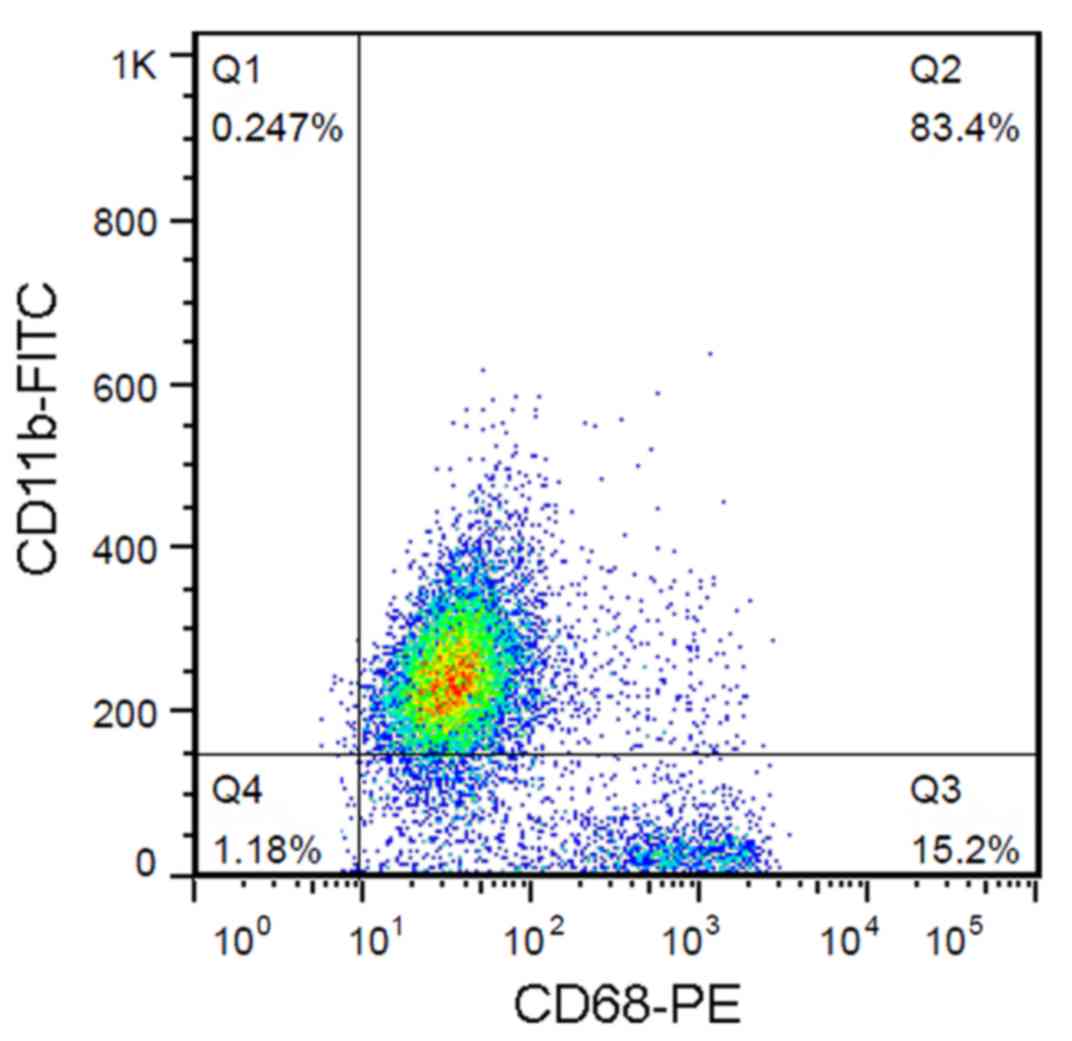
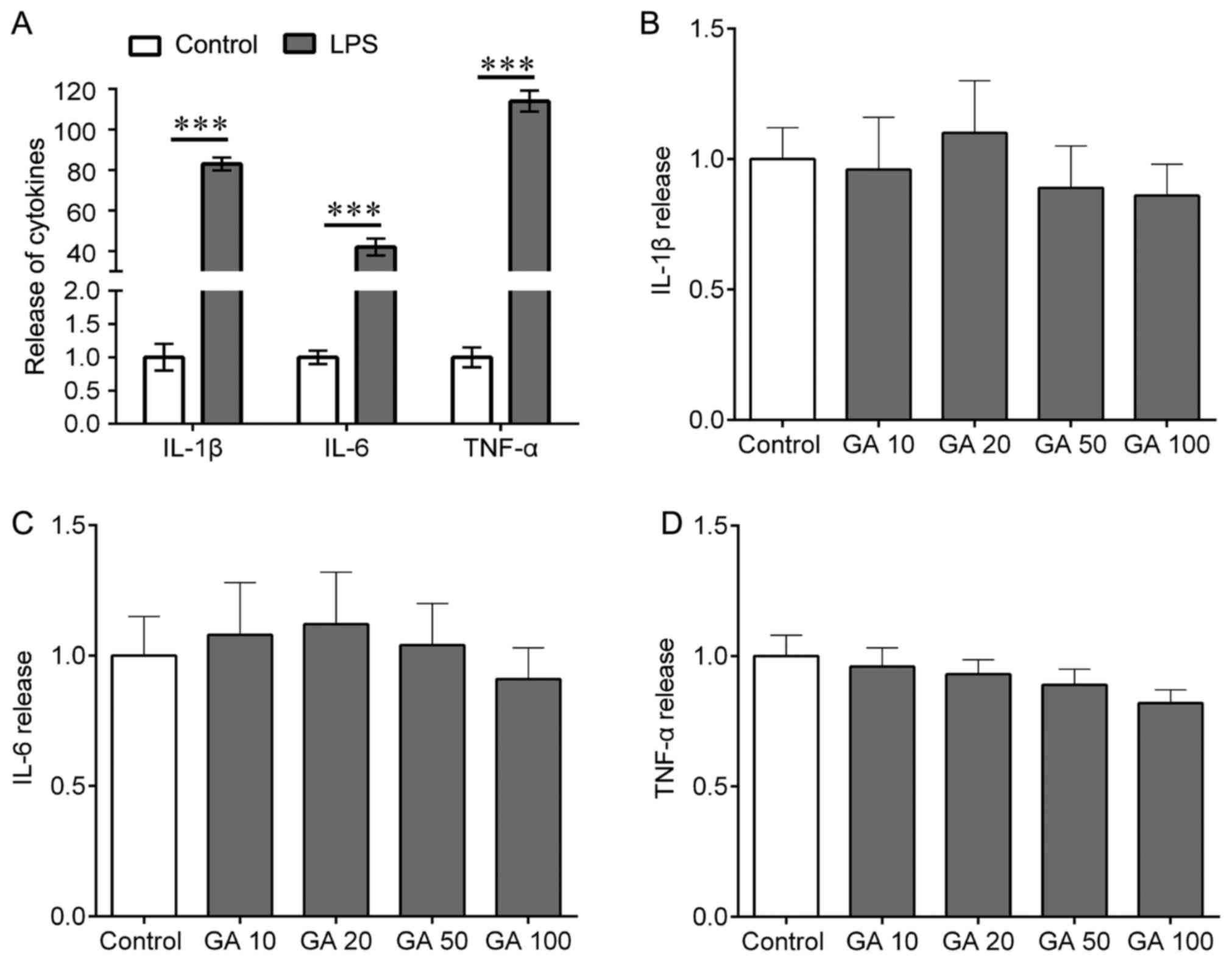
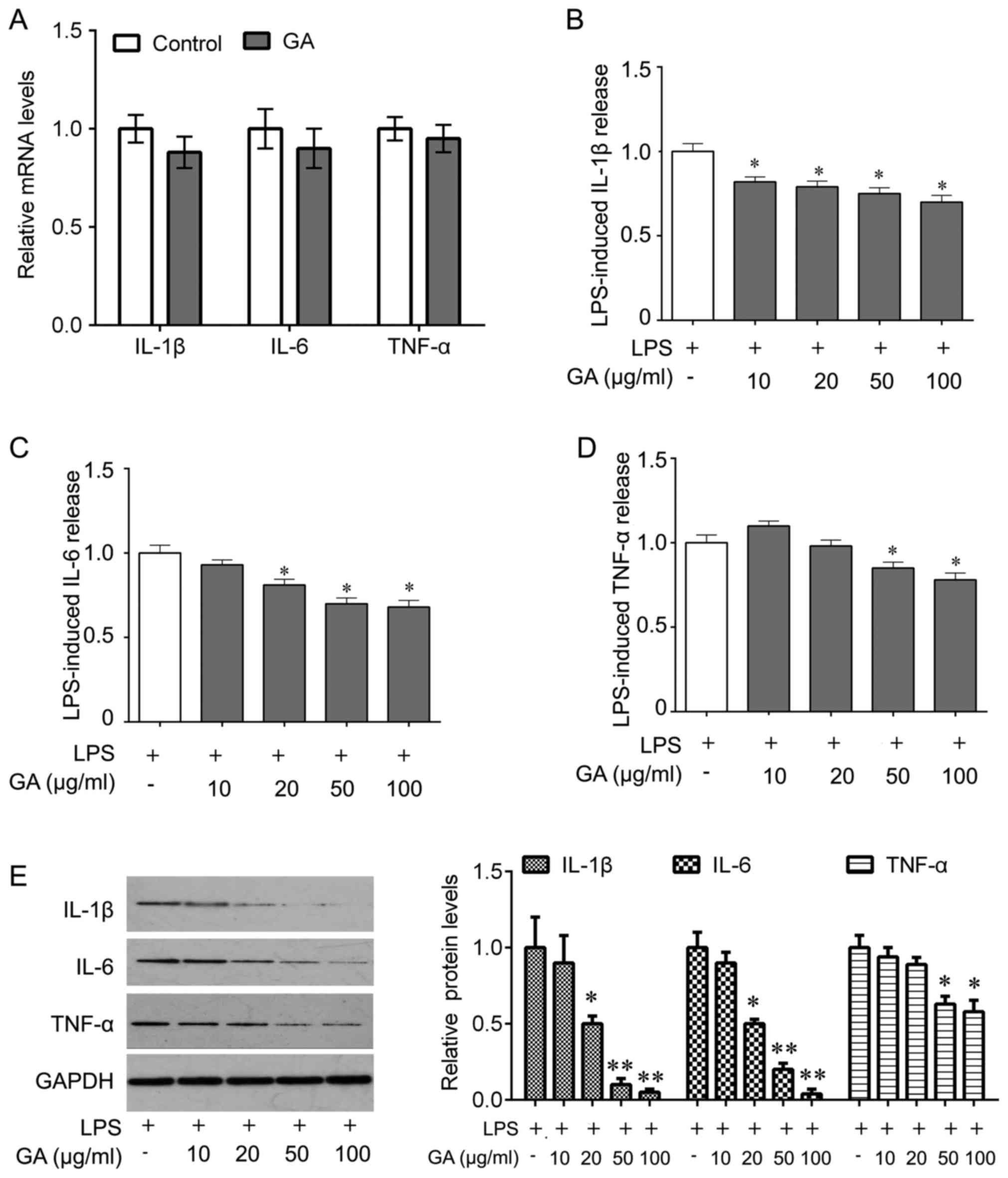
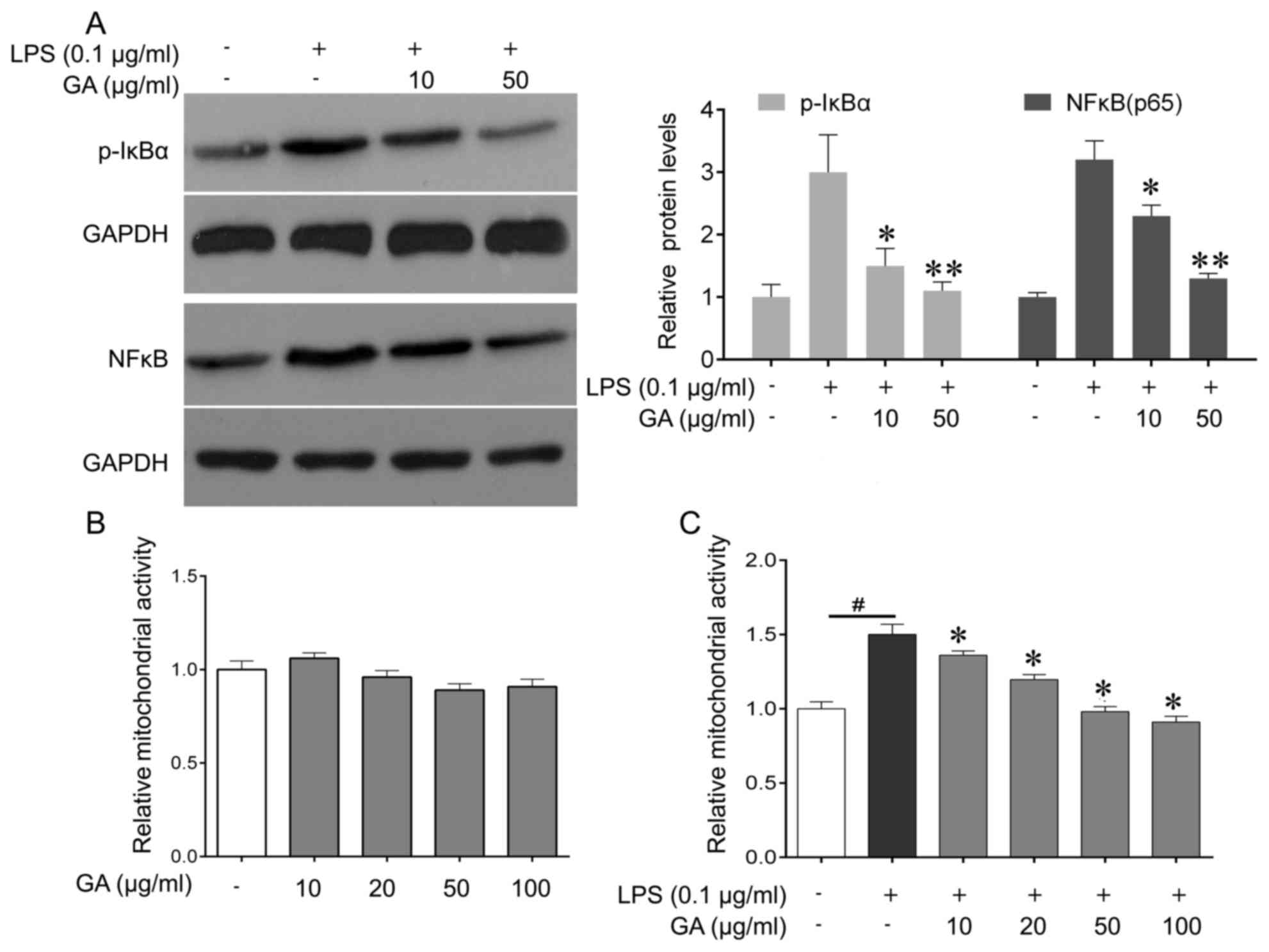
|
1
|
Bishop KS, Kao CH, Xu Y, Glucina MP,
Paterson RR and Ferguson LR: From 2000 years of Ganoderma lucidum
to recent developments in nutraceuticals. Phytochemistry.
114:56–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferreira IC, Heleno SA, Reis FS, Stojkovic
D, Queiroz MJ, Vasconcelos MH and Sokovic M: Chemical features of
Ganoderma polysaccharides with antioxidant, antitumor and
antimicrobial activities. Phytochemistry. 114:38–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dey A, Kang X, Qiu J, Du Y and Jiang J:
Anti-inflammatory small molecules to treat seizures and epilepsy:
From bench to bedside. Trends Pharmacol Sci. 37:463–484. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang B, Zou J, Han L, Rensing N and Wong
M: Microglial activation during epileptogenesis in a mouse model of
tuberous sclerosis complex. Epilepsia. 57:1317–1325. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfluger P, Viau CM, Coelho VR, Berwig NA,
Staub RB, Pereira P and Saffi J: Gamma-decanolactone inhibits iNOS
and TNF-alpha production by lipopolysaccharide-activated microglia
in N9 cells. Eur J Pharmacol. 780:38–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Avignone E, Lepleux M, Angibaud J and
Nägerl UV: Altered morphological dynamics of activated microglia
after induction of status epilepticus. J Neuroinflammation.
12:2022015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mecha M, Carrillo-Salinas FJ, Feliú A,
Mestre L and Guaza C: Microglia activation states and cannabinoid
system: Therapeutic implications. Pharmacol Ther. 166:40–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kiyomoto M, Shinoda M, Honda K, Nakaya Y,
Dezawa K, Katagiri A, Kamakura S, Inoue T and Iwata K: p38
phosphorylation in medullary microglia mediates ectopic orofacial
inflammatory pain in rats. Mol Pain. 11:482015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jebelli J, Su W, Hopkins S, Pocock J and
Garden GA: Glia: Guardians, gluttons, or guides for the maintenance
of neuronal connectivity? Ann N Y Acad Sci. 1351:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riazi K, Galic MA, Kuzmiski JB, Ho W,
Sharkey KA and Pittman QJ: Microglial activation and TNFalpha
production mediate altered CNS excitability following peripheral
inflammation. Proc Natl Acad Sci USA. 105:pp. 17151–17156. 2008;
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dambach H, Hinkerohe D, Prochnow N,
Stienen MN, Moinfar Z, Haase CG, Hufnagel A and Faustmann PM: Glia
and epilepsy: Experimental investigation of antiepileptic drugs in
an astroglia/microglia co-culture model of inflammation. Epilepsia.
55:184–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu C, Yang N, Song Y, Wang L, Zi J, Zhang
S, Dunkin D, Busse P, Weir D, Tversky J, et al: Ganoderic acid C1
isolated from the anti-asthma formula, ASHMI™ suppresses TNF-α
production by mouse macrophages and peripheral blood mononuclear
cells from asthma patients. Int Immunopharmacol. 27:224–231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu C, Dunkin D, Lai J, Song Y, Ceballos
C, Benkov K and Li XM: Anti-inflammatory effects of Ganoderma
lucidum triterpenoid in human crohn's disease associated with
downregulation of NF-κB signaling. Inflamm Bowel Dis. 21:1918–1925.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin X, Ruiz BJ, Sze DM and Chan GC:
Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane
Database Syst Rev. 4:CD0077312016.PubMed/NCBI
|
|
15
|
Gordon R, Hogan CE, Neal ML, Anantharam V,
Kanthasamy AG and Kanthasamy A: A simple magnetic separation method
for high-yield isolation of pure primary microglia. J Neurosci
Methods. 194:287–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon HM, Jang KJ, Han MS, Jeong JW, Kim
GY, Lee JH and Choi YH: Ganoderma lucidum ethanol extract inhibits
the inflammatory response by suppressing the NF-κB and toll-like
receptor pathways in lipopolysaccharide-stimulated BV2 microglial
cells. Exp Ther Med. 5:957–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rangarajan P, Karthikeyan A and Dheen ST:
Role of dietary phenols in mitigating microglia-mediated
neuroinflammation. Neuromolecular Med. 18:453–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zattoni M, Mura ML, Deprez F, Schwendener
RA, Engelhardt B, Frei K and Fritschy JM: Brain infiltration of
leukocytes contributes to the pathophysiology of temporal lobe
epilepsy. J Neurosci. 31:4037–4050. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnson AM, Sugo E, Barreto D, Hiew CC,
Lawson JA, Connolly AM, Somerville E, Hasic E, Bye AM and
Cunningham AM: The severity of gliosis in hippocampal sclerosis
correlates with pre-operative seizure burden and outcome after
temporal lobectomy. Mol Neurobiol. 53:5446–5456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vezzani A, Maroso M, Balosso S, Sanchez MA
and Bartfai T: IL-1 receptor/Toll-like receptor signaling in
infection, inflammation, stress and neurodegeneration couples
hyperexcitability and seizures. Brain Behav Immun. 25:1281–1289.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maroso M, Balosso S, Ravizza T, Liu J,
Bianchi ME and Vezzani A: Interleukin-1 type 1 receptor/Toll-like
receptor signalling in epilepsy: The importance of IL-1beta and
high-mobility group box 1. J Intern Med. 270:319–326. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Noe FM, Polascheck N, Frigerio F,
Bankstahl M, Ravizza T, Marchini S, Beltrame L, Banderó CR, Löscher
W and Vezzani A: Pharmacological blockade of IL-1β/IL-1 receptor
type 1 axis during epileptogenesis provides neuroprotection in two
rat models of temporal lobe epilepsy. Neurobiol Dis. 59:183–193.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dolga AM, Granic I, Blank T, Knaus HG,
Spiess J, Luiten PG, Eisel UL and Nijholt IM: TNF-alpha-mediates
neuroprotection against glutamate-induced excitotoxicity via
NF-kappaB-dependent up-regulation of K2.2 channels. J Neurochem.
107:1158–1167. 2008.PubMed/NCBI
|
|
25
|
Hwang JS, Jung EH, Kwon MY and Han IO:
Glioma-secreted soluble factors stimulate microglial activation:
The role of interleukin-1β and tumor necrosis factor-α. J
Neuroimmunol. 298:165–171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azab SF, Abdalhady MA, Almalky MA, Amin
EK, Sarhan DT, Elhindawy EM, Allah MA, Elhewala AA, Salam MM,
Hashem MI, et al: Serum and CSF adiponectin, leptin, and
interleukin 6 levels as adipocytokinesin Egyptian children with
febrile seizures: A cross-sectional study. Ital J Pediatr.
42:382016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lei HY, Yang DQ, Li YX, Wang LQ and Zheng
M: Association between human cytomegalovirus and onset of epilepsy.
Int J Clin Exp Med. 8:20556–20564. 2015.PubMed/NCBI
|
|
28
|
Galic MA, Riazi K, Heida JG, Mouihate A,
Fournier NM, Spencer SJ, Kalynchuk LE, Teskey GC and Pittman QJ:
Postnatal inflammation increases seizure susceptibility in adult
rats. J Neurosci. 28:6904–6913. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodgers KM, Hutchinson MR, Northcutt A,
Maier SF, Watkins LR and Barth DS: The cortical innate immune
response increases local neuronal excitability leading to seizures.
Brain. 132:2478–2486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Han X, Bao J, Liu Y, Ye A, Thakur M
and Liu H: Nicotine increases eclampsia-like seizure threshold and
attenuates microglial activity in rat hippocampus through the α7
nicotinic acetylcholine receptor. Brain Res. 1642:487–496. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kosonowska E, Janeczko K and Setkowicz Z:
Inflammation induced at different developmental stages affects
differently the range of microglial reactivity and the course of
seizures evoked in the adult rat. Epilepsy Behav. 49:66–70. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang F, Liu J and Shi JS:
Anti-inflammatory activities of resveratrol in the brain: Role of
resveratrol in microglial activation. Eur J Pharmacol. 636:1–7.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cattepoel S, Schaub A, Ender M, Gaida A,
Kropf A, Guggisberg U, Nolte MW, Fabri L, Adlard PA, Finkelstein
DI, et al: Intravenous immunglobulin binds beta amyloid and
modifies its aggregation, neurotoxicity and microglial phagocytosis
in vitro. PLoS One. 8:e631622013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Novak P, Williams A, Ravin P, Zurkiya O,
Abduljalil A and Novak V: Treatment of multiple system atrophy
using intravenous immunoglobulin. BMC Neurol. 12:1312012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Janke AD and Yong VW: Impact of IVIg on
the interaction between activated T cells and microglia. Neurol
Res. 28:270–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akihisa T, Nakamura Y, Tagata M, Tokuda H,
Yasukawa K, Uchiyama E, Suzuki T and Kimura Y: Anti-inflammatory
and anti-tumor-promoting effects of triterpene acids and sterols
from the fungus Ganoderma lucidum. Chem Biodivers. 4:224–231. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dudhgaonkar S, Thyagarajan A and Sliva D:
Suppression of the inflammatory response by triterpenes isolated
from the mushroom Ganoderma lucidum. Int Immunopharmacol.
9:1272–1280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang G, Zhao J, Liu J, Huang Y, Zhong JJ
and Tang W: Enhancement of IL-2 and IFN-gamma expression and NK
cells activity involved in the anti-tumor effect of ganoderic acid
Me in vivo. Int Immunopharmacol. 7:864–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rummel C: Inflammatory transcription
factors as activation markers and functional readouts in
immune-to-brain communication. Brain BehavImmun. 54:1–14. 2016.
|
|
40
|
Lin TY and Hsu HY: Ling Zhi-8 reduces lung
cancer mobility and metastasis through disruption of focal adhesion
and induction of MDM2-mediated Slug degradation. Cancer Lett.
375:340–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma B, Yu J, Xie C, Sun L, Lin S, Ding J,
Luo J and Cai H: Toll-like receptors promote mitochondrial
translocation of nuclear transcription factor nuclear factor of
activated T-cells in prolonged microglial activation. J Neurosci.
35:10799–10814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen H, Wang C, Wei X, Ding X and Ying W:
Malate-aspartate shuttle inhibitor aminooxyacetate acid induces
apoptosis and impairs energy metabolism of both resting microglia
and LPS-activated microglia. Neurochem Res. 40:1311–1318. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang W, Liu JW, Zhao WM, Wei DZ and Zhong
JJ: Ganoderic acid T from Ganoderma lucidum mycelia induces
mitochondria mediated apoptosis in lung cancer cells. Life Sci.
80:205–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen LW, Horng LY, Wu CL, Sung HC and Wu
RT: Activating mitochondrial regulator PGC-1α expression by
astrocytic NGF is a therapeutic strategy for Huntington's disease.
Neuropharmacology. 63:719–732. 2012. View Article : Google Scholar : PubMed/NCBI
|