Introduction
Oxidative stress results from a disruption of the
balance between the pro-oxidation and anti-oxidation systems. The
physiological level of reactive oxygen species (ROS) serves an
important role in female reproduction, including in ovarian
steroidogenesis, oocyte maturation, folliculogenesis, ovulation and
luteolysis (1,2). Production of physiological levels of
oxygen radicals at ovulation in response to luteinizing hormone
(LH) may signal differentiation of the oocyte. However,
overproduction may damage the oocytes and mitochondrial membrane
potential, reduce the mitochondrial DNA copy number and disrupt the
mitochondrial metabolism; all these changes are observed in
atresia. Subsequently, the lack of adenosine triphosphate causes
severe damage to the ultrastructure of mitochondrial cristae and
spindle formation in oocytes (3,4).
Endometriosis (EM) is an estrogen-dependent disease
characterized by the presence and growth of endometrial tissue
outside the uterine cavity (5). EM
is strongly associated with infertility, and has severe effects on
ovarian and tubal function, while it may also affect endometrial
receptivity (6–11). The development of in vitro
fertilization (IVF) is promising for infertile patients with EM;
however, the success rate of this procedure remains at a low level
(12). One of the main causes of
failure may be the poor quality of oocytes (13). Thus, research has been focusing on
determining the causes of poor oocyte quality in infertile women
with EM and improving their quality.
In recent years, the correlation between oxidative
stress and EM has received increasing attention. EM is considered
to be associated with oxidative stress, and several studies have
demonstrated that the follicular fluid (FF) of patients with EM
presented increased levels of ROS and a reduction in the total
antioxidant capacity (14–16). In addition, the FF of infertile women
with mild EM was demonstrated to greatly impair the meiotic spindle
of bovine oocytes matured in vitro (17). It has also been observed that
decreased percentage of mature oocytes, implantation rate and
clinical pregnancy rate were associated with the severity of EM
(18). Furthermore, the level of
myeloperoxidase as a potential oxidative stress target was revealed
to be an indicator of EM-associated infertility (18). The findings of these previous studies
suggest that the poor oocytes quality for EM may be associated with
oxidative stress.
Advanced oxidation protein products (AOPPs) are a
marker of oxidation-mediated protein damage that are usually
carried by plasma proteins (19).
AOPPs induce an oxidation stress reaction in vivo by
activating the neutrophil and monocyte oxidative metabolism
(20), and circulate for prolonged
periods in the patients' blood, since their degradation by cells
requires several hours or days since their degradation by cells
requires several hours or days (21). Due to the sensitivity, stability,
convenience and cost of detection, the role of AOPPs in predicting
the severity of oxidative stress and disease prognosis has become
increasingly important. AOPPs may accelerate renal fibrosis and
atherosclerosis, and may be detrimental to the progression of
chronic kidney disease (22–24). In postmenopausal women, AOPPs are
negatively associated with a reduced bone mineral density, which
increases an individual's risk of developing osteoporosis (25). As a key product in oxidative
reactions, AOPPs and their effects on the female reproductive
system have received increasing attention. It has been demonstrated
that the levels of serum AOPPs are significantly increased in women
with polycystic ovarian syndrome (26). In patients with uterine leiomyoma,
the serum level of AOPPs increases and the antioxidant capacity
decreases (27). In the peritoneal
fluid, the level of AOPPs is significantly higher in patients with
EM when compared with that in patients without EM (21). However, to the best of our knowledge,
there are no previous studies on AOPP levels in the FF of infertile
women with EM undergoing IVF.
The FF arises from the secretion of theca and
granulosa cells, and forms a direct microenvironment for the
development of oocytes, having crucial effects on the oocyte
quality (28). Estradiol
(E2) and progesterone (P4) in the FF are
closely associated with the quality of oocytes. In our previous
study, a high level of AOPPs in the FF resulted in adverse effects
on oocytes and early embryonic development, while it was negatively
associated with the outcome of IVF (29).
In the present study, the aim was to examine the
role of AOPPs in infertile women with EM undergoing IVF and whether
they are involved in altering the follicular environment of the
developing oocyte, subsequently affecting the outcome of IVF. The
levels of AOPPs, E2 and P4 in the FF were
detected, and the correlations between gonadal hormones levels,
prognosis of IVF and AOPP concentration were analyzed.
Materials and methods
Patients and sample collection
A total of 89 infertile women (age, 20–40 years)
were recruited between 01 April 2015 and 31 December 2016. All
patients were undergoing IVF at the Center for Reproductive
Medicine, Department of Obstetrics and Gynecology, Nanfang
Hospital, Southern Medical University (Guangzhou, China). Among the
89 women, 44 were diagnosed with EM (stages II, III and IV) that
was confirmed by laparoscopy and biopsy, while 45 non-EM women
because of male factors were enrolled into the control group. For
the patients with EM, the date of surgical diagnosis was within
~1–4 years, and the minimum period between the last surgery and IVF
was at least 6 months. Women in the control group were considered
to have no significant infertility factors according to the World
Health Organization guidelines (30). All women included in the present
study did not receive any medicinal treatment other than the
necessary treatment stipulated in the IVF protocol during the past
3 months. IVF stimulation protocols were compliant to the standard
treatment dictated by the patients' physicians.
Subsequent to obtaining informed consent from the
participants, FF samples were collected on the retrieval day (day
of transvaginal oocyte retrieval) from patients undergoing IVF.
Biochemical measurement of AOPP concentration was performed in all
samples. The outcome of the research did not affect the treatment
of participants. In addition, all the information collected in the
present study does not identify individual participants. The
approval of the study was obtained from the Institutional Research
Ethics Board of Nanfang Hospital of Southern Medical University
(Guangdong, China).
Oocyte evaluation
Oocyte maturity was examined by embryologists
subsequent to transvaginal oocyte retrieval or at the time of
intracytoplasmic sperm injection. The evaluation for oocytes was
performed according in the following parameters: Expanded cumulus,
appropriate cytoplasmic maturation, extruded first polar body and
arrest in metaphase II. The embryo quality was scored at day 3
following fertilization and prior to placement in the uterus, as
follows: Grade I, very good quality; grade II, good quality; grade
III, medium quality; and grade IV, poor embryo quality (13). A good embryo (grades I and II) was
defined as having seven or eight blastomeres, which were equally
sized, with <20% fragmentation and no multinucleation.
IVF-controlled ovarian
hyperstimulation protocol
Stimulation protocols were personalized, and
included a leuprolide acetate long protocol and a
gonadotropin-releasing hormone antagonist protocol (17). Controlled ovarian stimulation was
performed by administering recombinant follicle-stimulating hormone
(Gonal-F; EMD Serono, Inc., Rockland, MA, USA; or Puregon; MSD,
Kenilworth, NJ, USA). Ovulation was induced with 250 µg recombinant
human chorionic gonadotropin (Ovidrel, EMD Serono, Inc.)
subcutaneously or 6,000–10,000 U human chorionic gonadotropin (hCG;
Chorionic Gonadotropin for injection; Livzon Pharmaceutical Group
Inc., Zhuhai, China) intramuscularly at the appropriate time in
follicular development. At 34–36 h after hCG administration,
transvaginal oocyte retrieval was performed in patients under
intravenous sedation.
Collection of the FF
The FF was collected from 89 infertile women, 44
with EM and 45 non-EM with male infertility. At the time of oocyte
retrieval, the FF was carefully aspirated from the follicle and
stored in sterile containers preheated to 37°C. The FF sample was
collected only from a follicle that was >14 mm in diameter and
first punctured. The preparation of FF was conducted as described
by Giorgi et al (31).
Subsequently, the oocytes were microscopically removed from the
aspirated FF. Only FF samples with no blood contamination upon
visual inspection and presenting a mature oocyte were used. FF
samples containing >1 oocyte or from follicles with a diameter
of <14 mm were excluded. All FF samples included in the present
study were centrifuged at 1,500 × g for 10 min at 4°C to remove the
cellular components, and the clear supernate was stored at −80°C
for later analysis.
Measurements of the levels of AOPPs,
E2 and P4
The AOPP concentration in the FF samples was
determined according to the spectrophotometric method described by
Witko-Sarsat et al (32),
which was expressed in equivalents of chloramine-T. Furthermore,
the levels of E2 and P4 in the FF were
determined using a commercial Iodine (125I)
Radioimmunoassay kit (Beijing North Institute of Biological
Technology, Beijing, China), according to the manufacturer's
protocol. The assays were assessed using competitive
radioimmunoassay. The standard curve and assay sensitivity for
E2 was 5–4,000 and 2 pg/ml, respectively, while these
values for P4 were 0.2–100 and 0.2 ng/ml, respectively.
For both E2 and P4, the intravariabilities
were <10% and the interassay variabilities were <15%.
Statistical analysis
The fertilization rate was defined as follows:
Number of embryos/number of oocytes. The good embryo rate was
defined as follows: Number of grade I or grade II embryos/total
number of embryos. The blastocyst rate as defined as follows:
Number of embryos which were applied to culture blastocysts/number
of mature blastocysts. All values are presented as the mean ±
standard deviation. Considering the non-Gaussian distribution of
the parameters, statistical analysis between groups was performed
with the Mann-Whitney U test. Spearman's rank correlation test was
applied to analyze the correlations between the FF AOPP
concentration and the levels of hormones, correlations between the
FF AOPP concentration and the outcome parameters of IVF. A
statistically significant difference was defined as P<0.05. Bar
graphs were used to present differences between groups. Data were
analyzed by SPSS version 20.0 software (IBM Corp., Armonk, NY,
USA).
Results
Clinical characteristics, biochemical
and IVF outcome parameters
The clinical characteristics, biochemical and IVF
outcome parameters of patients are presented in Table I. There were no significant
differences between the two groups in the mean age, BMI, duration
of infertility, E2 concentration in the FF, as well as
the serum levels of the LH, E2 and P4 at the
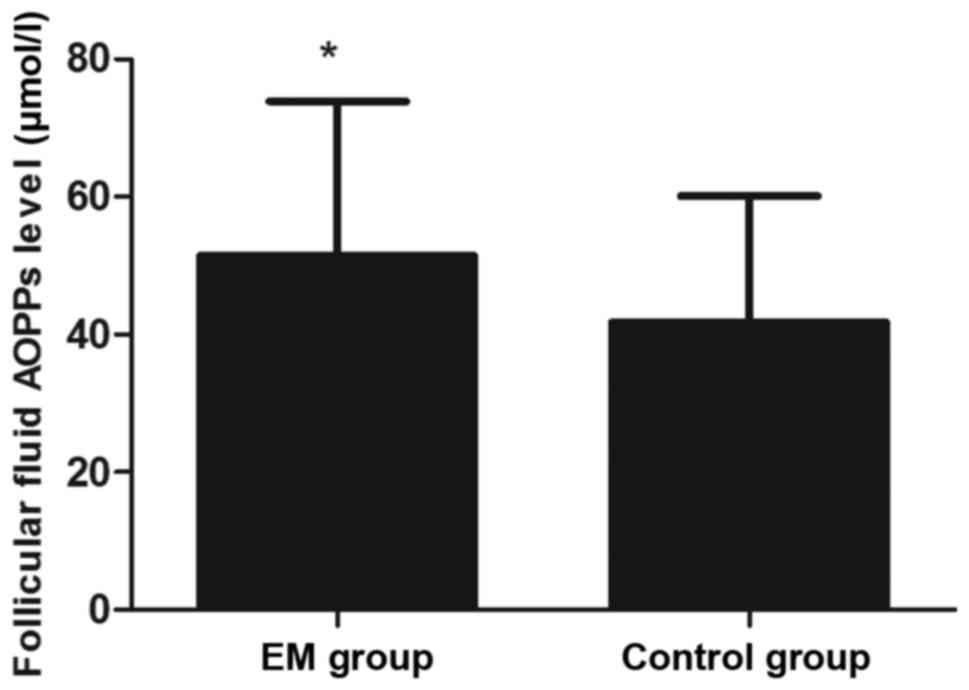
time of hCG administration (P>0.05; Table I). However, the AOPP concentration in
the FF was significantly higher in the EM group when compared with
that in the control group (P<0.05; Fig. 1). In addition, the levels of
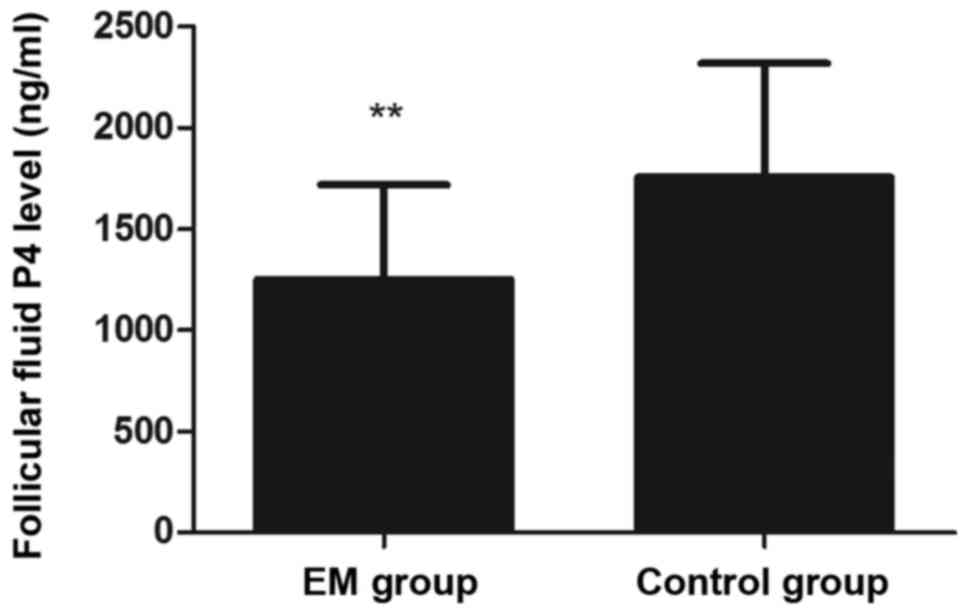
P4 in the FF were significantly lower in the EM group as
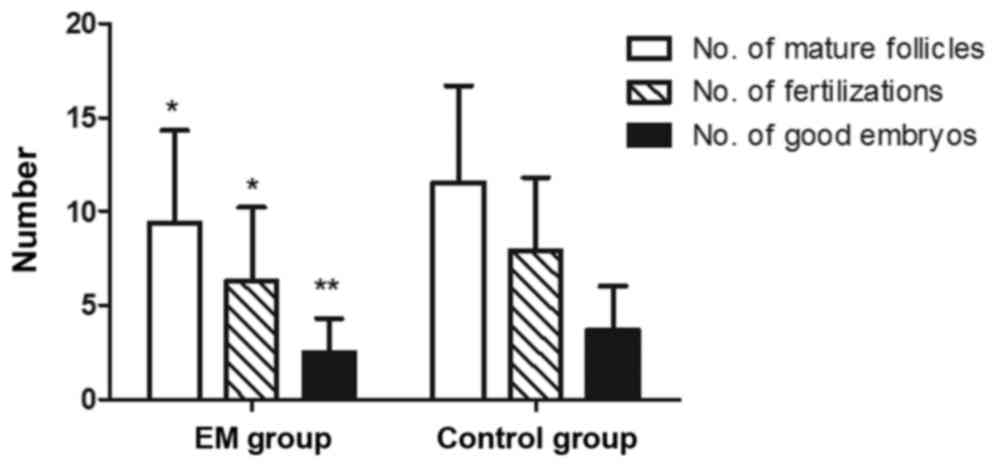
compared with the control group (P<0.01; Fig. 2). Regarding the outcome of IVF, the
EM group presented reduced numbers of mature follicles (diameter of
>14 mm), fertilizations and good embryos as compared with those
in the control group (P<0.05; Fig.
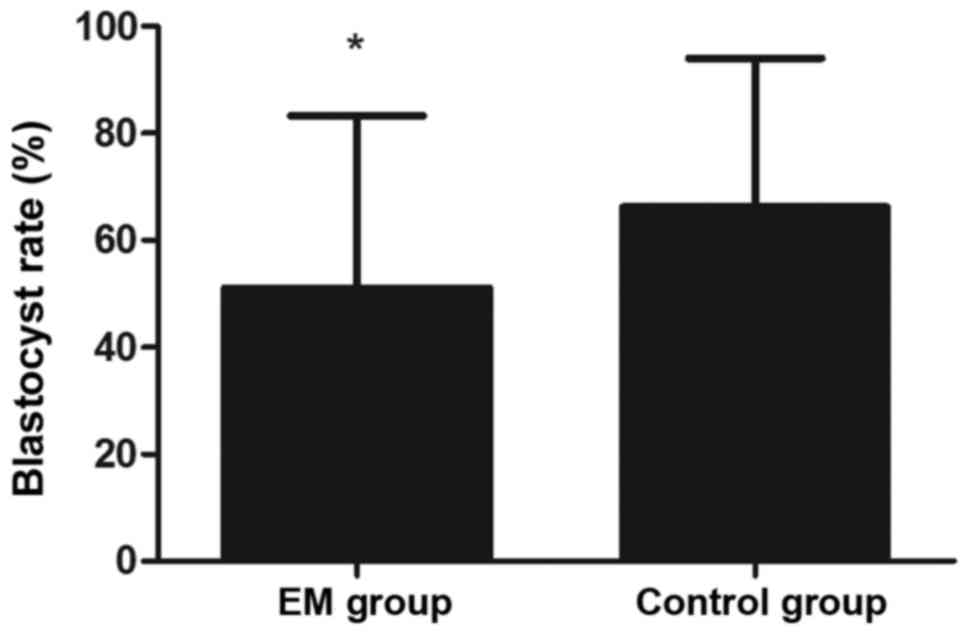
3). Furthermore, the blastocyst rate was markedly lower in the
EM group in comparison with that in the control group (P<0.05;
Fig. 4).
 | Table I.Clinical characteristics and in
vitro fertilization outcome parameters in each group. |
Table I.
Clinical characteristics and in
vitro fertilization outcome parameters in each group.
| Parameters | EM group
(n=44) | Control group
(n=45) | P-value |
|---|
| Age (years) |
31.3±4.0 |
30.6±3.3 | 0.542 |
| BMI
(kg/m2) |
21.9±2.9 |
21.2±2.2 | 0.275 |
| Duration of
infertility (years) |
3.7±2.3 |
3.6±2.1 | 0.980 |
| FF content |
|
|
|
| AOPP
level (µmol/l) |
51.5±22.4 |
41.8±18.3 | 0.044a |
| E2
level (ng/ml) |
14.3±5.9 |
15.4±4.8 | 0.112 |
| P4
level (ng/ml)b |
1,249.6±465.4 |
1,752.7±565.4 |
<0.001b |
| Prior to hCG
administration |
|
|
|
| LH
level (mU/ml) |
2.2±2.0 |
2.1±1.5 | 0.098 |
| E2
level (pg/ml) |
2,471.9±1333.8 |
2,903.8±1255.5 | 0.064 |
| P4
level (ng/ml) |
0.90±0.37 |
0.89±0.32 | 0.605 |
| No. of follicles
(diameter, >14 mm) |
9.4±4.9 |
11.5±5.2 | 0.032a |
| No. of
fertilizations |
6.3±3.9 |
7.9±3.9 | 0.024a |
| No. of good
embryos |
2.5±1.8 |
3.7±2.3 | 0.006b |
| Good embryo rate
(%) |
39.0±19.9 |
47.3±19.0 | 0.061 |
| Blastocyst rate
(%) |
51.1±32.2 |
66.2±27.8 | 0.022a |
Correlation between FF AOPP
concentration and hormone levels
The correlation between the AOPP concentration in
the FF and the levels of gonadal hormones is demonstrated in
Table II. The FF AOPP concentration
exhibited a significant negative correlation with the level of
P4 in the FF (EM group: r=−0.406, P=0.006; control
group: r=−0.315, P=0.035; total: r=−0.421, P<0.001). By
contrast, no significant correlations were identified between the
FF AOPP concentration and the serum levels of LH, E2,
and P4 at the time of hCG administration.
 | Table II.Correlations between the AOPP level
in the FF and the gonadal hormone levels. |
Table II.
Correlations between the AOPP level
in the FF and the gonadal hormone levels.
|
| AOPP level |
|---|
|
|
|
|---|
|
| EM group
(n=44) | Control group
(n=45) | Total (n=89) |
|---|
|
|
|
|
|
|---|
| Measurements | r | P-value | r | P-value | r | P-value |
|---|
| FF content |
|
E2 | −0.052 | 0.737 | −0.203 | 0.180 | −0.162 | 0.129 |
|
P4 | −0.406 | 0.006a | −0.315 | 0.035b | −0.421 |
<0.001a |
| Prior to hCG
administration |
| LH | 0.273 | 0.073 | 0.072 | 0.639 | 0.177 | 0.097 |
|
E2 | 0.130 | 0.399 | −0.141 | 0.355 | −0.046 | 0.668 |
|
P4 | −0.136 | 0.380 | −0.076 | 0.620 | −0.122 | 0.254 |
Correlation between AOPP concentration
in the FF and the outcome parameters of IVF
Regarding the outcome of IVF, a negative correlation
was observed between the FF AOPP concentration and the blastocyst
rate in the EM and the total groups (EM group: r=−0.376, P=0.012;
total: r=−0.367, P<0.001; Table
III). However, no significant correlation was obsrved between
the FF AOPP concentration and the fertilization rate, or between
the FF AOPP concentration and the good embryos rate.
 | Table III.Correlations between the AOPP levels
in the follicular fluid and the outcome parameters of in
vitro fertilization. |
Table III.
Correlations between the AOPP levels
in the follicular fluid and the outcome parameters of in
vitro fertilization.
|
| AOPP level |
|---|
|
|
|
|---|
|
| EM group
(n=44) | Control group
(n=45) | Total (n=89) |
|---|
|
|
|
|
|
|---|
| Parameters | r | P-value | r | P-value | r | P-value |
|---|
| No. of follicles
(diameter, >14 mm) | 0.066 | 0.670 | −0.052 | 0.736 | −0.055 | 0.609 |
| No. of
fertilizations | 0.082 | 0.598 | 0.027 | 0.862 | −0.019 | 0.862 |
| Fertilization rate
(%) | 0.093 | 0.547 | 0.107 | 0.483 | 0.028 | 0.797 |
| No. of good
embryos | 0.086 | 0.578 | −0.100 | 0.513 | −0.067 | 0.535 |
| Good embryo rate
(%) | 0.014 | 0.928 | −0.253 | 0.093 | −0.140 | 0.190 |
| Blastocyst rate
(%) | −0.376a | 0.012 | −0.161 | 0.292 | −0.367b | <0.001 |
Discussion
EM has been demonstrated to be a disease closely
associated with oxidative stress and the inflammatory reaction
(33). AOPPs are the products of the
oxidative stress reaction between chlorinated oxidants and plasma
albumin (32,34). In the peritoneal fluid and serum of
patients with EM, the AOPP concentration was significantly
increased (21,35). To date, the AOPPs in the FF of
patients with EM have not been widely investigated, although
certain other oxidative stress markers have been reported. For
instance, 8-hydroxy-2′-deoxyguanosine, ROS, nitric oxide and lipid
peroxidation were all reportedly higher in the FF of EM patients
when compared with the healthy individuals (36,37).
This suggests that there is more acute oxidative stress reaction in
the FF of EM patients. In the present study, the AOPP level in the
FF of infertile women with EM was markedly higher in comparison
with the controls, which was in agreement with previous
studies.
The quality of oocytes is a pivotal factor
associated with the success rate of IVF. Oocyte maturation depends
on the appropriate acquisition of cytoplasmic and nuclear
maturation, with the latter depending on the presence of a normal
cell spindle (38,39). The microenvironment in FF is closely
associated with the formation of spindles and the distribution of
chromosome (40–42). Certain inflammatory factors in the
FF, including tumor necrosis factor α and interleukin-17A, have
also been reported to adversely affect oocytes (43,44). The
FF in EM patients may induce oxidative stress reaction in oocytes
and cause DNA damage (45). In
addition, AOPPs in the plasma induce endoplasmic reticulum stress
in various cells (46–49), and this stress has been observed to
cause phenotypic alterations and death in cells (50,51).
Under an environment with acute oxidative stress, the oocyte
maturation is more likely to be arrested and the developmental
potency of embryos is inevitably decreased. As observed in the
current study, a reduced number of mature oocytes was retrieved and
fertilized in the infertile patients with EM, and consequently,
fewer good embryos could be used.
Another key factor for oocyte maturation is the
gonadal hormone level, such as the level of P4. Although
no correlation has been detected between the serum level of
P4 and the prognosis of assisted reproductive technology
(ART) (52), the P4 level
in the FF only secreted and synthesized by granulosa cells is more
closely correlated with the development of oocytes and the outcome
of IVF (53,54). In the present study, the EM group
with a higher FF AOPP concentration presented a lower FF
P4 level, thus a negative correlation was observed
between AOPPs and P4 levels. Santulli et al
(21) and Gomes et al
(55) reported that the peritoneal
fluid of EM patients containing a higher level of AOPPs reduced the
P4 release from granulosa cells. Therefore, the higher
level of AOPPs in the FF of EM patients may be the cause of the
decreased P4 level.
The negative influence of oxidative stress on
granulosa cells is unimportant; oxdative stress causes
mitochondrial and endoplasmic reticulum defects in granular cells
and the generation of ROS in granulosa cells is accompanied with
caspase3/7 expression, an important indicator for cells apoptosis
(56). Extreme oxidative stress is
able to induce granulosa cell apoptosis (57); however, the antioxidant factor would
exert a positive effect. L-DOPA in FF as an antioxidant factor that
exerts positive influences on granular cells, including decreasing
H2O2 production and promoting cell survival
(58). Based on these observations,
AOPPs may decrease P4 production via impairing the
function of granulosa cells. In addition, in mammalian preovulatory
follicles, P4 is one of the dominant steroid hormones
(59). P4 regulates
meiosis and ovulation by activating the progesterone receptors
(60). A high level of P4
in the FF increases the percentage of oocytes at the germinal
vesicle stage, and the maternal P4 level affects the
gene transcripts of cumulus-oocyte complexes (54). By contrast, upon the lack of the
necessary hormonal stimulation from P4, the development
and maturation of oocytes would be delayed or may even not reach an
acceptable level (61,62).
The present study observed that the EM patients with
fewer mature oocytes and fewer good-quality embryos presented a
lower blastocyst rate. In the EM group and in the total cohort of
patients, the correlation between AOPP concentration in the FF and
blastocyst rate presented a significant negative correlation. The
low success rate of ART may be explained by the low oocyte quality
and blockage of embryo development (63). In clinical practice, selecting
good-quality oocytes and embryos is key for promoting ART.
Blastocysts represent a vital stage in the embryo development, and
a good-quality blastocyst indicates a greater likelihood of
implantation and gives rise to live birth (64–67).
Oocytes, one of the origins of embryos, have been demonstrated to
be associated with the developmental potential of the embryo; thus,
good-quality oocytes are more likely to develop into blastocysts
following fertilization (68,69). As
described previously, excessive oxidative stress in FF is inversely
correlated with the prognosis of pregnancy in ART (70). Furthermore, oocytes with cytoplasmic
defects are more likely to occur in an acute oxidative stress
environment (71). While the
elevated AOPPs may inhibit the appearance of the first polar body
and cytoplasmic maturation, thus arresting oocyte maturation and
damaging the potential of embryos (45), AOPPs may also damage the function of
granulosa cells and decrease P4 production. Due to the
lack of sufficient hormonal support of P4, oocyte
maturation is arrested and the subsequent processes, including
fertilization, blastocyst formation and embryo development, are
negatively affected. This hypothesis is in accordance with previous
experiments on rhesus monkeys, which reported that a higher ratio
of P4 to E2 in the FF was beneficial for the
development of the embryos (72). In
addition, this is consistent with the findings of Gustafson et
al (73), suggesting that a
higher E2/P4 ratio in the FF was associated
with a lower IVF success rate. Therefore, an impairment of oocyte
quality, lower implantation and reduced pregnancy rates are
observed in patients with EM (74,75).
However, the current study had various limitations.
The major limitation is the overall small number of patients
included. Furthermore, no significant correlations between the FF
AOPP level and other gonadal hormones levels in the FF or serum
were observed; however, this does not indicate that there are no
correlations between the FF AOPP and other various gonadal hormones
A larger sample size may provide more substantive evidence in
further studies.
In conclusion, AOPP levels were significantly
elevated in the FF of infertile patients with EM in the present
study. In addition, the increased FF AOPP level was accompanied
with a decreased FF P4 concentration and blastocyst
rate. These findings indicate that AOPP may be a potentially
effective marker for predicting the oocyte quality and outcome of
IVF, particularly in infertile women with EM. This provides a novel
theoretical basis, suggesting that anti-oxidative treatments aimed
at reducing the AOPP levels may be a new effective strategy to
promote the maturation of oocytes and improve the success rate of
IVF.
Acknowledgements
The authors would like to thank Dr. Wei Cao and Dr.
Jianwei Tian (Department of Nephrology, Southern Medical
University, Guangzhou, China) for their technical assistance. The
present study was supported by the National Natural Science
Foundation of China (grant no. 81401180), the Population and Family
Planning Commission Scientific Research Project of Guangdong
Province in China (grant no. 20133058) and the Medical Scientific
Research Foundation of Guangdong Province in China (grant no.
A2013371).
Glossary
Abbreviations
Abbreviations:
|
AOPPs
|
advanced oxidation protein
products
|
|
EM
|
endometriosis
|
|
E2
|
estradiol
|
|
P4
|
progesterone
|
|
FF
|
follicular fluid
|
|
IVF
|
in vitro fertilization
|
|
ROS
|
reactive oxygen species
|
|
LH
|
luteinizing hormone
|
|
hCG
|
human chorionic gonadotropin
|
|
ART
|
assisted reproductive technology
|
References
|
1
|
Agarwal A, Gupta S, Abdel-Razek H, Krajcir
N and Athayde K: Impact of oxidative stress on gametes and embryos
in an ART Laboratory. Clin Embryol. 9:5–22. 2006.
|
|
2
|
Esfandiari N, Falcone T, Agarwal A,
Attaran M, Nelson DR and Sharma RK: Protein supplementation and the
incidence of apoptosis and oxidative stress in mouse embryos.
Obstet Gynecol. 105:653–660. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu XM, Zhang YP, Ji SY, Li BT, Tian X, Li
D, Tong C and Fan HY: Mitoguardin-1 and −2 promote maturation and
the developmental potential of mouse oocytes by maintaining
mitochondrial dynamics and functions. Oncotarget. 7:1155–1167.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Wu XQ, Lu S, Guo YL and Ma X:
Deficit of mitochondria-derived ATP during oxidative stress impairs
mouse MII oocyte spindles. Cell Res. 16:841–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
International Classification of Diseases
10th edition (ICD-10). World Human Organization; Apr 16–2015
|
|
6
|
D'Hooghe TM, Debrock S, Hill JA and
Meuleman C: Endometriosis and subfertility: Is the relationship
resolved? Semin Reprod Med. 21:243–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bérubé S, Marcoux S, Langevin M and Maheux
R: Fecundity of infertile women with minimal or mild endometriosis
and women with unexplained infertility. The Canadian collaborative
group on endometriosis. Fertil Steril. 69:1034–1041. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pellicer A, Navarro J, Bosch E, Garrido N,
Garcia-Velasco JA, Remohi J and Simón C: Endometrial quality in
infertile women with endometriosis. Ann N Y Acad Sci. 943:122–130.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mansour G, Sharma RK, Agarwal A and
Falcone T: Endometriosisinduced alterations in mouse metaphase II
oocyte microtubules and chromosomal alignment: A possible cause of
infertility. Fertil Steril. 94:1894–1899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fadhlaoui A, Bouquet de la Jolinière J and
Feki A: Endometriosis and infertility: How and when to treat? Front
Surg. 1:242014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lessey BA, Lebovic DI and Taylor RN:
Eutopic endometrium in women with endometriosis: Ground zero for
the study of implantation defects. Semin Reprod Med. 31:109–124.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Basile N, Mdel C Nogales, Bronet F,
Florensa M, Riqueiros M, Rodrigo L, Garcia-Velasco J and Meseguer
M: Increasing the probability of selecting chromosomally normal
embryos by time-lapse morphokinetics analysis. Fertil Steril.
101:699–704. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu B, Guo N, Zhang XM, Shi W, Tong XH,
Iqbal F and Liu YS: Oocyte quality is decreased in women with
minimal or mild endometriosis. Sci Rep. 5:107792015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goud PT, Goud AP, Joshi N, Puscheck E,
Diamond MP and AbuSoud HM: Dynamics of nitric oxide, altered
follicular microenvironment, and oocyte quality in women with
endometriosis. Fertil Steril. 102:151–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prieto L, Quesada JF, Cambero O, Pacheco
A, Pellicer A, Codoceo R and Garcia-Velasco JA: Analysis of
follicular fluid and serum markers of oxidative stress in women
with infertility related to endometriosis. Fertil Steril.
98:126–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh AK, Chattopadhyay R, Chakravarty B
and Chaudhury K: Markers of oxidative stress in follicular fluid of
women with endometriosis and tubal infertility undergoing IVF.
Reprod Toxicol. 42:116–124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Da Broi MG, Malvezzi H, Paz CC, Ferriani
RA and Navarro PA: Follicular fluid from infertile women with mild
endometriosis may compromise the meiotic spindles of bovine
metaphase II oocytes. Hum Reprod. 29:315–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santanam N, Zoneraich N and Parthasarathy
S: Myeloperoxidase as a potential target in women with
endometriosis undergoing IVF. Reprod Sci. 24:619–626. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Colombo G, Clerici M, Giustarini D,
Portinaro N, Badalamenti S, Rossi R, Milzani A and Dalle-Donne I: A
central role for intermolecular dityrosine cross-linking of
fibrinogen in high molecular weight advanced oxidation protein
product (AOPP) formation. Biochim Biophys Acta. 1850:1–12. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Witko-Sarsat V, Gausson V, Nguyen AT,
Touam M, Drüeke T, Santangelo F and Descamps-Latscha B:
AOPP-induced activation of human neutrophil and monocyte oxidative
metabolism: A potential target for N-acetylcysteine treatment in
dialysis patients. Kidney Int. 64:82–91. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Santulli P, Chouzenoux S, Fiorese M,
Marcellin L, Lemarechal H, Millischer AE, Batteux F, Borderie D and
Chapron C: Protein oxidative stress markers in peritoneal fluids of
women with deep infiltrating endometriosis are increased. Hum
Reprod. 30:49–60. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li HY, Hou FF, Zhang X, Chen PY, Liu SX,
Feng JX, Liu ZQ, Shan YX, Wang GB, Zhou ZM, et al: Advanced
oxidation protein products accelerate renal fibrosis in a remnant
kidney model. J Am Soc Nephrol. 18:528–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR,
Liu ZQ, Zhou ZM, Zhou M, Xie D, Wang GB and Zhang X: Advanced
oxidation protein products accelerate atherosclerosis through
promoting oxidative stress and inflammation. Arterioscler Thromb
Vasc Biol. 26:1156–1162. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao W, Xu J, Zhou ZM, Wang GB, Hou FF and
Nie J: Advanced oxidation protein products activate intrarenal
renin-angiotensin system via a CD36-mediated, redox-dependent
pathway. Antioxid Redox Signal. 18:19–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Q, Zhong ZM, Pan Y, Zeng JH, Zheng S,
Zhu SY and Chen JT: Advanced oxidation protein products as a novel
marker of oxidative stress in postmenopausal osteoporosis. Med Sci
Monit. 21:2428–2432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moti M, Amini L, Ardakani SS Mirhoseini,
Kamalzadeh S, Masoomikarimi M and Jafarisani M: Oxidative stress
and anti-oxidant defense system in Iranian women with polycystic
ovary syndrome. Iran J Reprod Med. 13:373–378. 2015.PubMed/NCBI
|
|
27
|
Santulli P, Borghese B, Lemaréchal H,
Leconte M, Millischer AE, Batteux F, Chapron C and Borderie D:
Increased serum oxidative stress markers in women with uterine
leiomyoma. PLoS One. 8:e720692013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jana SK, K NB, Chattopadhyay R,
Chakravarty B and Chaudhury K: Upper control limit of reactive
oxygen species in follicular fluid beyond which viable embryo
formation is not favorable. Reprod Toxicol. 29:447–451. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song YL, Quan S, Tian JW, Li H, Chen SM
and Xing FQ: Relationship between protein oxidation levels in the
follicular fluid and the outcome parameters of in vitro
fertilization-embryo transplantation. Nan Fang Yi Ke Da Xue Xue
Bao. 29:160–163. 2009.(In Chinese). PubMed/NCBI
|
|
30
|
World Health Organization, . WHO
Laboratory Manual for the Examination Human Semen and
Sperm-cervical Mucus Interaction. 4th. Cambridge University Press;
Oxford, UK: 2010
|
|
31
|
Giorgi VS, Da Broi MG, Paz CC, Ferriani RA
and Navarro PA: N-acetyl-cysteine and l-carnitine prevent meiotic
oocyte damage induced by follicular fluid from infertile women with
mild endometriosis. Repro Sci. 23:342–351. 2016. View Article : Google Scholar
|
|
32
|
Witko-Sarsat V, Friedlander M,
Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers
P and Descamps-Latscha B: Advanced oxidation protein products as a
novel marker of oxidative stress in uremia. Kidney Int.
49:1304–1313. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Agarwal A, Aponte-Mellado A, Premkumar BJ,
Shaman A and Gupta S: The effects of oxidative stress on female
reproduction: A review. Reprod Biol Endocrinol. 10:492012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Witko-Sarsat V, Friedlander M, Khoa T
Nguyen, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM,
Jungers P, Drüeke T and Descamps-Latscha B: Advanced oxidation
protein products as novel mediators of inflammation and monocyte
activation in chronic renal failure. J Immunol. 161:2524–2532.
1998.PubMed/NCBI
|
|
35
|
Jana SK, Dutta M, Joshi M, Srivastava S,
Chakravarty B and Chaudhury K: 1H NMR based targeted metabolite
profiling for understanding the complex relationship connecting
oxidative stress with endometriosis. Biomed Resint.
2013:3290582013.
|
|
36
|
Da Broi MG, de Albuquerque FO, de Andrade
AZ, Cardoso RL, Jordão Junior AA and Navarro PA: Increased
concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of
infertile women with endometriosis. Cell Tissue Res. 366:231–242.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Singh AK, Chattopadhyay R, Chakravarty B
and Chaudhury K: Markers of oxidative stress in follicular fluid of
women with endometriosis and tubal infertility undergoing IVF.
Repro Toxicol. 42:116–124. 2013. View Article : Google Scholar
|
|
38
|
Mattson BA and Albertini DF: Oogenesis:
Chromatin and microtubule dynamics during meiotic prophase. Mol
Reprod Dev. 25:374–383. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Albertini DF: Cytoplasmic microtubular
dynamics and chromatin organization during mammalian oogenesis and
oocyte maturation. Mutat Res. 296:57–68. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ma CH, Yan LY, Qiao J, Sha W, Li L, Chen Y
and Sun QY: Effects of tumor necrosis factor-alpha on porcine
oocyte meiosis progression, spindle organization, and chromosome
alignment. Fertil Steril. 93:920–926. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sabbaghi M, Aram R, Roustaei H, Islam M
Fadavi, Daneshvar M, Castaño AR and Haghparast A: IL-17A
concentration of seminal plasma and follicular fluid in infertile
men and women with various clinical diagnoses. Immunol Invest.
43:617–626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pauli SA, Session DR, Shang W, Easley K,
Wieser F, Taylor RN, Pierzchalski K, Napoli JL, Kane MA and Sidell
N: Analysis of follicular fluid retinoids in women undergoing in
vitro fertilization: Retinoic acid influences embryo quality and is
reduced in women with endometriosis. Reprod Sci. 20:1116–1124.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Opperman CM and Sishi BJ: Tumor necrosis
factor alpha stimulates p62 accumulation and enhances proteasome
activity independently of ROS. Cell Biol Toxicol. 31:83–94. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Żbikowska-Gotz M, Pałgan K,
Gawrońska-Ukleja E, Kuźmiński A, Przybyszewski M, Socha E and
Bartuzi Z: Expression of IL-17A concentration and effector
functions of peripheral blood neutrophils in food allergy
hypersensitivity patients. Int J Immunopathol Pharmacol. 29:90–98.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hamdan M, Jones KT, Cheong Y and Lane SI:
The sensitivity of the DNA damage checkpoint prevents oocyte
maturation in endometriosis. Sci Rep. 6:369942016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yuan F, Liu SX and Tian JW: Advanced
oxidation protein products induce reactive oxygen species
production in endothelial cells. Di Yi Jun Yi Da Xue Xue Bao.
24:1350–1352. 2004.(In Chinese). PubMed/NCBI
|
|
47
|
Zhou QG, Zhou M, Lou AJ, Xie D and Hou FF:
Advanced oxidation protein products induce inflammatory response
and insulin resistance in cultured adipocytes via induction of
endoplasmic reticulum stress. Cell Physiol Biochem. 26:775–786.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rong G, Tang X, Guo T, Duan N, Wang Y,
Yang L, Zhang J and Liang X: Advanced oxidation protein products
induce apoptosis in podocytes through induction of endoplasmic
reticulum stress. J Physiol Biochem. 71:455–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liang X, Duan N, Wang Y, Shu S, Xiang X,
Guo T, Yang L, Zhang S, Tang X and Zhang J: Advanced oxidation
protein products induce endothelial-to-mesenchymal transition in
human renal glomerular endothelial cells through induction of
endoplasmic reticulum stress. J Diabetes Complications. 30:573–579.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bouvier N, Flinois JP, Gilleron J, Sauvage
FL, Legendre C, Beaune P, Thervet E, Anglicheau D and Pallet N:
Cyclosporine triggers endoplasmic reticulum stress in endothelial
cells: A role for endothelial phenotypic changes and death. Am J
Physiol Renal Physiol. 296:F160–F169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang XY, Yang SM, Zhang HP, Yang Y, Sun
SB, Chang JP, Tao XC, Yang TY, Liu C and Yang YM: Endoplasmic
reticulum stress mediates the arsenic trioxide-induced apoptosis in
human hepatocellular carcinoma cells. Int J Biochem Cell Biol.
68:158–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Timur H, Yimaz N, Kahyaoglu I, Inal HA and
Erkaya S: The effect of serum and follicular fluid
amyloid-associated protein levels on in vitro fertilization outcome
in patients with polycystic ovary syndrome. J Assist Reprod Genet.
32:1637–1642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wen X, Li D, Tozer AJ, Docherty SM and
Iles RK: Estradiol, progesterone, testosterone profiles in human
follicular fluid and cultured granulosa cells from luteinized
pre-ovulatory follicles. Reprod Biol Endocrinol. 8:1172010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bogacki M, Wasielak M, Kitewska A, Bogacka
I and Jalali BM: The effect of hormonal estrus induction on
maternal effect and apoptosis-related genes expression in porcine
cumulus-oocyte complexes. Reprod Biol Endocrinol. 12:322014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gomes FM, Navarro PA, de Abreu LG,
Ferriani RA, dos Reis RM and de Moura MD: Effect of peritoneal
fluid from patients with minimal/mild endometriosis on progesterone
release by human granulosa-lutein cells obtained from infertile
patients without endometriosis: A pilot study. Eur J Obstet Gynecol
Reprod Biol. 138:60–65. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bar-Joseph H, Ben-Ami I, Ron-El R, Shalgi
R and Chuderland D: Pigment epithelium-derived factor exerts
antioxidative effects in granulosa cells. Fertil Steril.
102:891–898.e3. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Saller S, Kunz L, Berg D, Berg U, Lara H,
Urra J, Hecht S, Pavlik R, Thaler CJ and Mayerhofer A: Dopamine in
human follicular fluid is associated with cellular uptake and
metabolism-dependent generation of reactive oxygen species in
granulosa cells: Implications for physiology and pathology. Hum
Reprod. 29:555–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Blohberger J, Buck T, Berg D, Berg U, Kunz
L and Mayerhofer A: L-DOPA in the hu man ovarian follicular fluid
acts as an antioxidant factor on granulosa cells. J Ovarian Res.
9:622016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Su YQ, Sugiura K, Woo Y, Wigglesworth K,
Kamdar S, Affourtit J and Eppig JJ: Selective degradation of
transcripts during meiotic maturation of mouse oocytes. Dev Biol.
302:104–117. 2017. View Article : Google Scholar
|
|
60
|
Salehnia M and Zavareh S: The effects of
progesterone on oocyte maturation and embryo development. Int J
Fertil Steril. 7:74–81. 2013.PubMed/NCBI
|
|
61
|
Bertoldo M, Holyoake PK, Evans G and
Grupen CG: Oocyte developmental competence is reducedin sows during
the seasonal infertility period. Reprod Fertil Dev. 22:1222–1229.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Grupen CG and Armstrong DT: Relationship
between cumulus cell apoptosis, progesterone production and porcine
oocyte developmental competence: Temporal effects of follicular
fluid during IVM. Reprod Fertil Dev. 22:1100–1109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Catala MG, Izquierdo D, Rodriguez-Prado M,
Hammami S and Paramio MT: Effect of oocyte quality on blastocyst
development after in vitro fertilization (IVF) and intracytoplasmic
sperm injection (ICSI) in a sheep model. Fertil Steril.
97:1004–1008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Braude P, Bolton V and Moore S: Human gene
expression first occurs between the four- and eight-cell stages of
preimplantation development. Nature. 332:459–461. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lagalla C, Barberi M, Orlando G, Sciajno
R, Bonu MA and Borini A: A quantitative approach to blastocyst
quality evaluation: Morphometric analysis and related IVF outcomes.
J Assist Reprod Genet. 32:705–712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Dokras A, Sargent IL and Barlow DH: Human
blastocyst grading: An indicator of developmental potential? Hum
Reprod. 8:2119–2127. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
De Kock A, Smuts MP, Madden JD, Rodriguez
AJ, Chantilis SJ and Meintjes M: Digital image analysis of
blastocysts. Morphometrics correlations with pregnancy outcome.
Fertil Steril. 86 Suppl:S51–S52. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Van Blerkom J, Davis PW and Lee J: ATP
content of human oocytes and developmental potential and outcome
after in-vitro fertilization and embryo transfer. Hum Reprod.
10:415–424. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Stojkovic M, Machado SA, Stojkovic P,
Zakhartchenko V, Hutzler P, Goncalves PB and Wolf E: Mitochondrial
distribution and adenosine triphosphate content of bovine oocytes
before and after in vitro maturation: Correlation with
morphological criteria and developmental capacity after in vitro
fertilization and culture. Biol Reprod. 64:904–909. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bedaiwy MA, Elnashar SA, Goldberg JM,
Sharma R, Mascha EJ, Arrigain S, Agarwal A and Falcone T: Effect of
follicular fluid oxidative stress parameters on intracytoplasmic
sperm injection outcome. Gynecol Endocrinol. 28:51–55. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Van Blerkom J, Antczak M and Schrader R:
The developmental potential of the human oocyte is related to the
dissolved oxygen content of follicular fluid: Association with
vascular endothelial growth factor levels and perifollicular blood
flow characteristics. Hum Reprod. 12:1047–1055. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Morgan PM, Boatman DE and Bavister BD:
Relationships between follicular fluid steroid hormone
concentrations, oocyte maturity, in vitro fertilization and
embryonic development in the rhesus monkey. Mol Reprod Dev.
27:145–151. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Gustafson O, Nylund L and Carlström K:
Does hyperandrogenism explain lower in vitro fertilization (IVF)
success rates in smokers? Acta Obstet Gynecol Scand. 75:149–156.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kuivasaari P, Hippelainen M, Anttila M and
Heinonen S: Effect of endometriosis on IVF/ICSI outcome: Stage
III/IV endometriosis worsens cumulative pregnancy and live-born
rates. Hum Reprod. 20:3130–3135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jancar N, Kopitar AN, Ihan A, Virant KI
and Bokal EV: Effect of apoptosis and reactive oxygen species
production in human granulosa cells on oocyte fertilization and
blastocyst development. J Assist Reprod Genet. 24:91–97. 2007.
View Article : Google Scholar : PubMed/NCBI
|