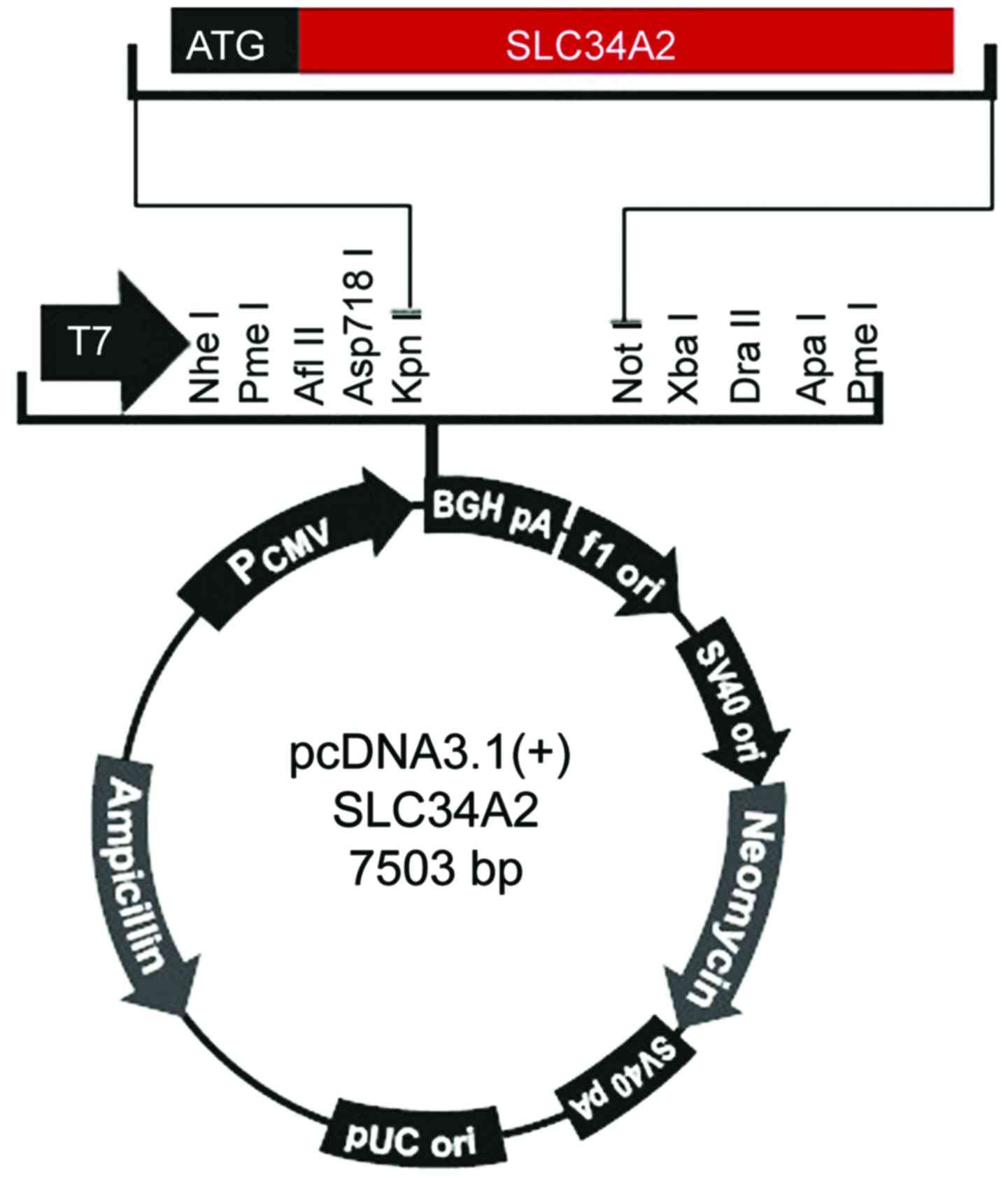
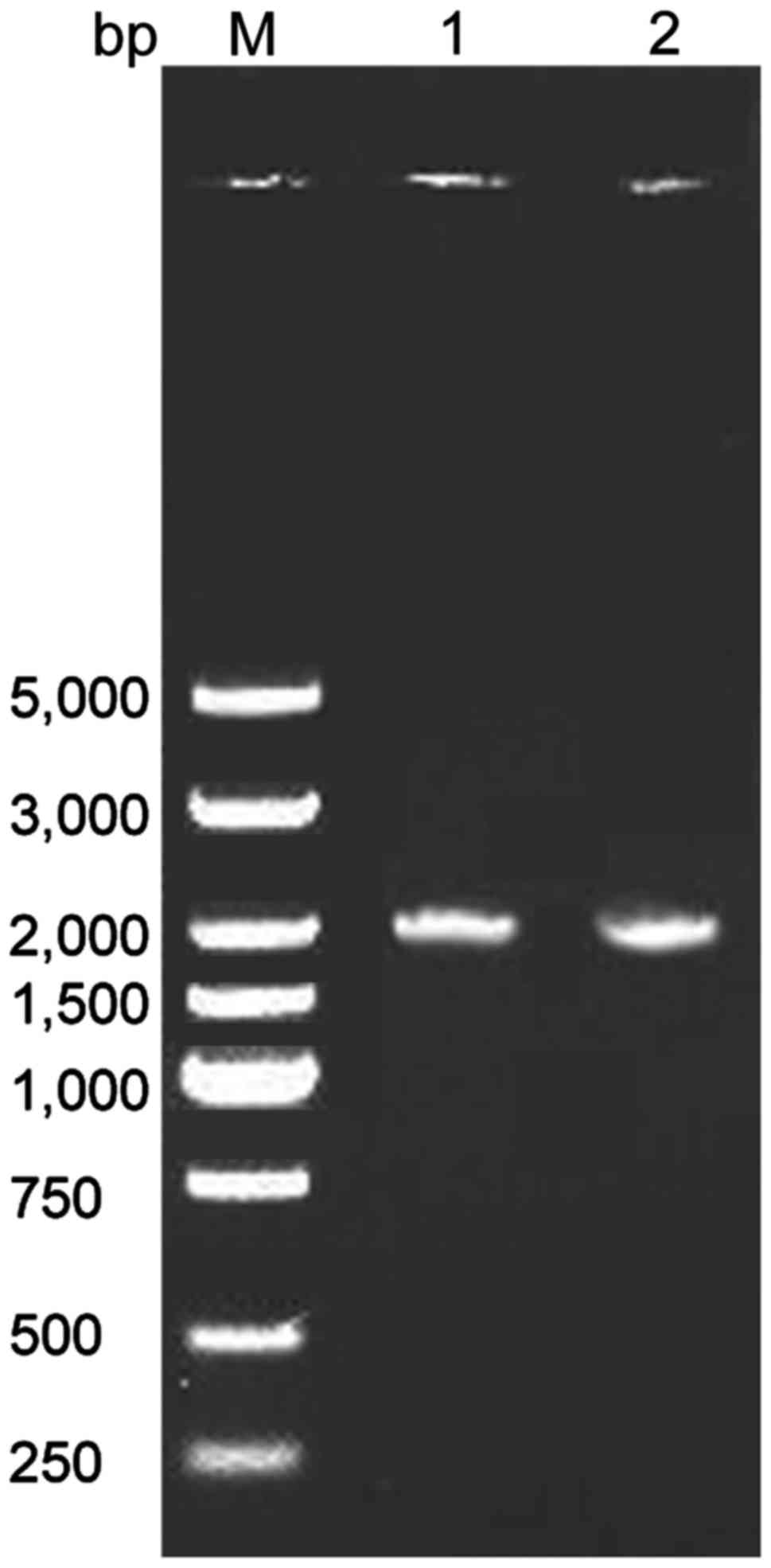
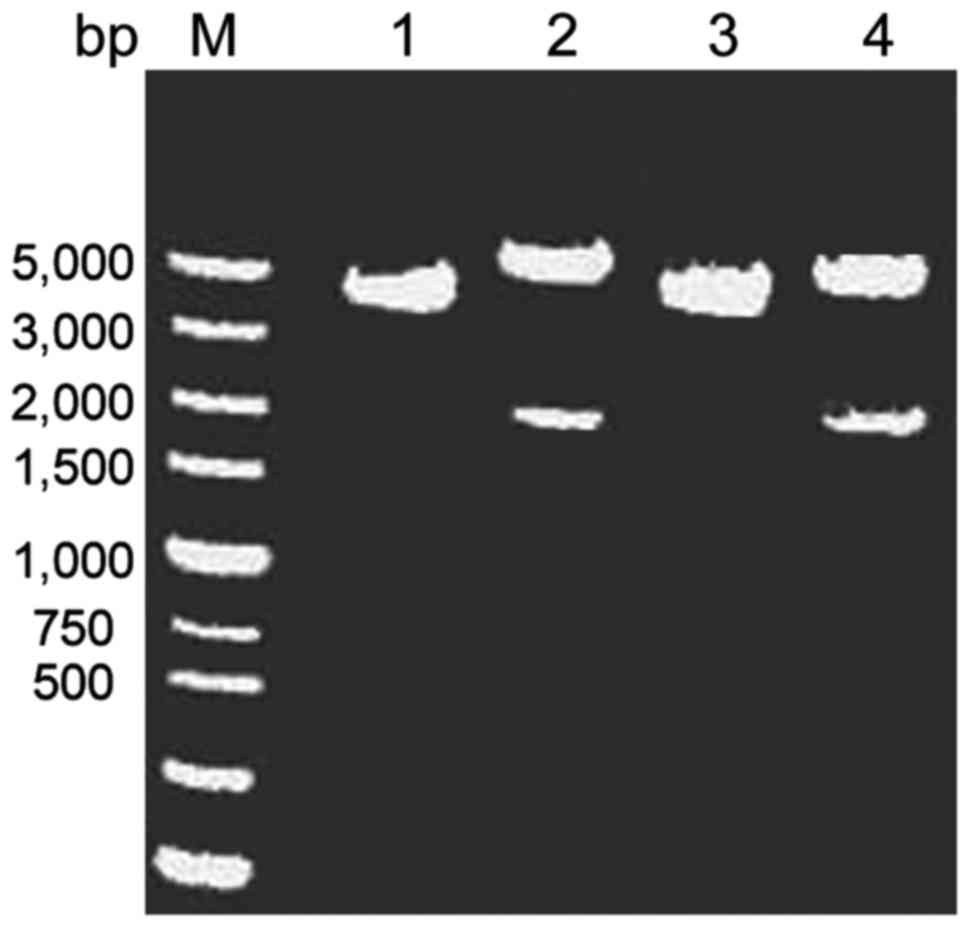
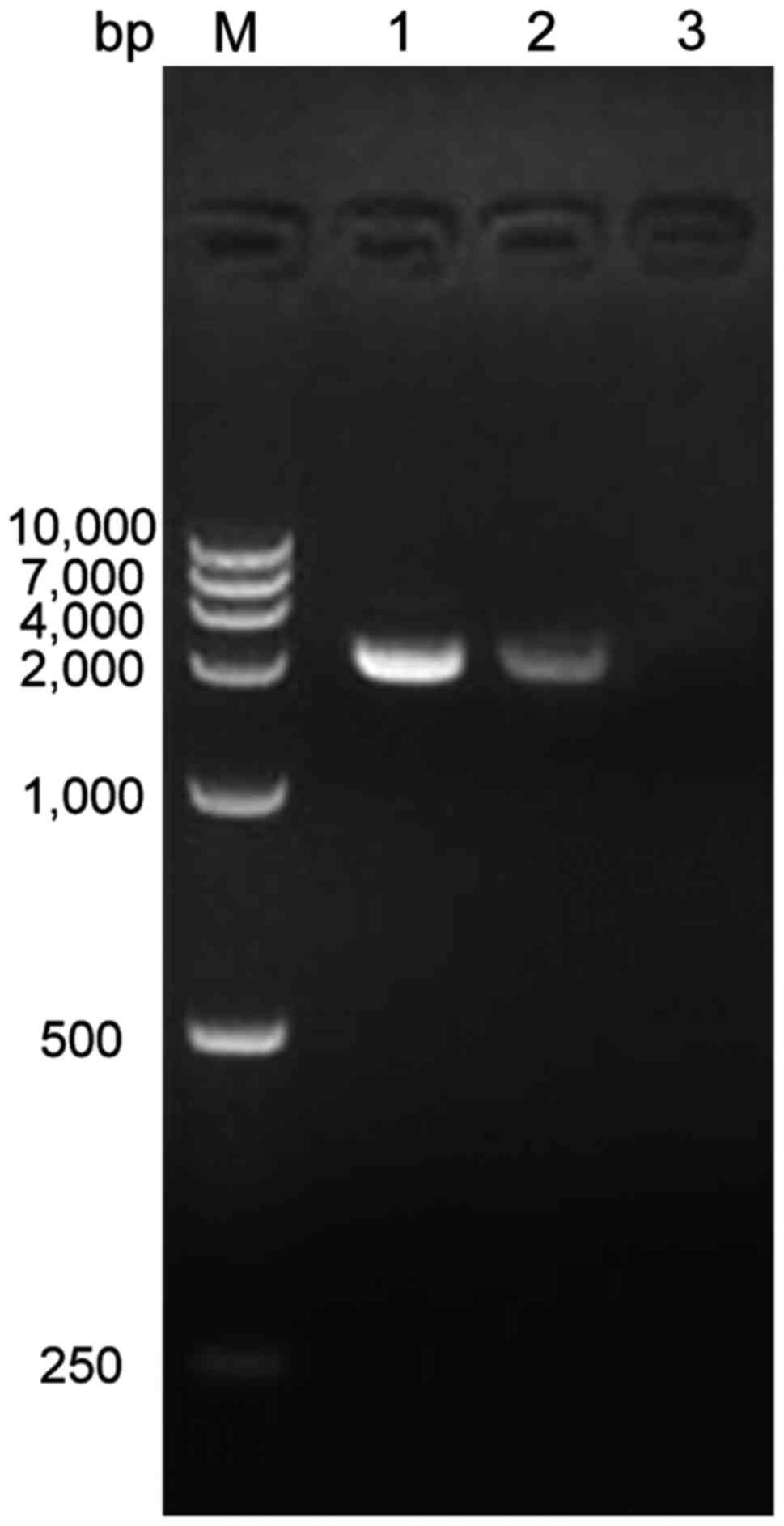
|
1
|
Samano MN, Waisberg DR, Canzian M, Campos
SV, Pêgo-Fernandes PM and Jatene FB: Lung transplantation for
pulmonary alveolar microlithiasis: A case report. Clinics (Sao
Paulo). 65:233–236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jankovic S, Pavlov N, Ivkosic A, Erceg I,
Glavina-Durdov M, Tocilj J, Dragisic-Ivulic S and Primorac D:
Pulmonary alveolar microlithiasis in childhood: Clinical and
radiological follow-up. Pediatr Pulmonol. 34:384–387. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Proesmans M, Boon M, Verbeken E, Ozcelik
U, Kiper N, Van de Casseye W and De Boeck K: Pulmonary alveolar
microlithiasis: A case report and review of the literature. Eur J
Pediatr. 171:1069–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ishihara Y, Hagiwara K, Zen K, Huqun,
Hosokawa Y and Natsuhara A: A case of pulmonary alveolar
microlithiasis with an intragenetic deletion in SLC34A2 detected by
a genome-wide SNP study. Thorax. 64:365–367. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hagiwara K, Johkoh T and Tachibana T:
Molecular basis of lung disease, insight from rare lung
disordersPulmonary Alveolar Microlithiasis. Panos R, Trapnell B and
McCormack F: Humana Press; Totowa, NJ: pp. 154–156. 2009
|
|
6
|
Izumi S Huqun, Miyazawa H, Ishii K,
Uchiyama B and Ishida T: The autozygous segments predicted by a
genome-wide SNP typing revealed mutations in the type IIb sodium
phosphate co-transporter (SLC34A2) causing pulmonary alveolar
microlithiasis. Proc Am Thorac Soc. 3:pp. A1022006;
|
|
7
|
Corut A, Senyigit A, Ugur SA, Altin S,
Ozcelik U, Calisir H, Yildirim Z, Gocmen A and Tolun A: Mutations
in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly
associated with testicular microlithiasis. Am J Hum Genet.
79:650–656. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izumi S Huqun, Miyazawa H, Ishii K,
Uchiyama B, Ishida T, Tanaka S, Tazawa R, Fukuyama S, Tanaka T, et
al: Mutations in the SLC34A2 gene are associated with pulmonary
alveolar microlithiasis. Am J Respir Crit Care Med. 175:263–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Izumi H, Kurai J, Kodani M, Watanabe M,
Yamamoto A, Nanba E, Adachi K, Igishi T and Shimizu E: A novel
SLC34A2 mutation in a patient with pulmonary alveolar
microlithiasis. Hum Genome Var. 4:160472017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poelma DL, Ju MR, Bakker SC, Zimmermann
LJ, Lachmann BF and van Iwaarden JF: A common pathway for the
uptake of surfactant lipids by alveolar cells. Am J Respir Cell Mol
Biol. 30:751–758. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dogan OT, Ozsahin SL, Gul E, Arslan S,
Koksal B, Berk S, Ozdemir O and Akkurt I: A frame-shift mutation in
the SLC34A2 gene in three patients with pulmonary alveolar
microlithiasis in an inbred family. Intern Med. 49:45–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin X, Wang H, Wu D, Zhao G, Shao J and
Dai Y: SLC34A2 Gene mutation of pulmonary alveolar microlithiasis:
Report of four cases and review of literatures. Respir Med.
107:217–222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monabati A, Ghayumi MA and Kumar PV:
Familial pulmonary alveolar microlithiasis diagnosed by
bronchoalveolar lavage. A case report. Acta Cytol. 51:80–82. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhong YQ, Hu CP, Cai XD and Nie HP: A
novel mutation of the SLC34A2 gene in a Chinese pedigree with
pulmonary alveolar microlithiasis. Zhonghua Yi Xue Yi Chuan Xue Za
Zhi. 26:365–368. 2009.(In Chinese). PubMed/NCBI
|
|
15
|
Tachibana T, Hagiwara K and Johkoh T:
Pulmonary alveolar microlithiasis: Review and management. Curr Opin
Pulm Med. 15:486–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozcelik U, Yalcin E, Ariyurek M, Ersoz DD,
Cinel G, Gulhan B and Kiper N: Long-term results of disodium
etidronate treatment in pulmonary alveolar microlithiasis. Pediatr
Pulmonol. 45:514–517. 2010.PubMed/NCBI
|