Introduction
Congenital diaphragmatic hernia (DH) is a birth
defect of the diaphragm is known that is exhibited in 1/3,000 live
births worldwide (1). In DH cases,
~85% exhibit the left-sided posterolateral defect, 40% of whom
exhibited an isolated defect (2). DH
is lethal in ~35% of cases in the neonatal period and surviving
patients suffer from a number of resulting chronic disorders such
as decreased respiratory function and asthma (3). Furthermore, in patients with DH, lung
development is altered, which may lead to medial hyperplasia,
decreased pulmonary vessels displaying adventitial thickening,
reduced surface area for gas exchange, an increase in the thickness
of alveolar walls and a decrease in the number of bronchial
branches (4). At present, effective
management of DH is limited and highly specialized equipped center
is required for fetal endoscopic tracheal occlusion. In fetal
endoscopic tracheal occlusion, a detachable endoluminal balloon is
inserted endoscopically and subsequently changes shape to provide
effective occlusion of the growing trachea without causing tracheal
damage (5). Furthermore, management
of pulmonary hypoplasia is achieved through transplacental or
intra-amniotic injection of several agents with pulmonary delivery
facilitated by fetal breathing movements rather than fetal tracheal
instrumentation (6–7). A number of drugs, including
tetrandrine, estrogen, ghreline, sildenafil and tadalafil, have
previously been used in animal models to explore the treatment of
DH (8). However, in the clinical
application of these drugs, the results of these preclinical
experiments have not been replicated.
Baicalin is a glycoside that is isolated from the
leaves of Scutellaria galericulata, and is reported to exert
anti-anxiety actions by acting on the GABA receptor without
producing sedation (9). Furthermore,
baicalin exerts an inhibitory effect on prolyl endopeptidase and
has been demonstrated to effectively manage pancreatic cancer by
inducing apoptosis (10–11). In addition, a previous study on
baicalin has suggested that it improves the fetal lung growth by
increasing fetal lung surfactant phospholipids (12). Given these previous findings, the aim
of the present study was to evaluate the effect of baicalin on
fetal lung development in DH.
Materials and methods
Animals
Female New Zealand rabbits (3–4 kg; 9–12 months old)
were used in the present study and male New Zealand rabbits were
also procured from Shanghai Institutes for Biological
Sciences, Shanghai, China). Rabbits (n=12) were housed in
standard laboratory conditions, including a 12-h light-dark cycle
and at room temperature 20–25°C. Rabbits were acclimatized to these
conditions for 10 days prior to the onset of experiments. All the
protocols used in the present study were approved by the Animal
Ethics Committee of Yantai Yuhuangding Hospital of Qingdao
University (Yantai, China).
Experimental procedure
Rabbits were acclimatized in the animal house for 10
days and later housed individually following mating in the
conditions detailed above with ad libitum access to water
and chow. Blood glucose level and body weight of each rabbit was
measured prior to gestational day (GD) 23 using a GOD-POD kit
(Accuplex Diagnostics Ltd., Maynooth, Ireland). Rabbits were
anesthetized by administering ketamine (35 mg/kg; Dechra,
Northwich, UK) and xylazine (6 mg/kg) intramuscularly (Shenzhen
Sendi Biotechnology Co., Ltd., Guangdong, China). Buprenorphine
(0.03 mg/kg; BioDelivery Sciences International, Inc., Raleigh, NC,
USA), medroxyprogesterone (0.9 mg/kg; Pfizer, New York, NY, USA)
and penicillin G (300,000 U; Hebei New Century Pharmaceutical Co.,
Ltd., Hebei, China) were administered subcutaneously and 1.5%
isoflurane (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used
with oxygen (2 ml/min) to maintain anesthesia. The surgical area
(abdomen) was subsequently shaved and sterile conditions were
maintained for all surgical procedures. Lidocaine (Xylocaine; Astra
Zeneca, Cambridge, UK) at a concentration of 1% was administered to
the subcutaneous tissue prior to surgery. Laparotomy was performed
to exteriorized uterine horns according to a previous study
(13), thus establishing a
left-sided diaphragmatic hernia in one additional fetus and in two
ovarian ends. Briefly, a purse string suture (6-0 Prolene) was
inserted, and the left fetal arm was exteriorized. The diaphragm
was exposed through a low left lateral thoracotomy using
purpose-designed retractors and the membraneous part was opened
with scissors. The uterus was protected from dehydration and
hypothermia via regular washing with warm saline throughout the
surgical procedure. Subsequently, a 2-0 polyglactine suture and a
2-0 nylon were used to close the rectus fascia and the skin,
respectively.
Baicalin administration and tissue
collection
Rabbits were separated randomnly into two groups
(n=6) on GD24. The control group was administered an equal volume
of saline solution and the baicalin-treated group were administered
5 mg/kg/day baicalin (Sigma-Aldrich; Merck KGaA) for one week, i.e.
until the GD31. For each case, one surviving littermate acted as an
internal nonhypoplastic control. Therefore, fetuses used in the
present study were separated into the following four groups (n=7):
Control with DH, control without DH, baicalin with DH and baicalin
without DH.
One day following the end of treatment, weight and
blood glucose was measured and rabbits were subsequently
anesthetized using ketamine and xylazine as described above.
Confirmation of fetus viability was performed at cesarean section
and later rabbits were sacrificed by administering 1 ml otetracaine
(5 mg), mebezonium (50 mg) and embutramide mixture (200 mg; all
Marion Roussel, Brussels, Belgium) intravenously on gestational day
31. Fetuses were collected and anesthetized via intraperitoneal
injection of ketamine (50 mg/kg) and blood was collected via
cardiac puncture. Fetuses were sacrificed using the same method as
mothers. The weight of fetuses was subsequently measured, lungs
were isolated and weighed and lung-to-body weight ratios (LBWRs)
were calculated. The lung contralateral to the diaphragmatic defect
was ligated at the hilum, snap frozen in liquid nitrogen and stored
at −80°C. The trachea was cannulated and the left lung
(ipsilateral) was perfused with 10% formalin under 25 cm
H2O pressure for 1 day at room temperature and embedded
in paraffin.
Lung morphometry
Paraffin-embedded lung tissues were cut into 4-µm
thick sections and stained at room temperature for 10 min with
hematoxylin and eosin. Sections were observed using a trinaocular
light microscope (Zeiss AG, Oberkochen, Germany) at ×200
magnification for the microscopic evaluation of lung tissue. Each
fetal lung tissue sample was separated into 20 equal and
non-overlapping areas. Mean terminal bronchial density (MTBD) is
inversely proportional to the number of alveoli present in each
bronchiole, mean wall transection length (Lmw; an index of the
thickness of alveolar septa) and mean linear intercept (Lm), which
is associated with airspace size. Furthermore, vascular morphometry
was observed by staining lung tissues with Miller's elastic stain
and viewing under light microscopy at ×400 magnification. Lung
morphology was evaluated by measuring the external (ED), internal
(ID) and adventitial (AD) diameter along the shortest axis. Medial
thickness and adventitial thickness were calculated by using these
parameters and the following formulae: Percentage medial thickness
(%MT) = ED-ID / ED ×100.Percentage adventitial thickness (%AT) =
AD-ED / ED ×100.Peripheral muscularization was determined as
previously described (14).
Immunohistochemistry
Histological study of lung tissue was performed by
cutting 4 µm sections of lung tissue and staining them with
anti-α-smooth muscle actin (SMA), -Ki-67 and -surfactant protein B
(SPB) antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Endogenous peroxidase was blocked using 0.5%
H2O2 in methanol for Ki67 staining and α-SMA
staining for 30 min at room temperature. Deparaffinized tissue
sections were incubated at 37°C with mouse polyclonal anti-SPB
(1:75) (sc-7702) and human monoclonal anti-Ki-67 antibodies (1:50)
(sc-3752; both Santa Cruz Biotechnology, Inc.) at 4°C, and human
monoclonal anti-α-SMA (1:200) (sc-4961; Santa Cruz Biotechnology,
Inc.) antibodies at 27°C for 2 h. Sections (n=6) used for Ki-67
staining were washed with TBS/Tween-20 (0.1%) and incubated with
rabbit serum, anti-mouse peroxidase (1:100; X-5832; Jackson
ImmunoResearch Europe, Ltd., Newmarket, UK) and
3,3′-Diaminobenzidine (DAB) for 10 min at 37°C. Subsequently,
tissues were counterstained with hematoxylin for 30 min at room
temprature, dehydrated for 10 min with ethanol and mounted. SPB
staining tissues (n=7) were incubated with rabbit serum and
biotin-conjugated anti-goat secondary antibody (1:250; sc-2489;
Santa Cruz Biotechnology, Inc.) for 30 min at 37°C, washed in PBS
and incubated for 20 min at 37°C with streptavidin alkaline
phosphatase conjugate (1:1,000; Roche Diagnostics, Basel,
Switzerland). Samples were then washed again, counterstained for 2
min with nitro blue tetrazolium and methyl green at room
temperature and mounted. Sections used for α-SMA staining (n=7)
were incubated with anti-mouse horseradish peroxidase-conjugated
antibody (1:100; Jackson ImmunoResearch) and rabbit serum for 20
min at 37°C. Sections were subsequently washed with PBS and
incubated with DAB for 10 min at 37°C. Hematoxylin was used to
counterstain sections for 10 min at room temperature, which were
then mounted. ImageJ software (v1.47, National Institutes of
Health, Bethesda, MD, USA) was used for evaluation. Slides were
observed with a light microscope (Zeiss AG) at ×400 magnification
to record 10 non-overlapping images in each slide. The number of
Ki-67-positive cells were counted and Ki-67 density percentage was
measured. For SPB staining, 10 non-overlapping images were also
captured at ×400 magnification and the number of SPB stained cells
present per mm2 of total tissue was calculated (%).
Statistical analysis
Data are presented as the mean ± standard deviation
(n=7). Results were analyzed statistically via one-way analysis of
variance with a post hoc Dunnett test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Fetal survival
Effects of baicalin treatment on the survival of
fetuses are presented in Table I. In
the present study 17 rabbits were used, 1 of which developed
incisional hernia and another exhibited chronioamnionitis at fetal
collection. Of the remaining 15, 6 rabbits, which produced 56
fetuses, were administered saline and considered as controls, and
the remaining 9, which produced 84 fetuses, were administered
baicalin. Of the total 140 fetuses, DH was developed in 48 fetuses,
~34%.
 | Table I.Fetal survival in baicalin-treated
rabbits. |
Table I.
Fetal survival in baicalin-treated
rabbits.
|
| Fetal survival
(n) |
|
|---|
|
|
|
|
|---|
| Group | GD21 | GD31 | Survival (%) | Lung samples used for
histology (n) |
|---|
| Control with DH | 18 | 11a | 61 | 8 |
| Control without
DH | 38 | 24 | 63 | 8 |
| Baicalin with DH | 30 | 10a | 33 | 6 |
| Baicalin without
DH | 54 | 20 | 37 | 6 |
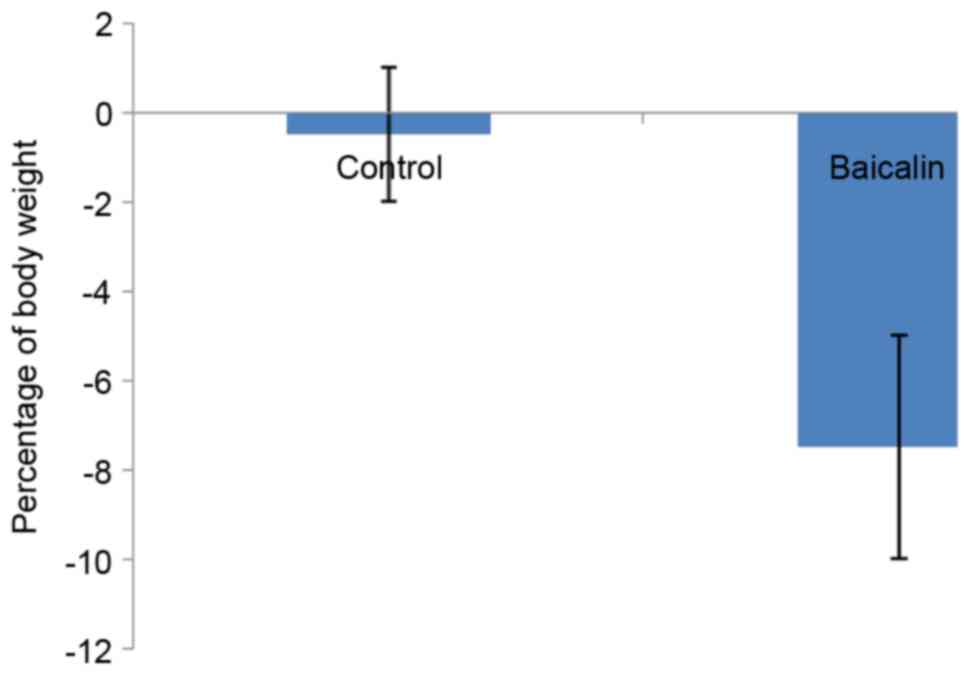
Effect of baicalin on weight loss in
mother rabbits
The effect of baicalin treatment on the percentage
of weight loss in mother rabbits is presented in Fig. 1. It was observed that treatment with
baicalin markedly increased the percentage of weight loss to 7.4%,
compared with 0.25% observed in the control group, during the
period of GD23-GD31.
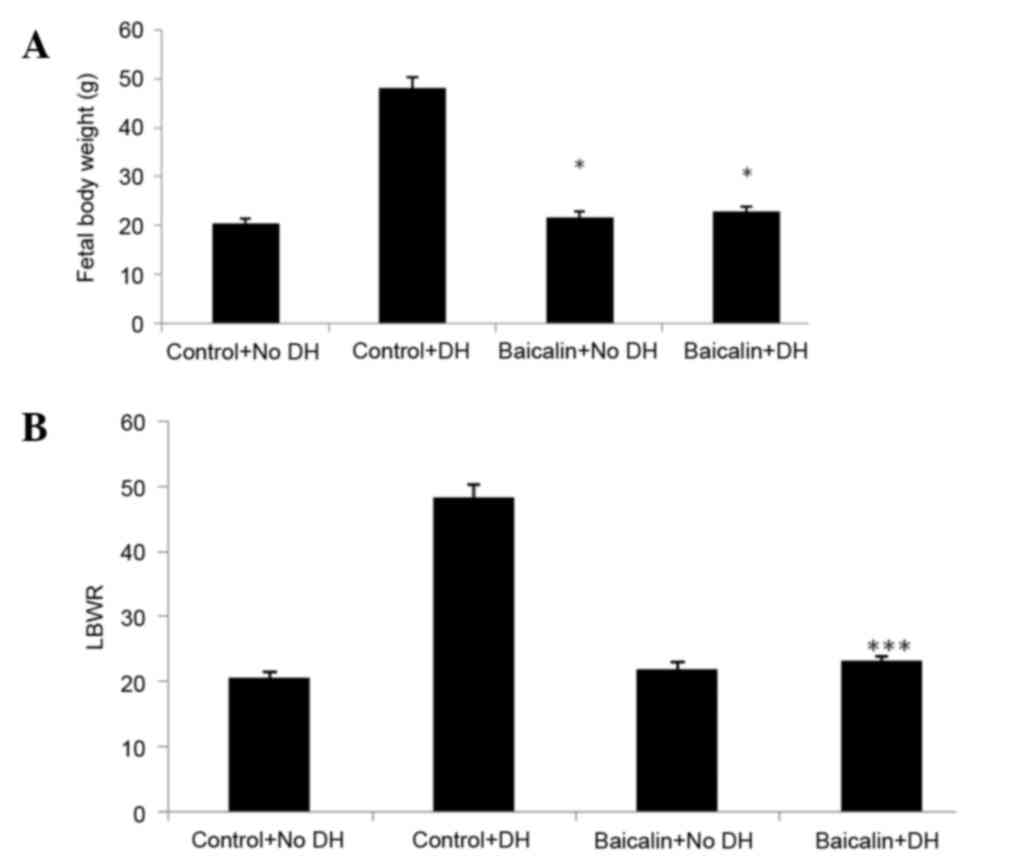
Effect of baicalin on fetal body
weight and LBWR
A significant decrease was observed in the body
weight of fetuses in both baicalin groups compared with control
groups (Fig. 2A). LBWR was found to
be significantly decreased in the baicalin with DH group compared
with the control with DH group. However there was no significant
difference between the LBWR of the control without DH and baicalin
without DH groups (Fig. 2B).
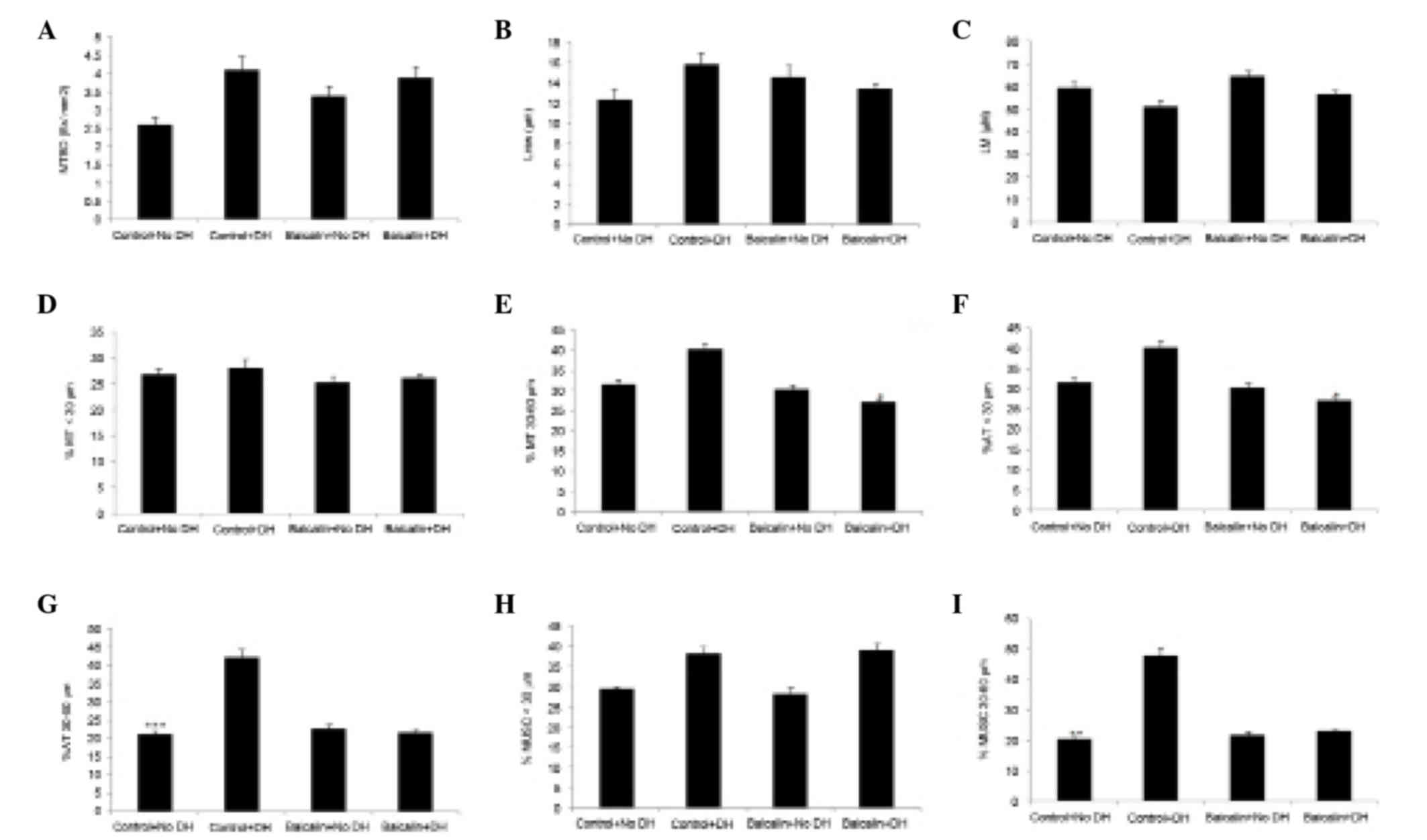
Effect of baicalin on lung
morphometry
Effects of baicalin on lung morphometry in the
rabbit model of DH are presented in Fig.
3. Significant differences were observed in the airway
morphometry of DH groups. It was observed that in control groups,
MTBD and Lmw were markedly increased with DH, which was not
observed in baicalin treated groups. However, no significant
changes in Lm were observed between any groups. Marked changes in
the %MT were observed in 30–60 µm vessel size and not in <30 µm.
It was also observed that %AT <30 and %MT 30–60 µm were
significantly decreased in the baicalin with DH compared with the
control with DH group.
There were no significant changes observed in the
muscularization of vessels <30 µm in the baicalin with DH
groupcompared with the control group. However, treatment with
baicalin markedly decreased the muscularization of vessels in the
30–60 µm category in DH groups.
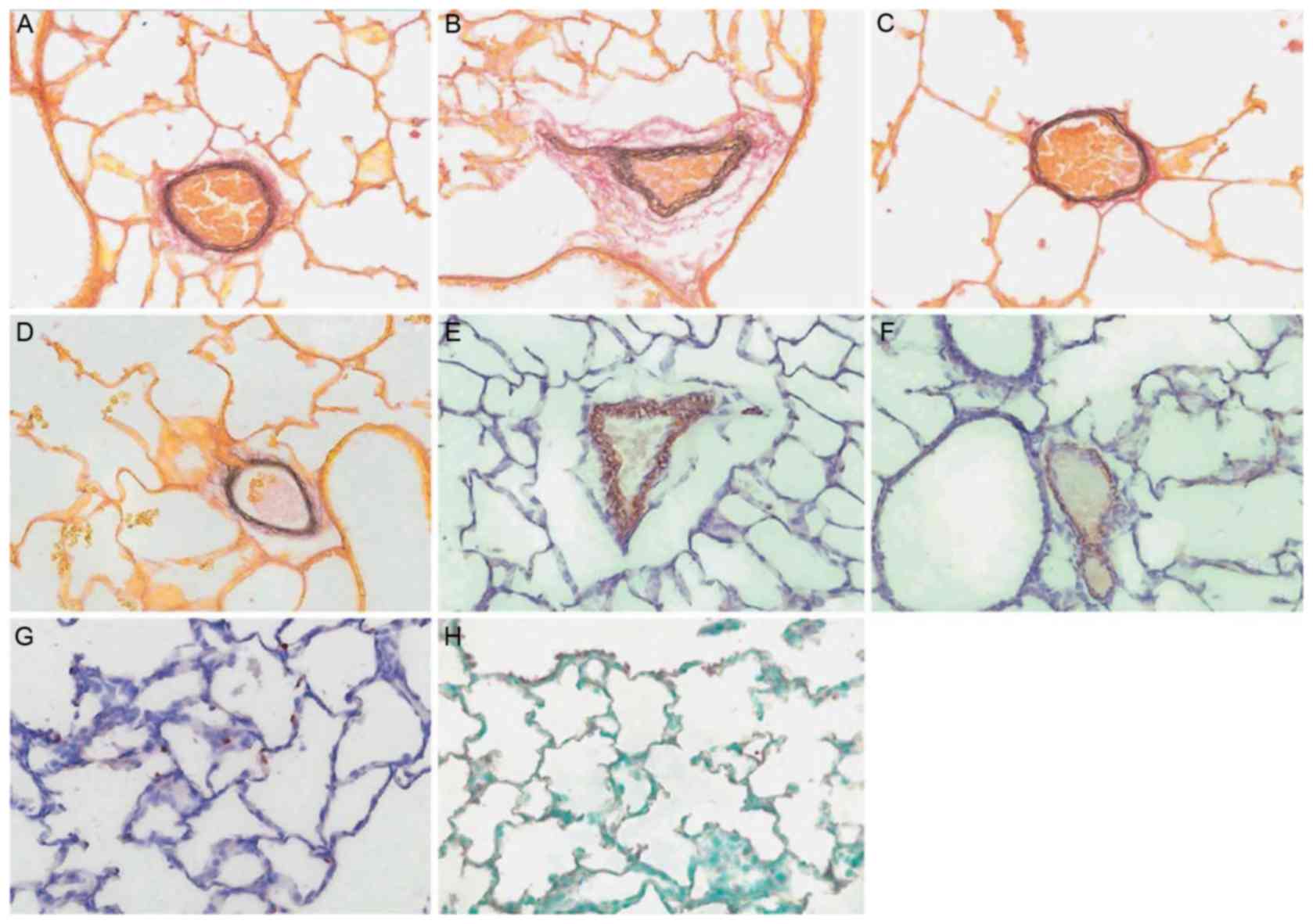
Effect of baicalin on the expressions
of Ki-67 and SPB
Treatment with baicalin was demonstrated to markedly
enhance the expression of Ki-67 in lungs compared with control
groups. However there was no significant difference observed
between the expression of SPB in the lungs of the baicalin treated
and control groups (Table II). As
presented in Fig. 4, no marked
differences in SPB was observed between any groups and Ki-67 stain
shows positive nuclear stain in brown (Fig. 4G). A representative stain of SPB with
positive brown protein coating the alveolar wall is also
presented.
 | Table II.Effect of baicalin on the expressions
of Ki-67 and surfactant protein B. |
Table II.
Effect of baicalin on the expressions
of Ki-67 and surfactant protein B.
| Sr. No. | Group | Ki-67 | SPB |
|---|
| 1 | Control with DH |
0.0385±0.0012 |
2.23±0.15 |
| 2 | Control without
DH |
0.0332±0.0011 |
2.05±0.11 |
| 3 | Baicalin with DH |
0.0531±0.0021 |
2.48±0.23 |
| 4 | Baicalin without
DH |
0.0410±0.0019 |
1.92±0.09 |
Discussion
Baicalin is a naturally isolated glycoside that is
reported to increase fetal lung surfactant phospholipids and
thereby enhance lung growth in fetal development (12). Furthermore, it also exhibits
anti-inflammatory, antiviral, anticancer and analgesic effects, as
documented previously (15–16). Baicalin significantly protects
against lipopolysaccharide induced lung injury as it exhibits
anti-inflammatory actions by blocking the communication between the
CX3CL1-CX3CR1 axis and the nuclear factor-κB signaling pathway
(17). Furthermore, it protects
against lung injury via its antiviral activity (18). Therefore, the aim of the present
study was to evaluate the effect of baicalin treatment on fetal
lung development in a rabbit model of DH. The effects of baicalin
were evaluated by determining fetal LBWR, lung morphometry analysis
and immunohistochemistry of lung tissue via α-SMA, Ki-67 and SPB
staining.
In the present study, baicalin administration in
mother rabbits induced no significant effect in the morphologic,
anatomic and proliferation indices following surgically-induced DH.
A recent study suggested that DH was associated with significant
loss of body weight of mother and fetus, increased fetal mortality
rates (19). The findings of the
present study demonstrated that maternal and fetal weight loss and
mortality was higher. However LBWR was demonstrated to be
significantly decreased in the baicalin-treated DH group compared
with the control + DH group.
In a previous study, baicalin treatment was
demonstrated to improve lung development in fetal rats by
increasing pulmonary surfactant phospholipids (12). Baicalin was also reported to improve
the level of growth hormone in the mother, which may have also
improved fetal lung development (12). However, a previous study on DH
demonstrated that ghrelin, which is a physiologic growth hormone,
significantly improved lung development in neonates (20). However, the failure to demonstrate an
improvement in airway morphometry may be due to differences in the
model, particularly in the stages of lung development. Lung
hypoplasia in the nitrofen rat model represents an embryonic defect
in lung development, and this animal is also born in the late
canalicular or early saccular phase (20). Conversely, rabbits are in the late
pseudoglandular phase at the time of surgically-induced DH, yet the
final three stages of lung development occur rapidly in
utero. Transplacental treatment in the rat model was
administered in the mid-pseudoglandular or early saccular phase of
lung development, whereas in the rabbit it occurred in the early
canalicular and alveolar phase (21). Regardless, clinical treatment may
only be initiated following the time of diagnosis, which is
typically following 20 weeks' gestation, hence in the canalicular
stage of lung development, thus the rabbit is a more representative
model (22).
Lung maturity of lung is recognized by the enhanced
expression of SPB (23) and the
present results did not reveal any significant change in SPB
expression in the baicalin with DH group. Furthermore, Ki-67 is a
marker of lung proliferation (17)
and no significant changes in Ki-67 expression were observed
following baicalin treatment.
The present study investigated the treatment of
defect in lung development in large animals via a natural,
non-invasive fetal therapy. Large animals like rabbit exhibit the
same pattern of lung development as humans (24). Furthermore, the present study avoided
the use of invasive therapies that are responsible for infection,
preterm labor and premature rupture of membrane. However, the
present study tested the highest-tolerated dose that is effective
in lung development by enhancing the surfactant.
In conclusion, the present study suggests that
baicalin was able to improved lung morphology in rabbits. However,
due to limited effects in airways, the present study concludes that
baicalin is not suitable for the management of DH.
References
|
1
|
Clark RH, Hardin WD Jr, Hirschl RB, Jaksic
T, Lally KP, Langham MR Jr and Wilson JM: Current surgical
management of congenital diaphragmatic hernia: A report from the
congenital diaphragmatic hernia study group. J Pediatr Surg.
33:1004–1009. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stoll C, Alembik Y, Dott B and Roth MP:
Associated malformations in cases with congenital diaphragmatic
hernia. Genet Couns. 19:331–339. 2008.PubMed/NCBI
|
|
3
|
Tsao K and Lally KP: The congenital
diaphragmatic hernia study group: A voluntary international
registry. Semin Pediatr Surg. 17:90–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verbelen T, Lerut T, Coosemans W, De Leyn
P, Nafteux P, Van Raemdonck D, Deprest J and Decaluwé H: Antireflux
surgery after congenital diaphragmatic hernia repair: A plea for a
tailored approach. Eur J Cardiothorac Surg. 44:263–268. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dekoninck P, Gratacos E, Van Mieghem T,
Richter J, Lewi P, Ancel AM, Allegaert K, Nicolaides K and Deprest
J: Results of fetal endoscopic tracheal occlusion for congenital
diaphragmatic hernia and the set up of the randomized controlled
TOTAL trial. Early Hum Dev. 87:619–624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trudinger BJ and Knight PC: Fetal age and
patterns of human fetal breathing movements. Am J Obstet Gynecol.
137:724–728. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davey MG, Danzer E, Schwarz U, Robinson L,
Shegu S, Adzick NS, Flake AW and Hedrick HL: Prenatal
glucocorticoids improve lung morphology and partially restores
surfactant mRNA expression in lambs with diaphragmatic hernia
undergoing fetal tracheal occlusion. Pediatr Pulmonol.
41:1188–1196. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewis NA, Holm BA, Rossman J, Swartz D and
Glick PL: Late administration of antenatal vitamin A promotes
pulmonary structural maturation and improves ventilation in the
lamb model of congenital diaphragmatic hernia. Pediatr Surg Int.
27:119–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li BQ, Fu T, Gong WH, Dunlop N, Kung H,
Yan Y, Kang J and Wang JM: The flavonoid baicalin exhibits
anti-inflammatory activity by binding to chemokines.
Immunopharmacology. 49:295–306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tarragó T, Kichik N, Claasen B, Prades R,
Teixidó M and Giralt E: Baicalin, a prodrug able to reach the CNS,
is a prolyl oligopeptidase inhibitor. Bioorg Med Chem.
16:7516–7524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shieh DE, Cheng HY, Yen MH, Chiang LC and
Lin CC: Baicalin-induced apoptosis is mediated by Bcl-2-dependent,
but not p53-dependent, pathway in human leukemia cell lines. Am J
Chin Med. 34:245–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CM, Wang LF and Cheng KT: Maternal
baicalin treatment increases fetal lung surfactant phospholipids in
rats. Evid Based Complement Alternat Med. 2011:4087142011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Yamamoto H, Gratacos E, Ge X,
Verbeken E, Sueishi K, Hashimoto S, Vanamo K, Lerut T and Deprest
J: Lung development following diaphragmatic hernia in the fetal
rabbit. Human Reprod. 15:2483–2488. 2000. View Article : Google Scholar
|
|
14
|
Hattori Y, Jojima T, Tomizawa A, Satoh H,
Hattori S, Kasai K and Hayashi T: A glucagon-like peptide-1 (GLP-1)
analogue, liraglutide, upregulates nitric oxide production and
exerts anti-inflammatory action in endothelial cells. Diabetologia.
53:2256–2263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
16
|
Bonham M, Posakony J, Coleman I,
Montgomery B, Simon J and Nelson PS: Characterization of chemical
constituents in Scutellaria baicalensis with antiandrogenic and
growth-inhibitory activities toward prostate carcinoma. Clin Cancer
Res. 11:3905–3914. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang KL, Chen CS, Hsu CW, Li MH, Chang H,
Tsai SH and Chu SJ: Therapeutic effects of baicalin on
lipopolysaccharide-induced acute lung injury in rats. Am J Chin
Med. 36:301–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eastwood MP, Kampmeijer A, Jimenez J, Zia
S, Vanbree R, Verbist G, Toelen J and Deprest JA: The effect of
transplacental administration of glucagon-like peptide-1 on fetal
lung development in the rabbit model of congenital diaphragmatic
hernia. Fetal Diagn Ther. 39:125–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santos M, Bastos P, Gonzaga S, Roriz JM,
Baptista MJ, Nogueira-Silva C, Melo-Rocha G, Henriques-Coelho T,
Roncon-Albuquerque R Jr, Leite-Moreira AF, et al: Ghrelin
expression in human and rat fetal lungs and the effect of ghrelin
administration in nitrofen-induced congenital diaphragmatic hernia.
Pediatr Res. 59:531–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keijzer R, Liu J, Deimling J, Tibboel D
and Post M: Dual-hit hypothesis explains pulmonary hypoplasia in
the nitrofen model of congenital diaphragmatic hernia. Am J Pathol.
156:1299–1306. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garne E, Haeusler M, Barisic I, Gjergja R,
Stoll C and Clementi M; Euroscan Study Group, : Congenital
diaphragmatic hernia: Evaluation of prenatal diagnosis in 20
European regions. Ultrasound Obstet Gynecol. 19:329–333. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Romaní-Pérez M, Outeiriño-Iglesias V,
Gil-Lozano M, González-Matías LC, Mallo F and Vigo E: Pulmonary
GLP-1 receptor increases at birth and exogenous GLP-1 receptor
agonists augmented surfactant-protein levels in litters from normal
and nitrofen-treated pregnant rats. Endocrinology. 154:1144–1155.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mendelson CR and Boggaram V: Hormonal and
developmental regulation of pulmonary surfactant synthesis in fetal
lung. Baillieres Clin Endocrinol Metab. 4:351–378. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamaruzaman NA, Kardia E, Kamaldin N',
Latahir AZ and Yahaya BH: The rabbit as a model for studying lung
disease and stem cell therapy. Biomed Res Int. 2013:6918302013.
View Article : Google Scholar : PubMed/NCBI
|