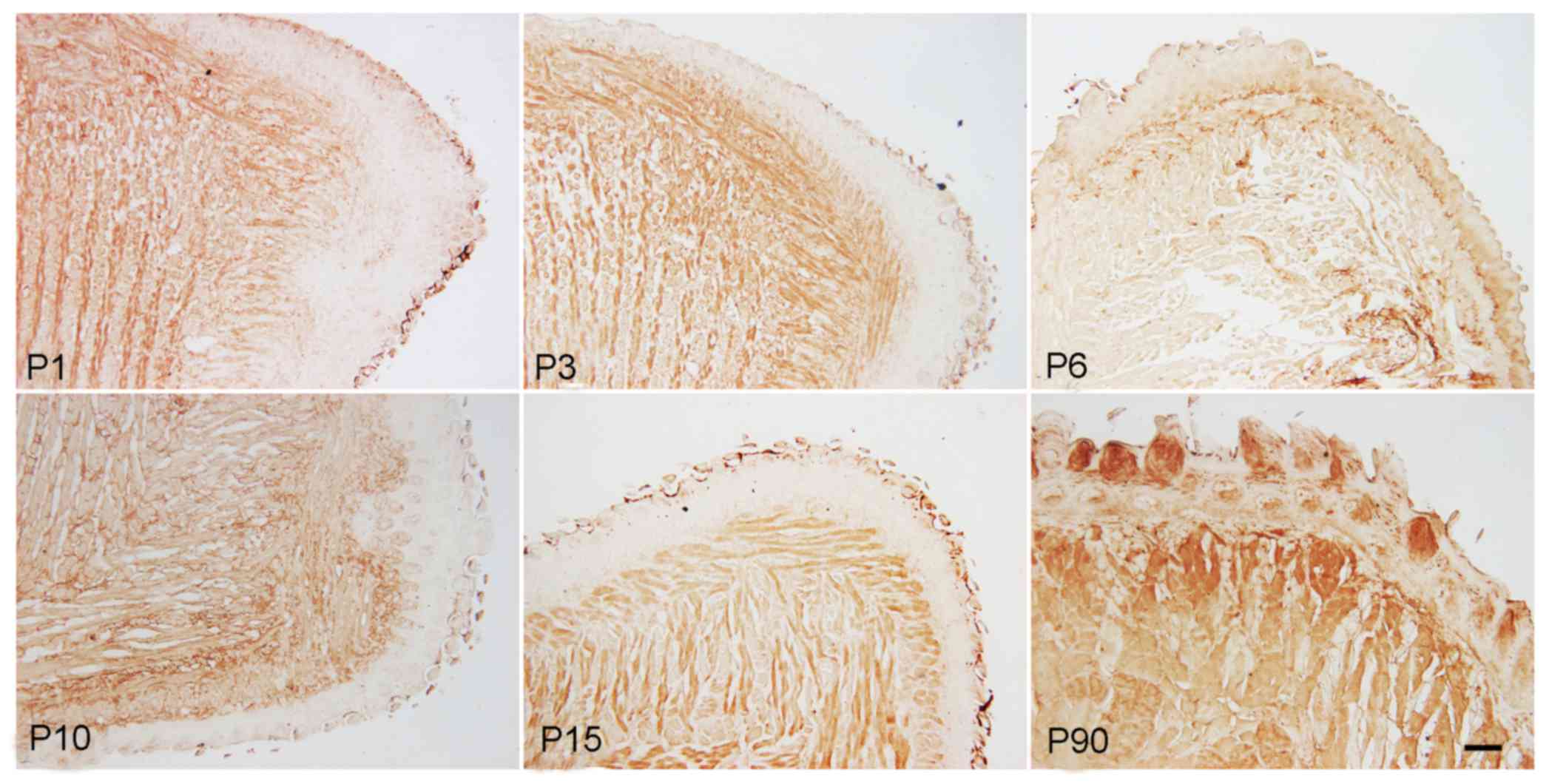
Immunohistochemical staining results demonstrated
that C-kit, a marker of mesenchymal progenitor cells, was expressed
in muscle cells as well as in foliate and fungiform papilla cells.
The expression was weaker in foliate papilla and fungiform papilla
cells when compared with that in muscle cells. The expression of
C-kit in muscle and papilla cells was gradually downregulated from
the newborn to the adult stage, but was markedly increased at P90
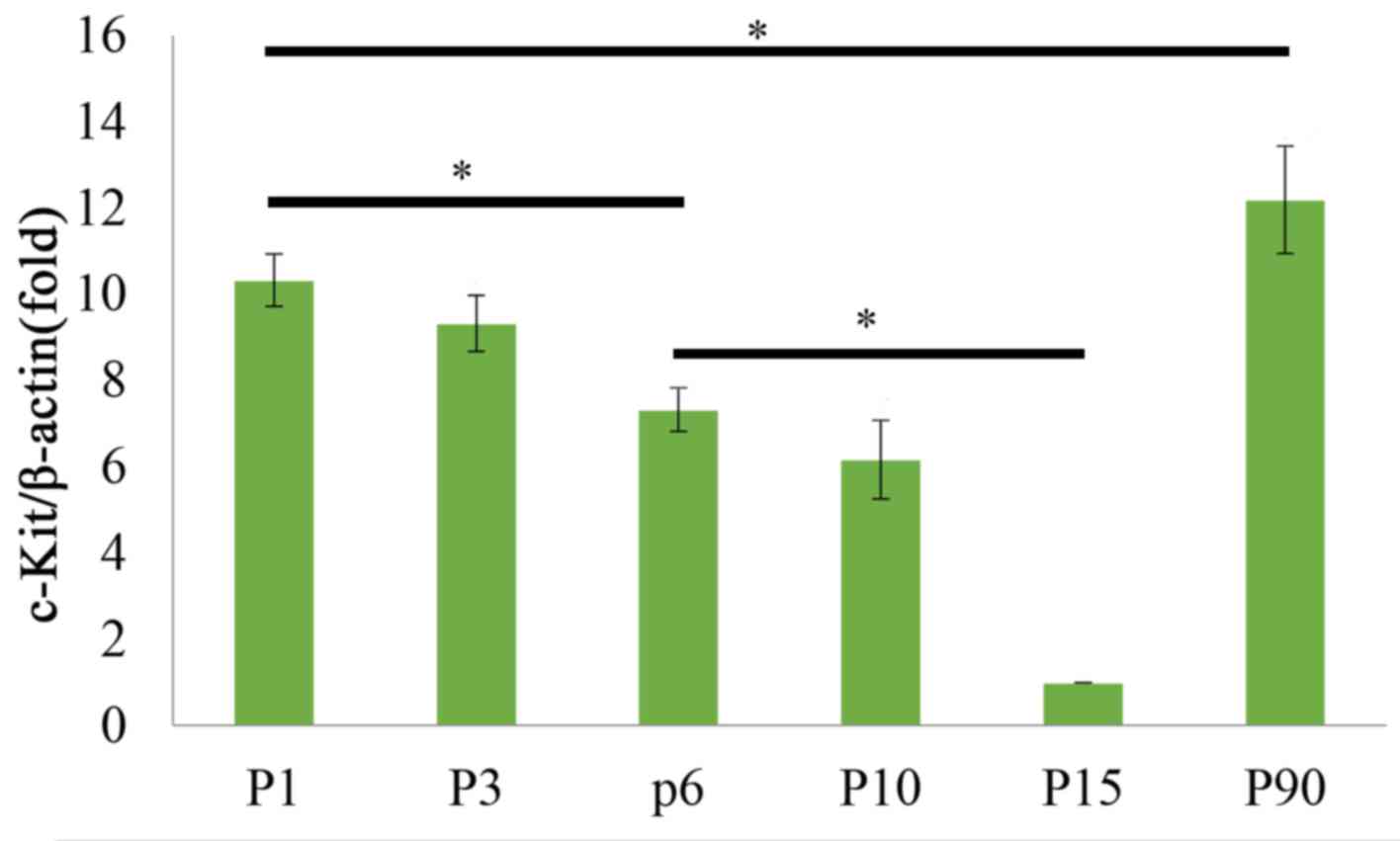
(Fig. 2). The qPCR results also
demonstrated that c-kit mRNA levels displayed dynamic changes
during postnatal development of the tongue, as they were decreased
from P1 to P15, but then increased at P90 (Fig. 3). Duncan's multiple range test
indicated that the expression levels of c-kit exhibited no
significant difference between the P1 and P3 stage, or between the
P6 and P10 stage, whereas they differed significantly between the
any other two stages (P<0.05; Fig.
3). These results implied that at the early postnatal stage,
c-kit expression and the amount of c-kit-expressing cells was
higher to promote tongue development, and in the adult stage c-kit
was expressed to maintain the homeostasis and functional adaptation
of the tongue.
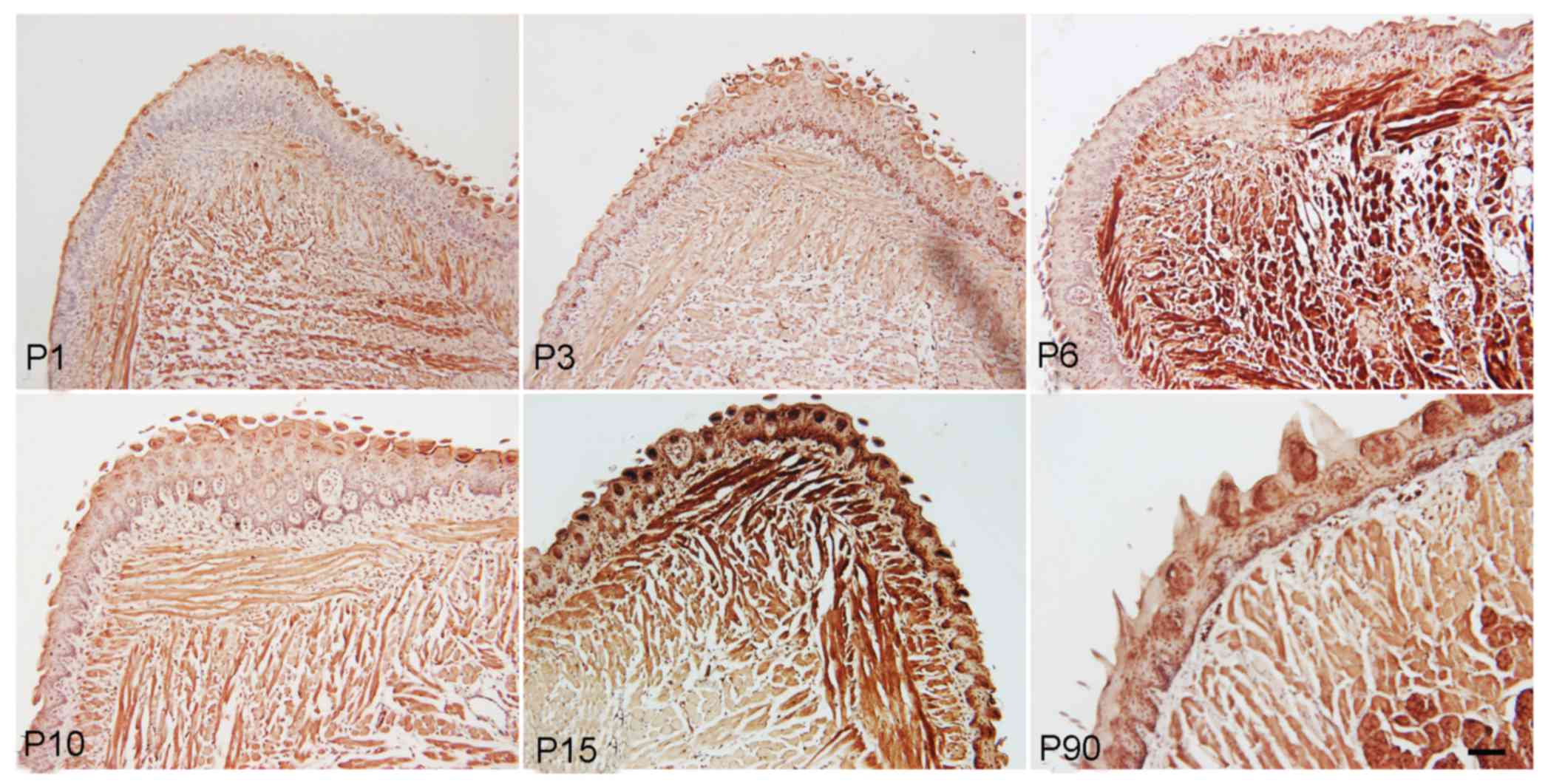
The immunohistochemical staining results also
indicated that Ki-67, a marker of cell proliferation, was expressed
in muscle cells and papilla cells from the newborn to the adult
stage, with relatively higher expression level at P6 and P15,
suggesting increased cell proliferation at this stage (Fig. 4).
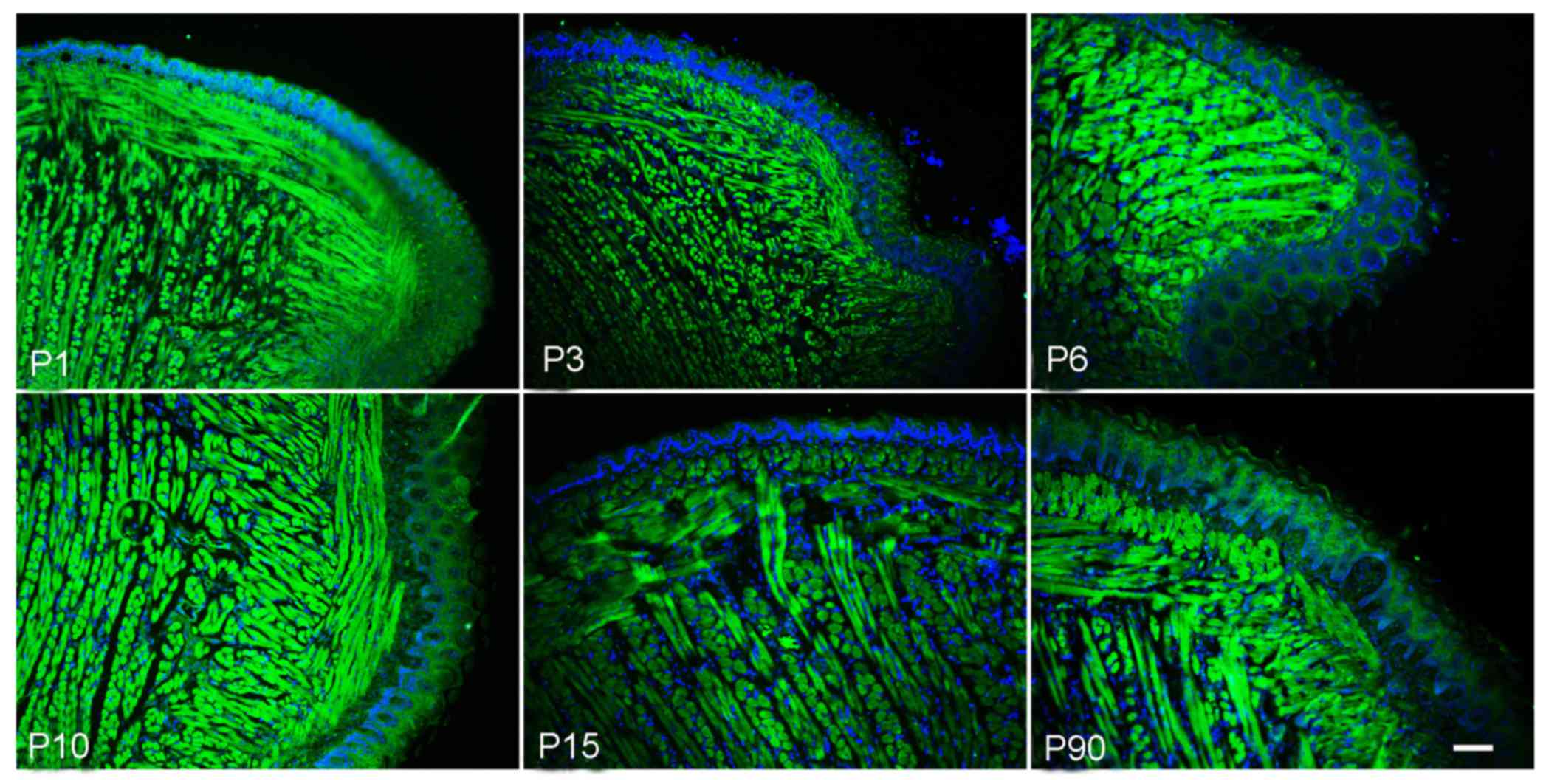
Expression of Msx2 in postnatal mouse
tongues
Msx2, a homeobox transcription factor, has a crucial
role in vascular smooth muscle and skeletal muscle development. The
present study aimed to explore the expression of Msx2 in the tongue
muscle. The immunofluorescence staining results indicated that Msx2
was expressed in postnatal tongue muscle cells, but almost no
expression was present in papilla cells. In addition, there was
relatively high expression level at the P1 to P6 stage, which
gradually decreased from P15 (Fig.
5).
Discussion
During the embryonic development of the mammalian
tongue, early tongue movement is adapted for certain functional
activities, including suckling, swallowing and chewing. During
postnatal stages, the tongue also continually develops, the tongue
size increases and the papilla become mature. However, the number,
position and ratio of different types of papillae gradually changes
to adopt different functions (7,8). The
results of the present study also indicated that tongue muscle
fibers became larger, mature and stronger during development from
newborn to adult mice. The foliate and fungiform papillae also
became mature, but the former gradually decreased from the newborn
to the adult stage, which may have been for the purpose of
acquiring a gustation function as well as adapting to oral
functions such as vocalization and eating.
Numerous factors, including trauma, myophagism and
tumors, may lead to tongue defects. The implementation of various
flaps, such as the forearm flap, are the major repair methods for
tongue defects (9). However, these
methods are not able to repair the tongue muscle and gustation
function. Thus, intensive research into tongue development may
offer useful clues for the development of novel tongue regeneration
strategies based on tissue engineering and stem cell therapy.
Previous studies have indicated that muscle regeneration after
injury exhibits similarities to muscle development, and that
quiescent myogenic cells, also called satellite cells, which are
situated beneath the basal lamina that surrounds each myofiber,
were activated to proliferate, differentiate and fuse to form
multinucleated myofibers (10–12).
Other types of non-muscle stem cells may also participate in this
process. The results of the present study indicated that C-kit, a
marker of mesenchymal progenitor cells (13,14), and
Ki-67, a marker of cell proliferation (15), were expressed in muscle cells,
foliate papilla cells and fungiform papilla cells from mouse
tongues from newborn to adult stages. It was implied that precursor
cells may exist among tongue muscle and papilla cells that express
c-kit to promote tongue muscle and papilla cell development or
maintain the homeostasis and functional adaptation of the tongue.
These findings also offered certain clues regarding c-kit being a
potential target for tongue regeneration, self-repair or treatments
for tongue diseases with a postnatal onset. In addition, the
expression profile of Ki-67 suggests that cell proliferation was
increased at P6 and P15, indicating that taste bud development may
be promoted during this stage.
The mechanisms underlying myogenesis in vertebrate
organisms have been extensively investigated, and numerous factors,
including interleukin (IL)-4, IL-6, MyoD, Myf5, Pax3 and Pax-7,
have important roles in muscle development and tongue muscle
satellite cell activity (16–18).
However, the exact molecular mechanisms of muscle remodeling have
remained elusive. During the development of muscle, the Msx family
of transcription factors also has crucial roles. A previous study
has indicated that Msx1 and histone H1b cooperate to inhibit muscle
differentiation in cell culture and in Xenopus ectodermal explants
(19). Overexpression of LIM
homeobox 2 (Lhx2) completely inhibited the myotube-forming capacity
of C2C12 cells and primary myoblasts, and the muscle
dedifferentiation factors Msx1 and Msx2 were strongly induced by
the Lhx2 overexpression or inhibited by decreased Lhx2 expression
(20). The results of the present
study indicated that Msx2 was highly expressed in tongue muscle
cells during postnatal development, but almost no expression was
detected in papilla cells. These results implied that Msx2 may have
an important role in the postnatal development of tongue muscles,
and may also be a marker for tongue muscle regeneration and
self-repair or for tongue muscle diseases with a postnatal
onset.
In conclusion, the present study indicated that
muscle fibers of mouse tongues became larger, mature and stronger,
and the foliate and fungiform papillae also became mature during
development from newborn to adult. C-kit was dynamically expressed
in muscle cells as well as in foliate and fungiform papilla cells
from newborn to adult stages, suggesting that the number of
c-kit-expressing cells increased to promote tongue development at
the early postnatal stage and to maintain homeostasis and
functional adaptation of the tongue in the adult stage. Ki-67 was
also dynamically expressed in muscle cells, as well as in foliate
and fungiform papilla cells, from newborn to adult stages, with
high expression l at P6 and P15, which suggests increased cell
proliferation at this stage In addition, Msx2 was also dynamically
expressed in postnatal tongue muscle cells, but almost no
expression was detected in papilla cells. These results implied the
presence of precursor cells among tongue muscle and papilla cells,
and Msx2 may have an important role in postnatal tongue muscle
development. The present study also suggests that c-kit and Msx2
may be cell markers for postnatal tongue regeneration and
self-repair, and may provide an approach for developing treatment
methods for tongue diseases with a postnatal onset.
References
|
1
|
Cong W, Liu B, Liu S, Sun M, Liu H, Yang
Y, Wang R and Xiao J: Implications of the Wnt5a/CaMKII pathway in
retinoic acid-induced myogenic tongue abnormalities of developing
mice. Sci Rep. 4:60822014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwata J, Suzuki A, Pelikan RC, Ho TV and
Chai Y: Noncanonical transforming growth factor β (TGFβ) signaling
in cranial neural crest cells causes tongue muscle developmental
defects. J Biol Chem. 288:29760–29770. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shuler CF and Dalrymple KR: Molecular
regulation of tongue and craniofacial muscle differentiation. Crit
Rev Oral Biol Med. 12:3–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maciejewski A, Szymczyk C and Wierzgoń J:
Triple skin island fibula free flap: A good choice for combined
mandible and tongue defect reconstruction. J Reconstr Microsurg.
24:461–468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Qi S, Wang J, Xia D, Qin L, Zheng
Z, Wang L, Zhang C, Jin L, Ding G, et al: Spatial and temporal
expression of c-Kit in the development of the murine submandibular
gland. J Mol Histol. 45:381–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miller IJ Jr and Smith DV: Proliferation
of taste buds in the foliate and vallate papillae of postnatal
hamsters. Growth Dev Aging. 52:123–131. 1988.PubMed/NCBI
|
|
8
|
Liu HX, Ermilov A, Grachtchouk M, Li L,
Gumucio DL, Dlugosz AA and Mistretta CM: Multiple shh signaling
centers participate in fungiform papilla and taste bud formation
and maintenance. Dev Biol. 382:82–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song XM, Ye JH, Yuan Y, Zhang SY, Jiang HB
and Wu YN: Radial forearm free flap for reconstruction of a large
defect after radical ablation of carcinoma of the tongue and floor
of the mouth: Some new modifications. ORL J Otorhinolaryngol Relat
Spec. 72:106–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hindi SM and Kumar A: TRAF6 regulates
satellite stem cell self-renewal and function during regenerative
myogenesis. J Clin Invest. 126:151–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collins CA, Olsen I, Zammit PS, Heslop L,
Petrie A, Partridge TA and Morgan JE: Stem cell function,
self-renewal, and behavioral heterogeneity of cells from the adult
muscle satellite cell niche. Cell. 122:289–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wagers AJ and Conboy IM: Cellular and
molecular signatures of muscle regeneration: Current concepts and
controversies in adult myogenesis. Cell. 122:659–667. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Noda S, Horiguchi K, Ichikawa H and
Miyoshi H: Repopulating activity of ex vivo-expanded murine
hematopoietic stem cells resides in the CD48-c-Kit+Sca-1+lineage
marker- cell population. Stem Cells. 26:646–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandstedt J, Jonsson M, Dellgren G,
Lindahl A, Jeppsson A and Asp J: Human C-kit+CD45- cardiac stem
cells are heterogeneous and display both cardiac and endothelial
commitment by single-cell qPCR analysis. Biochem Biophys Res
Commun. 443:234–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang YK, Han XY and Che ZY: Effects of
buyang huanwu tang combined with bone marrow mesenchymal stem cell
transplantation on the expression of VEGF and Ki-67 in the brain
tissue of the cerebral ischemia-reperfusion model rat. J Tradit
Chin Med. 30:278–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamane A, Mayo M, Shuler C, Crowe D,
Ohnuki Y, Dalrymple K and Saeki Y: Expression of myogenic
regulatory factors during the development of mouse tongue striated
muscle. Arch Oral Biol. 45:71–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawabe Y, Wang YX, McKinnell IW, Bedford
MT and Rudnicki MA: Carm1 regulates Pax7 transcriptional activity
through MLL1/2 recruitment during asymmetric satellite stem cell
divisions. Cell Stem Cell. 11:333–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kirkpatrick LJ, Allouh MZ, Nightingale CN,
Devon HG, Yablonka-Reuveni Z and Rosser BW: Pax7 shows higher
satellite cell frequencies and concentrations within intrafusal
fibers of muscle spindles. J Histochem Cytochem. 56:831–840. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee H, Habas R and Abate-Shen C: MSX1
cooperates with histone H1b for inhibition of transcription and
myogenesis. Science. 304:1675–1678. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kodaka Y, Tanaka K, Kitajima K,
Tanegashima K, Matsuda R and Hara T: LIM homeobox transcription
factor Lhx2 inhibits skeletal muscle differentiation in part via
transcriptional activation of Msx1 and Msx2. Exp Cell Res.
331:309–319. 2015. View Article : Google Scholar : PubMed/NCBI
|