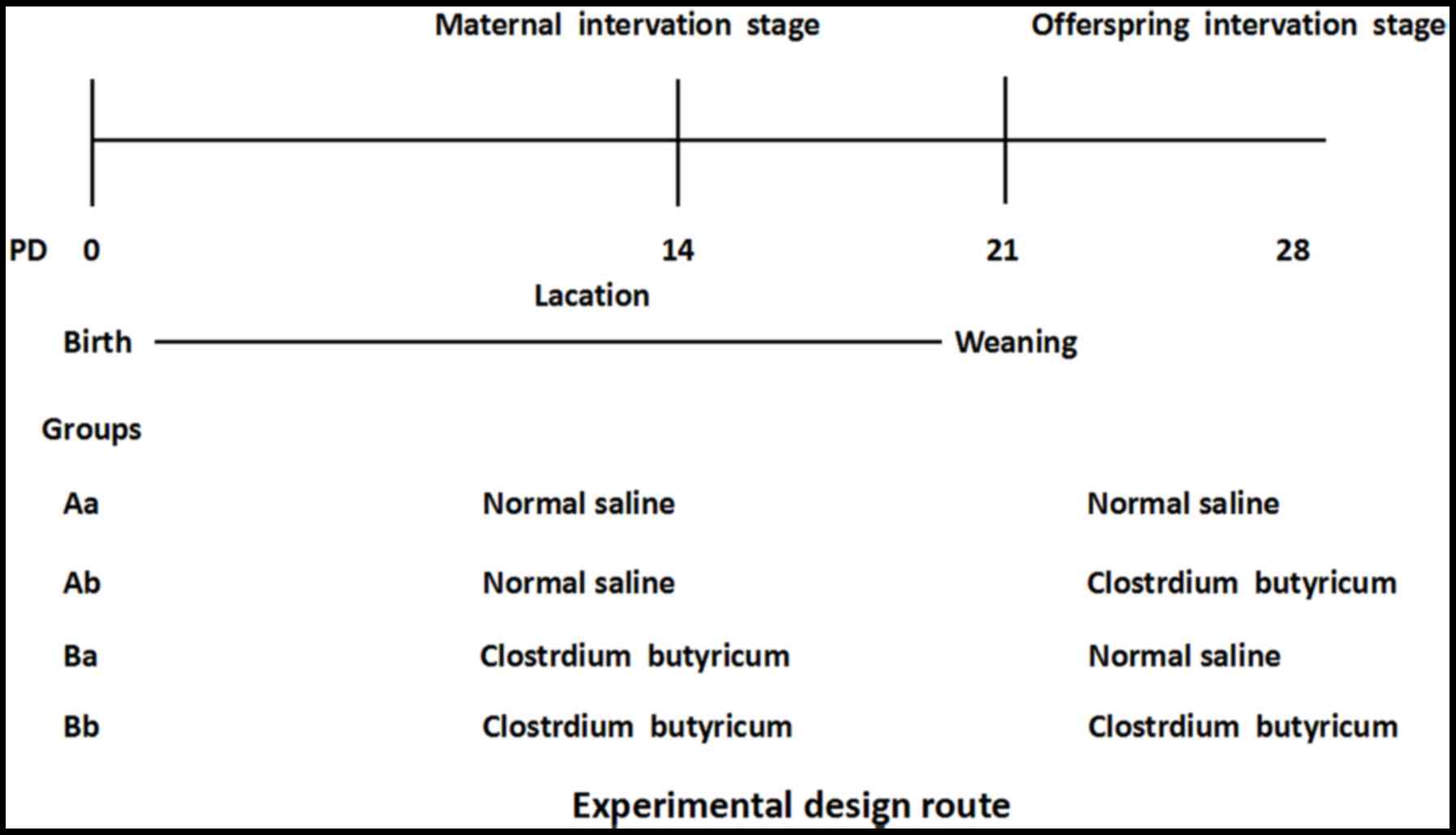
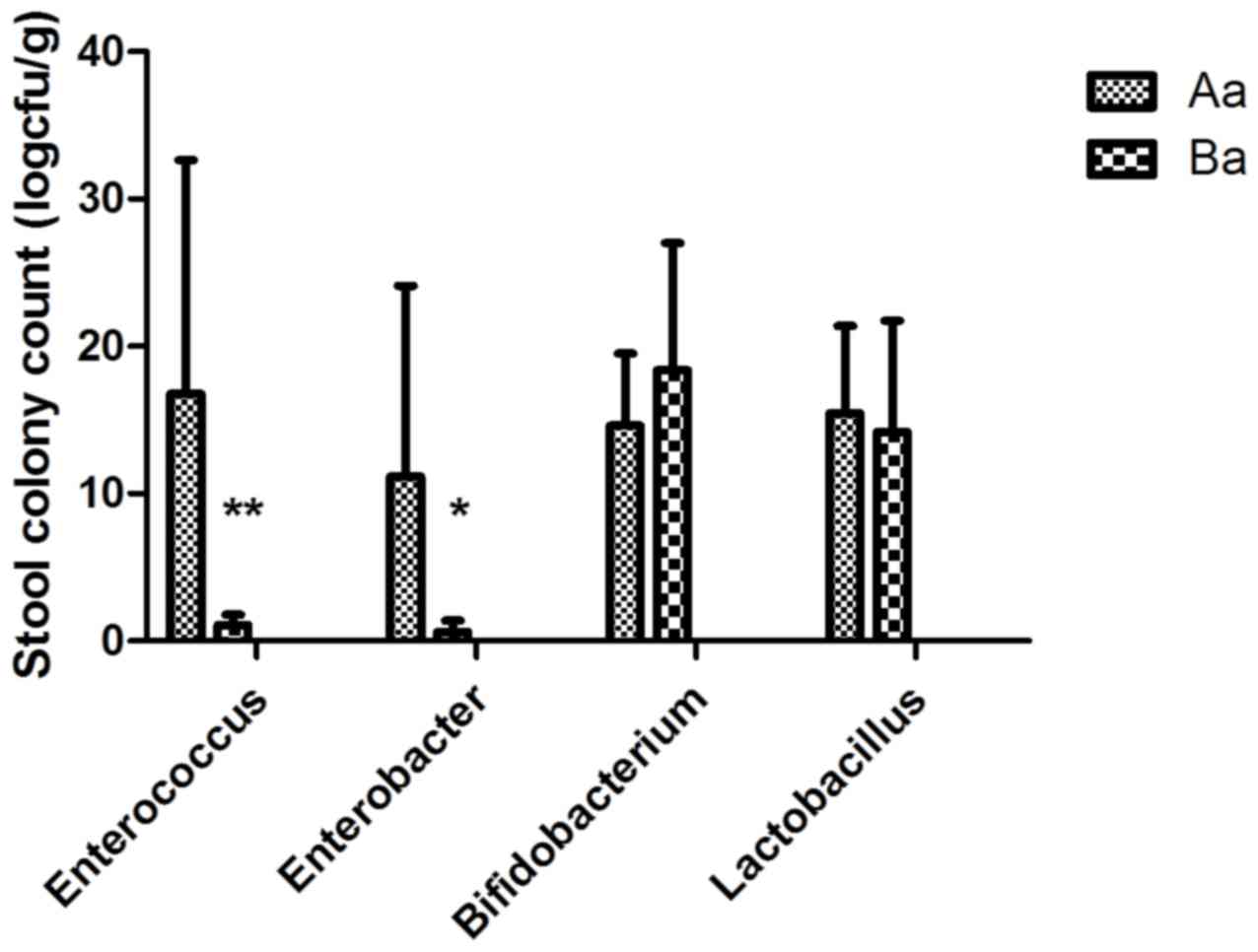
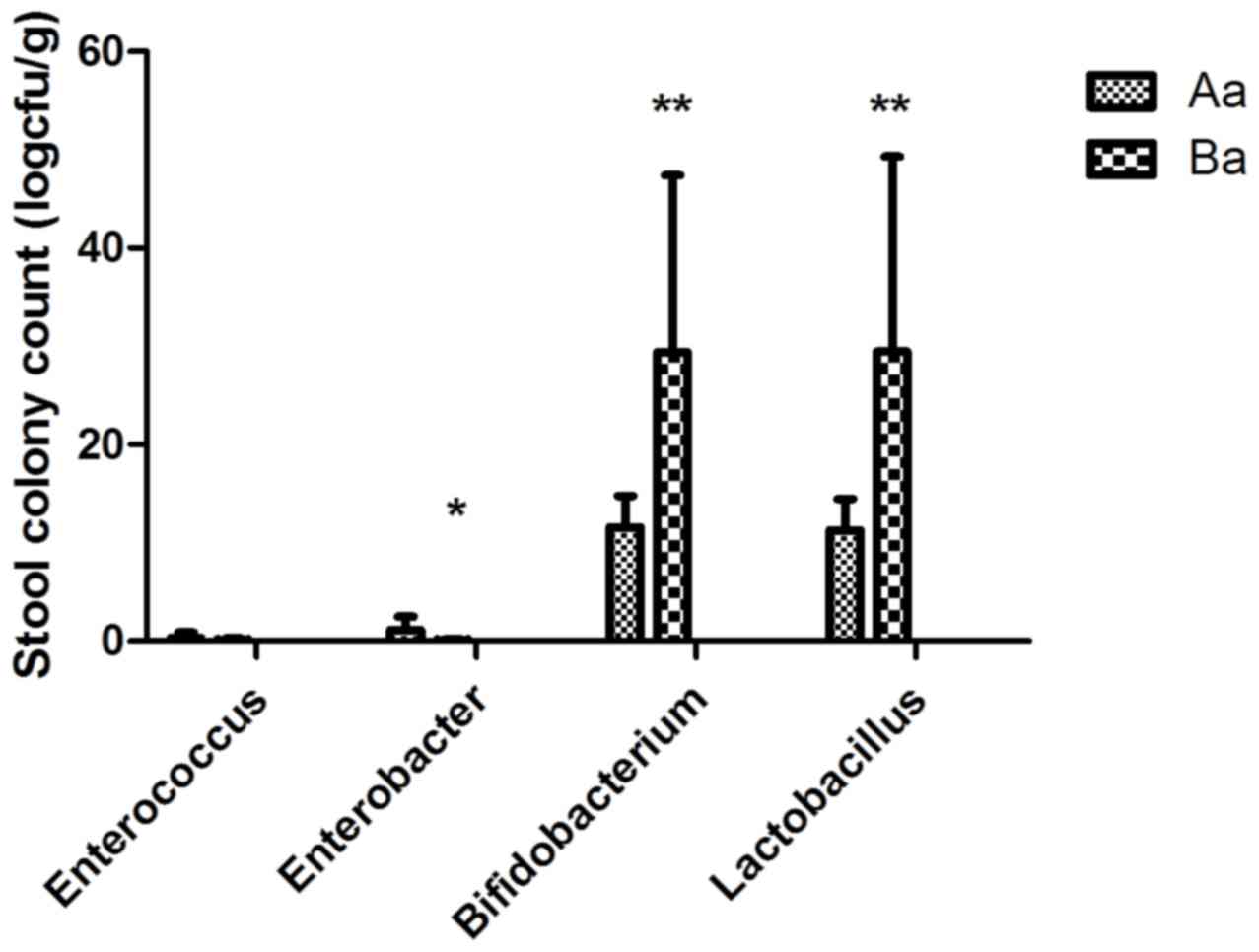
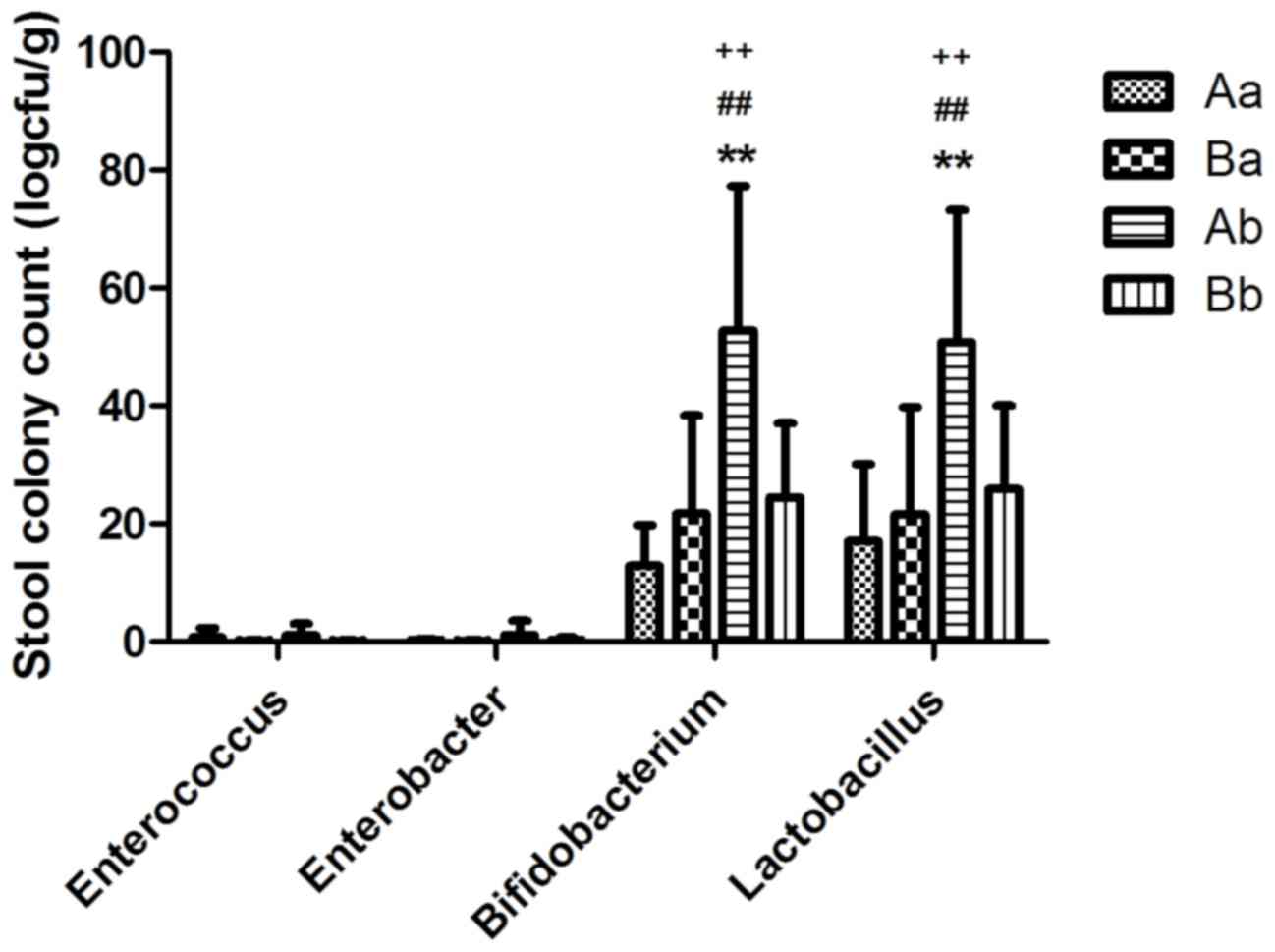
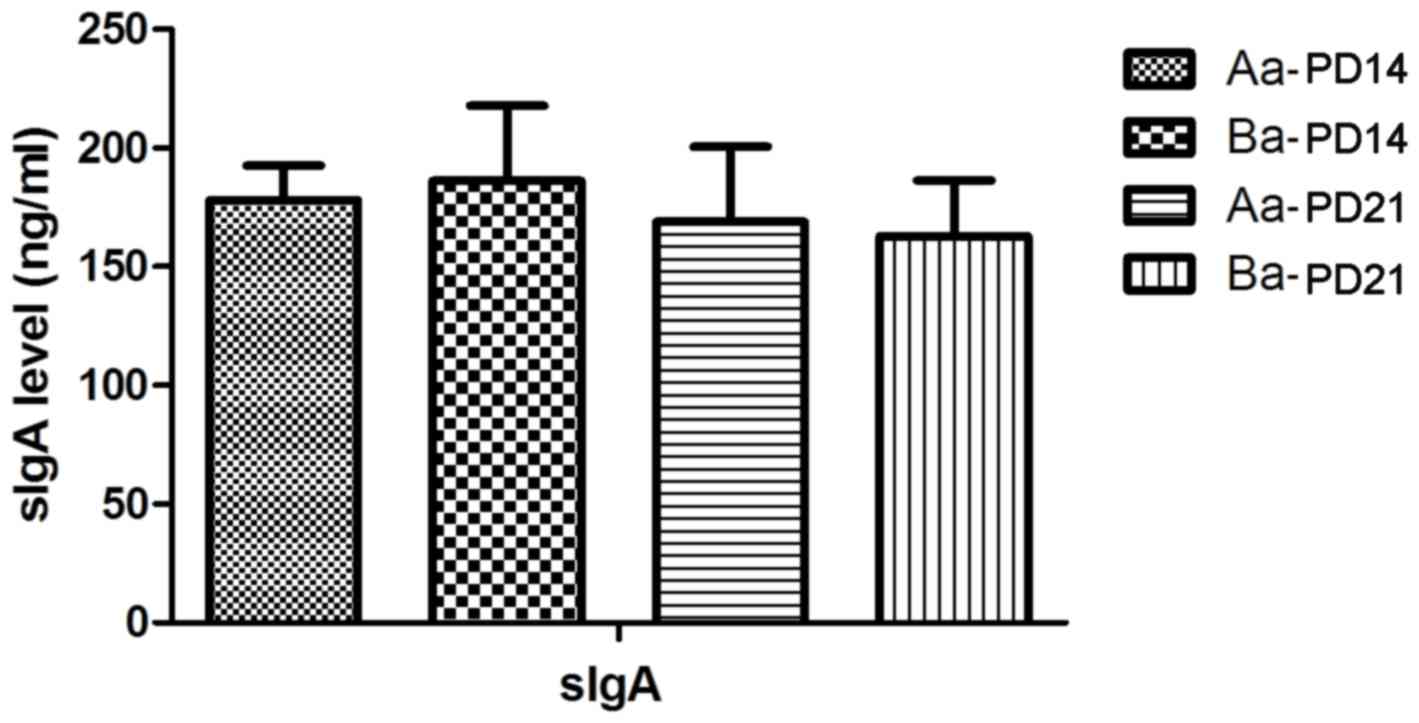
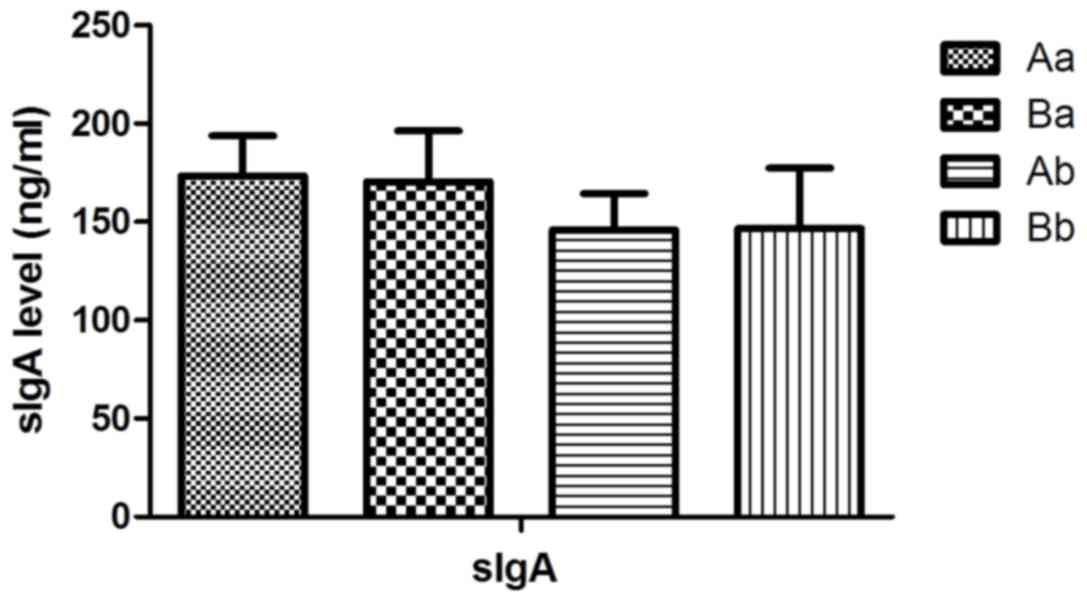
|
1
|
Schuijt TJ, van der Poll T, de Vos WM and
Wiersinga WJ: The intestinal microbiota and host immune
interactions in the critically ill. Trends Microbiol. 21:221–229.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez JM, Murphy K, Stanton C, Ross
RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, et
al: The composition of the gut microbiota throughout life, with an
emphasis on earlylife. Microb Ecol Health Dis.
26:260502015.PubMed/NCBI
|
|
3
|
Kidd P: Th1/Th2 balance: The hypothesis,
its limitations, and implications for health and disease. Altern
Med Rev. 8:223–246. 2003.PubMed/NCBI
|
|
4
|
Fujiwara D, Inoue S, Wakabayashi H and
Fujii T: The anti-allergic effects of lactic acid bacteria are
strain dependent and mediated by effects on both Th1/Th2 cytokine
expression and balance. Int Arch Allergy Immunol. 135:205–215.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vandenplas Y: Healthy gut microbiota and
long term health. Benef Microbes. 6:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bustos Fernandez LM, Lasa JS and Man F:
Intestinal microbiota: Its role in digestive diseases. J Clin
Gastroenterol. 48:657–666. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Björkstén B, Naaber P, Sepp E and
Mikelsaar M: The intestinal microflora in allergic Estonian and
Swedish 2-year-old children. Clin Exp Allergy. 29:342–346. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valsecchi C, Marseglia A, Montagna L,
Tagliacarne SC, Elli M, Licari A, Marseglia GL and Castellazzi AM:
Evaluation of the effects of a probiotic supplementation with
respect to placebo on intestinal microflora and secretory IgA
production, during antibiotic therapy, in children affected by
recurrent airway infections and skin symptoms. J Biol Regul Homeost
Agents. 28:117–124. 2014.PubMed/NCBI
|
|
9
|
Edwards CA and Parrett AM: Intestinal
flora during the first months of life: New perspectives. Br J Nutr.
88 Suppl 1:S11–S18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshimatsu Y, Yamada A, Furukawa R, Sono
K, Osamura A, Nakamura K, Aoki H, Tsuda Y, Hosoe N, Takada N and
Suzuki Y: Effectiveness of probiotic therapy for the prevention of
relapse in patients with inactive ulcerative colitis. World J
Gastroenterol. 21:5985–5994. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsai YT, Cheng PC and Pan TM: The
immunomodulatory effects of lactic acid bacteria for improf oving
immune functions and benefits. Appl Microbiol Biotechnol.
96:853–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakanishi S, Kataoka K, Kuwahara T and
Ohnishi Y: Effects of high amylose maize starch and Clostridium
butyricum on metabolism in colonic microbiota and formation of
azoxymethane-induced aberrant crypt foci in the rat colon.
Microbiol Immunol. 47:951–958. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scheppach W: Effects of short chain fatty
acids on gut morphology and function. Gut. 35 1 Suppl:S35–S38.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ichikawa H, Kuroiwa T, Inagaki A, Shineha
R, Nishihira T, Satomi S and Sakata T: Probiotic bacteria stimulate
gut epithelial cell proliferation in rat. Dig Dis Sci.
44:2119–2123. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Seki H, Shiohara M, Matsumura T, Miyagawa
N, Tanaka M, Komiyama A and Kurata S: Prevention of
antibiotic-associated diarrhea in children by Clostridium butyricum
MIYAIRI. Pediatr Int. 45:86–90. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Küster T, Zumkehr B, Hermann C, Theurillat
R, Thormann W, Gottstein B and Hemphill A: Voluntary ingestion of
antiparasitic drugs emulsified in honey represents an alternative
to gavage in mice. J Am Assoc Lab Anim Sci. 51:219–223.
2012.PubMed/NCBI
|
|
17
|
Mitsou EK, Kirtzalidou E, Oikonomou I,
Liosis G and Kyriacou A: Fecal microflora of Greek healthy
neonates. Anaerobe. 14:94–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Falk PG, Hooper LV, Midtvedt T and Gordon
JI: Creating and maintaining the gastrointestinal ecosystem: What
we know and need to know from gnotobiology. Microbiol Mol Biol Rev.
62:1157–1170. 1998.PubMed/NCBI
|
|
19
|
Purchiaroni F, Tortora A, Gabrielli M,
Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E and
Gasbarrini A: The role of intestinal microbiota and the immune
system. Eur Rev Med Pharmacol Sci. 17:323–333. 2013.PubMed/NCBI
|
|
20
|
Kaetzel CS: Cooperativity among secretory
IgA, the polymeric immunoglobulin receptor, and the gut microbiota
promotes host-microbial mutualism. Immunol Lett. 162:10–21. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Jong R, Brouwer M, Kuiper HM, Hooibrink
B, Miedema F and van Lier RA: Maturation- and
differentiation-dependent responsiveness of human CD4+ T helper
subsets. J Immunol. 149:2795–2802. 1992.PubMed/NCBI
|
|
22
|
Pessi T, Sütas Y, Hurme M and Isolauri E:
Interleukin-10 generation in atopic children following oral
Lactobacillus rhamnosus GG. Clin Exp Allergy. 30:1804–1808. 2000.
View Article : Google Scholar : PubMed/NCBI
|