Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare
acquired clonal disorder caused by a somatic mutation in the
phosphatidylinositol N-acetylglucosaminyltransferase subunit A gene
of multipotent hematopoietic stem cells (1). This leads to defects in the
biosynthesis of glycosylphosphatidylinositol (GPI) and GPI-linked
proteins, including complement decay-accelerating factor and the
cluster of differentiation (CD)59, which are particularly sensitive
to complement regulation (1–3). As a consequence, the absence of
GPI-linked proteins induces intravascular hemolysis, bone marrow
failure and life-threatening venous thrombosis (4–6).
Thrombosis, which can occur in veins and arteries, is the most
frequent complication of PNH and has a high mortality rate. Venous
thrombosis can occur in the majority of organs, including the
liver, lung, brain, kidney, spleen and bowel (7). A previous study demonstrated that a
number of factors contributed to thrombosis in patients with PNH,
including free hemoglobin, nitric oxide (NO) depletion, damaged
endothelial cells, deregulation of the fibrinolytic system and
platelet activation (8). However,
the mechanism of thrombosis in patients with PNH is complex and
remains unclear.
The glycoprotein CD177 antigen (NB1) is a GPI-linked
protein that belongs to the lymphocyte antigen 6 superfamily, which
also includes CD59 (9,10). NB1 was first reported in isoimmune
neonatal neutropenia (11), but was
later identified in transfusion-related acute lung injury,
myeloproliferative neoplasms, gastric cancer and Wegener's
granulomatosis associated with vasculitis (12–16). NB1
binds to platelet endothelial cell adhesion molecule to promote
neutrophil migration and is involved in the inflammatory response
(17,18). Proteinase 3 (PR3) is a
neutrophil-derived serine proteinase that is primarily stored in
azurophil granules in polymorphonuclear leukocytes. PR3 degrades a
variety of matrix proteins, including fibronectin, laminin,
vitronectin and collagen type IV (19), and regulates platelet activation
through the cleavage and inactivation of proteinase-activated
receptor 1 (PAR1), which is expressed on plasma membranes and is
associated with NB1 (20,21). The present study aimed to investigate
the expression of PR3 and NB1 in neutrophils, and explore the
association between PR3 and thrombosis in patients with PNH or
PNH-aplastic anemia (AA) syndrome.
Patients and methods
Patients
A total of 21 patients with classical PNH, 6
patients with PNH-AA syndrome and 25 healthy controls were enrolled
in the present study. The patients with PNH and PNH-AA syndrome
consisted of 14 males and 13 females, with median age of 29 years
old (range, 21–43 years). All patients were recruited from the
Department of Hematology of Tianjin Medical University General
Hospital (Tianjin, China) between November 2014 and February 2016,
and diagnosed according to the criteria for PNH set out by the
Chinese Medical Association (22).
Table I presents the
clinicopathological characteristics of the patients included in the
present study. There were 7 newly diagnosed patients (6 with PNH
and 1 with PNH-AA). All patients exhibited the typical clinical
manifestations of PNH and an abnormal expression of CD59
(CD59− granulocytes were >10% of total granulocytes),
as detected by flow cytometry. Thrombosis was investigated by
spiral computed tomography, magnetic resonance imaging or Doppler
ultrasound wherever appropriate. A total of 2 patients had cerebral
embolisms and 3 patients had portal vein thrombosis, in which 1
patient also had lower limb venous thrombosis.
 | Table I.Clinicopathological characteristics
of the patients included in the present study. |
Table I.
Clinicopathological characteristics
of the patients included in the present study.
| Patient no. | Age (years) | Gender | Diagnosis | PNH clone
granulocytes (%) | WBC
(×109/1) | Hb (g/1) | PLT
(×109/1) | RET (%) |
|---|
| 1 | 38 | F | PNH-AA | 19.33 |
5.96 | 106 | 113 |
5.15 |
| 2 | 43 | F | PNH-AA | 30.23 |
3.56 | 98 | 110 |
6.12 |
| 3 | 21 | F | PNH-AA | 20.83 |
3.30 | 87 | 80 |
5.40 |
|
4b | 55 | M | PNH-AA | 33.43 |
2.70 | 70 | 254 |
6.01 |
| 5 | 26 | M | PNH | 50.68 |
4.58 | 133 | 80 |
0.80 |
| 6 | 13 | F | PNH | 88.84 |
3.34 | 91 | 42 |
7.84 |
| 7 | 33 | F | PNH | 63.21 |
7.27 | 81 | 223 |
9.16 |
|
8a,b | 25 | F | PNH | 85.46 |
4.73 | 76 | 71 |
7.88 |
| 9 | 25 | F | PNH | 78.11 |
2.88 | 83 | 92 |
6.70 |
| 10a,b | 24 | F | PNH | 97.98 |
1.34 | 67 | 36 |
4.95 |
| 11a | 58 | M | PNH | 86.75 |
3.62 | 114 | 38 |
3.92 |
| 12 | 50 | F | PNH | 45.54 |
4.20 | 85 | 123 |
5.70 |
| 13 | 46 | F | PNH | 62.32 |
5.50 | 87 | 219 |
4.20 |
| 14 | 24 | F | PNH | 57.88 |
3.90 | 84 | 111 |
3.80 |
| 15a,b | 20 | M | PNH | 92.57 |
3.91 | 76 | 51 |
3.37 |
| 16 | 46 | M | PNH-AA | 31.23 |
3.55 | 111 | 75 |
5.35 |
| 17a,b | 27 | F | PNH | 59.41 |
1.40 | 50 | 28 |
4.90 |
| 18 | 11 | M | PNH | 55.60 |
6.10 | 78 | 102 |
5.10 |
| 19 | 27 | M | PNH | 66.38 | 19.34 | 110 | 68 |
4.26 |
| 20b | 29 | M | PNH | 93.60 |
5.90 | 61 | 78 |
8.50 |
| 21 | 15 | M | PNH-AA | 10.98 |
4.80 | 108 | 310 |
1.10 |
| 22b | 43 | M | PNH | 56.37 | 10.59 | 63 | 56 |
8.36 |
| 23 | 33 | M | PNH | 80.64 | 10.32 | 89 | 92 |
7.76 |
| 24 | 16 | F | PNH | 92.00 |
7.69 | 98 | 158 | 10.30 |
| 25 | 22 | M | PNH | 50.34 |
7.10 | 79 | 88 |
5.89 |
| 26 | 45 | M | PNH | 87.11 |
5.60 | 87 | 77 |
5.21 |
| 27 | 32 | M | PNH | 98.20 |
6.10 | 91 | 67 |
8.90 |
All patients were treated with corticosteroids
(prednisone 0.5 mg/kg/day, oral administration; Zhejiang Xianju
Pharmaceutical Co., Ltd., Zhejiang, China) and vitamin E (300
mg/day, oral administration; Hebei Tiancheng Pharmaceutical Co.,
Ltd., Hebei, China) if they exhibited hemolysis. Patients with
PNH-AA also received cyclosporine (3 mg/kg/day, oral
administration; Huabei Pharmaceutical Co., Ltd., Huabei, China) for
≥6 months. A total of 5 patients with thrombosis were treated with
low molecular weight heparin (0.1 ml/10 kg/day for 7–14 days,
subcutaneous injection; Qilu Pharmaceutical Co., Ltd., Qilu, China)
and warfarin (2.5–5 mg/day for 6–12 months, oral administration;
Qilu Pharmaceutical Co., Ltd.).
The healthy controls consisted of 15 healthy donors
and 10 patients with iron deficiency anemia, 13 males and 12
females, with a median age of 31 years (range, 27–58 years). The
present study was approved by the Ethical Committee of Tianjin
Medical University (Tianjin, China). Written informed consent was
obtained from the patients for the publication of the present
study.
Flow cytometry
Fresh peripheral blood (100 µl) was collected in
EDTA-anticoagulation tubes and incubated with 20 µl of antibodies
directed against CD59 [conjugated with phycoerythrin (PE); 1:5; cat
no. 555764; BD Pharmingen; BD Biosciences, San Diego, CA, USA], NB1
(conjugated with allophycocyanin; 1:5; cat no. ab77230) and PR3
[conjugated with fluorescein isothiocyanate (FITC); 1:5; cat no.
ab65255; both Abcam, Cambridge, MA, USA]. Their isotype control
antibodies (1:5; BD Biosciences) were used as the negative
controls. Following incubation in the dark for 30 min at room
temperature, red blood cells were lyzed with 10 ml erythrocytolysin
solution (BD Biosciences) and then centrifuged at room temperature
at 150 × g for 5 min. The cells were then washed twice with PBS.
Finally, the cells were resuspended in 300 ul PBS. Flow cytometry
was performed using a BD FACSCalibur™ Flow Cytometer (BD
Biosciences) and ≥20,000 events were acquired for each sample. All
results were analyzed using CellQuest™ Pro Software
4.0.2 (BD Biosciences).
ELISA
The levels of PR3, NB1 and PAR1 in the serum were
measured by ELISA. Briefly, 100 µl of diluted (1:100) capture
antibodies directed against PR3 (Human proteinase-antineutrophil
cytoplasmic antibody; PR3-ANCA ELISA kit; cat no. fk1344Y; R&D
Systems, Inc, Minneapolis, MN, USA), NB1 (CD177 ELISA kit; cat no.
EH1752; Cusabio Biotech Co., Ltd., Wuhan, China) or PAR1 (Human
Protease Activated Receptor 1 ELISA kit; cat no. SEC939Hu;
Cloud-Clone Corp., Katy, TX, USA) were added to each well and the
plates were incubated at 4°C overnight. The plates were washed
three times, then 200 µl assay diluent was added to each well, and
the plates were incubated for 1 h at room temperature. The plates
were then washed three times, and diluted standards and the sera
(100 µl) of the patients and controls were added to the wells in
duplicate, after which the plates were incubated for 2 h at room
temperature. Following another wash, 100 µl of a diluted (20 ng/ml)
working detector was added to each well and the plates were
incubated for 1 h at room temperature. The wells were washed seven
times. Then, 3,3′,5,5′-tetramethylbenzidine 1:100 substrate
solution was added to each well and the samples were incubated in
the dark at room temperature for 30 min. Stop solution was added
and the optical density at 450 nm was measured within 30 min using
a VersaMax™ ELISA Microplate Reader (Molecular Devices,
LLC, Sunnyvale, CA, USA).
Neutrophil isolation
Neutrophils were isolated from the peripheral blood
of patients with PNH/PNH-AA and healthy controls. Briefly, 5 ml
peripheral blood was collected into a tube containing 2 mM EDTA.
Then, the blood was layered over a Ficoll Paque Plus solution (cat
no. 17-1440-02; GE Healthcare, Chicago, IL, USA) and centrifuged at
room temperature for 20 min at 700 × g according to the
manufacturer's protocol. Neutrophils were isolated from the buffy
coat layer and washed with PBS without calcium or magnesium. The
cells were then washed twice with PBS and centrifuged at room
temperature at 200 × g for 10 min. If the neutrophil solution mixed
with red blood cells, Red Blood Cell Lysis Buffer (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) was used to lyse the red blood
cells before the neutrophils were quantified.
PNH clone sorting by
magnetic-activated cell sorting (MACS)
In 90 µl autoMACS Running buffer (Miltenyi Biotec
GmbH, Bergisch Gladbach, Germany) 10,000,000 cells were
resuspended, according to manufacturer's protocol. Then, 20 µl
CD59-PE and 20 µl PE MicroBeads (Miltenyi Biotec GmbH) were added
and the cells were incubated at 4°C in the dark for 20 min.
Following a wash with 2 ml of the buffer, the cells were
centrifuged at room temperature at 300 × g for 5 min. The cells
were then resuspended in 500 µl of the buffer. The LD column was
placed in the magnetic field of a suitable Quadro MACS separator
(both Miltenyi Biotec GmbH). Following the preparation of the
column by rinsing it with 2 ml of the buffer, the cells were
applied to the column. The flow through containing unlabeled cells
was collected. Finally, the column was washed with 2 ml of the
buffer. The purity of the PNH clone (CD59− cells) was
detected by flow cytometry.
Immunofluorescence (IF)
The sorted PNH cells were collected for smear. The
cells were smeared on a coverslip and then the smear was rinsed
with PBS three times (5 min each) and blocked with 1% BSA
(Sigma-Aldrich; Merck KGaA) at room temperature for 30 min. Then
the coverslips were incubated with anti-PR3 (1:50; cat no. ab65255)
and anti-NB1 (1:50; cat no. ab26013; both Abcam) antibodies
conjugated with FITC at 4°C overnight and washed with PBS three
times. Then, the coverslips were stained at room temperature with
hematoxylin for 1 min and rinsed with ammonium hydroxide. Following
the coverslips being washed two times with PBS (3 min each), the
coverslips were blocked for 3 days at room temperature, with
glycerin and viewed under an oil immersion lens (magnification,
×1000; Olympus Corporation, Tokyo, Japan).
Isolation of total RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA was extracted from the neutrophils using
TRIzol™ reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). From the purified RNA, 1 µg was used for
the RT-qPCR analysis using the SuperScript™ First-Strand
Synthesis system for RT-PCR (Invitrogen; Thermo Fisher Scientific,
Inc.). The RT-qPCR was performed using SYBR® Premix
Ex Taq™ (Tli RNaseH Plus), ROX plus and the Thermal
Cycler Dice Real Time system (both Takara Bio, Inc., Otsu, Japan)
in a 96-well plate according to the manufacturer's protocol. The
amplification utilized 45 cycles at 95°C for 30 sec and 56.7°C for
30 sec, with the extension at 72°C for 30 sec. The primers used for
the RT-qPCR were as follows: PR3 forward (F), 5′-ACG CGG AGA ACA
AAC TGA AC-3 and reverse (R), 5′-AGG GAC GAA AGT GCA AAT GT-3; and
NB1 F, 5′-GCAGAGACTTCAGGGTGCTC-3′ and R,
5′-CGACACATTTCTAACGACACG-3. Human β-actin was used as a
housekeeping gene for quantity normalization with the following
primer sequences: F, 5′-CTGGAACGGTGAAGGTGACA-3 and R,
5′-AAGGGACTTCCTGTAACAATGCA-3′. The PR3 and NB1 levels were
calculated using the 2−ΔΔCq method (23) following normalization of the
data.
Western blotting
Isolated neutrophils were lysed in RIPA buffer
(R&D Systems, Inc., Minneapolis, MN, USA) supplemented with
complete protease inhibitor and phosphatase inhibitors (both Roche
Diagnostics, Basel, Switzerland). Protein levels were detected
using bicinchoninic acid assay kit (Thermo Fisher Scientific,
Inc.). A total 40 µg protein/lane were separated by SDS-PAGE on a
12% gel and transferred to nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% milk (BD
Biosciences) for 1 h at room temperature, followed by incubation
with primary antibodies, anti-PR3 (1:50; cat no. ab65255, Abcam)
and GAPDH (1:1,000; Anti-GAPDH Monoclonal Antibody; cat no. A01020;
Abbkine Scientific Co., Ltd., Wuhan, China) at 4°C overnight. The
membranes were washed with Tris-buffered saline with Tween-20 (20
mM Tris-HCl buffer, pH=7.4, containing 150 mM NaCl and 0.05%
Tween-20) three times and then incubated with secondary antibodies
horseradish peroxidase-labeled goat anti-mouse Immunoglobulin G
(1:2,500; cat no. ab6789; Abcam) at room temperature for 2 h. The
reaction was detected with Super ECL Plus Detection Reagent (Thermo
Fisher Scientific, Inc.). Protein levels were normalized to
GAPDH.
Statistical analysis
Results for each group are expressed as the median
(serum level of D-Dimer) or mean ± standard error of the mean (PR3,
NB1 and PAR1 levels). Statistical analysis was performed using
one-way analysis of variance followed by a Dunnett's post hoc test.
The t-test was performed for two groups. Correlations between
different percentages of PR3 and all variables was determined using
Spearman's correlation coefficient. Data were analyzed using
GraphPad Prism software (version 5.0; GraphPad Software, San Diego,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
PR3 and NB1 expression on the
neutrophil plasma membranes of patients with PNH/PNH-AA is
decreased but not correlated
The mRNA levels of PR3 and NB1 were detected in 27
patients with PNH/PNH-AA and 25 healthy controls by flow cytometry
(Fig. 1A and B). The expression of
NB1 on CD59− neutrophils
(CD59−NB1+/CD59−) in patients with
PNH/PNH-AA (20.61±26.07%) was significantly lower compared with the
CD59+ neutrophils
(CD59+NB1+/CD59+) in patients with
PNH/PNH-AA (72.25±25.62%, P=0.001) and healthy controls
(67.72±19.6%, P=0.001) (Fig. 1C1).
The expression of PR3 on CD59− neutrophils
(CD59−PR3+/CD59−) in patients with
PNH/PNH-AA (70.40±29.86%) was significantly lower compared with the
healthy control group (93.28±10.53%, P=0.001) and CD59+
neutrophils (CD59+PR3+/CD59+) in
patients with PNH/PNH-AA (85.68±22.21%, P=0.011) (Fig. 1C2). The expression of PR3 in the two
latter demonstrated no significant differences (P=0.252).
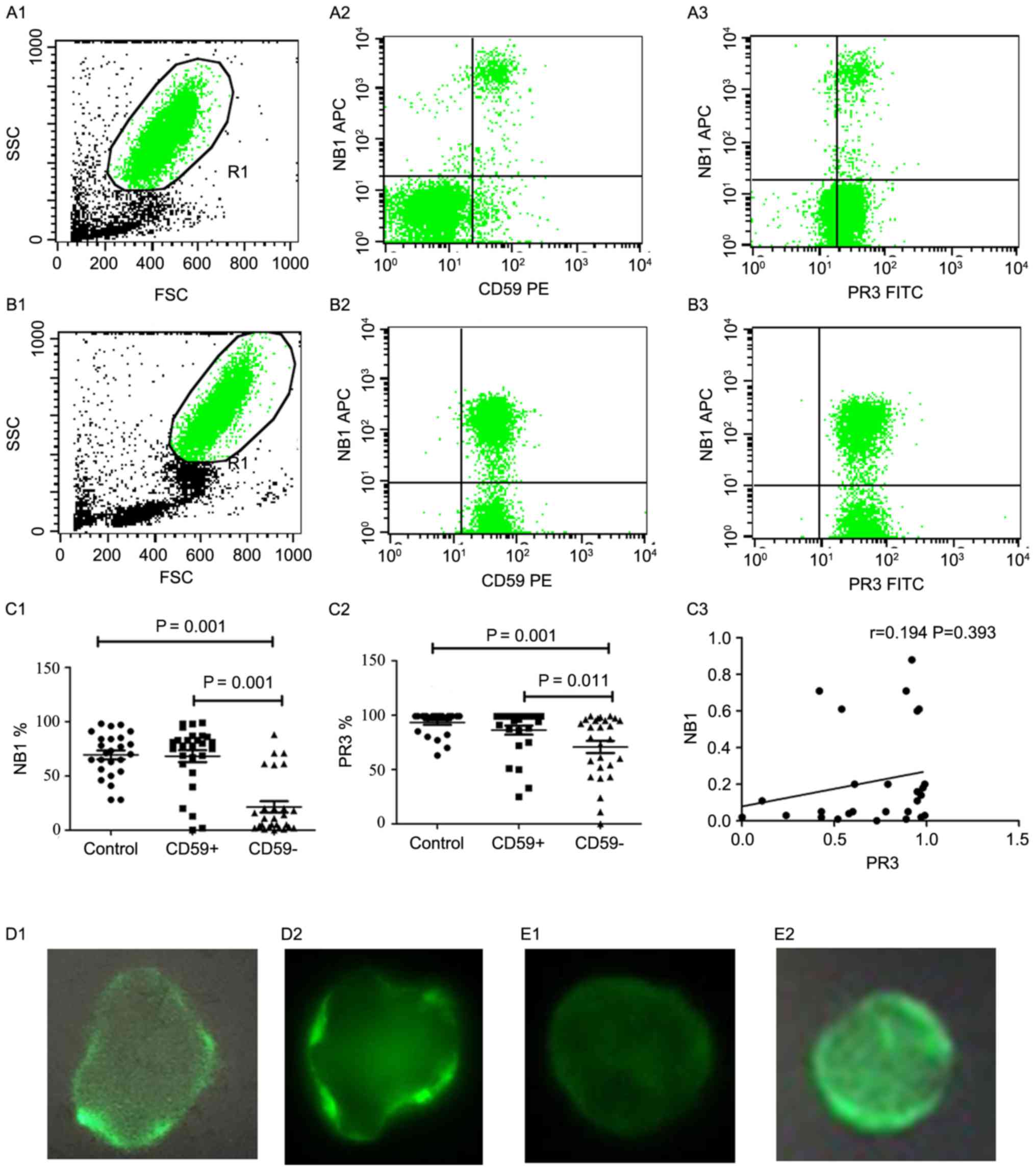 | Figure 1.PR3 and NB1 expression on the
neutrophil plasma membranes of patients with PNH or PNH-AA is
decreased. The expression of PR3 and NB1 were detected by flow
cytometry in (A) PNH/PNH-AA patients and (B) healthy controls
(presented as the mean ± standard error of the mean). (A1 and B1)
The neutrophils were gated as R1 and then (A2 and B2) NB1
expression was investigated on CD59−/CD59+
neutrophils. (A3 and B3) PR3 and NB1 were demonstrated to be
expressed on CD59−/CD59+ neutrophils. (C)
Quantification and analysis of the flow cytometry results. (C1 and
C2) The results demonstrated that the expression of NB1 and PR3 on
CD59− neutrophils significantly decreased compared with
CD59+ neutrophils in patients with PNH/PNH-AA and the
healthy controls. (C3) No correlation was identified between PR3
and NB1 expression in patients with PNH/PNH-AA. Furthermore, the
expression of these two proteins were measured by
immunofluorescence. PR3 was partially expressed on CD59−
neutrophils of (D1) patients with PNH/PNH-AA compared with (D2)
healthy controls, while no NB1 expression was identified on
CD59− neutrophils of (E1) patients with PNH/PNH-AA
compared with (E2) healthy controls. SSC, side-scattered light;
FSC, forward-scattered light; CD, cluster of differentiation; NB1,
CD177 antigen; APC, allophycocyanin; PE, phycoerythrin; PR3,
proteinase 3; FITC, fluorescein isothiocyanate; PNH, paroxysmal
nocturnal hemoglobinuria; AA, aplastic anemia. |
Notably, PR3 mRNA expression did not correlate with
NB1 mRNA expression (r=0.194; P=0.393; Fig. 1C3), indicating that PR3 expression is
not associated with NB1. In order to explore the association
between PR3 and NB1, their expression levels on CD59−
neutrophils were detected by IF. The results demonstrated that PR3
was partially expressed in patients with PNH/PNH-AA (Fig. 1D), whereas NB1 was not expressed in
patients with PNH/PNH-AA due to a defect in the GPI anchor
(Fig. 1E).
PR3 in the serum of patients with
PNH/PNH-AA is decreased, which is negatively correlated with PAR1
and D-Dimer levels
PAR1 and D-Dimer are associated with thrombosis,
thus their protein levels were investigated. The serum level of
PAR1 in patients with PNH/PNH-AA (1.38±0.96 µg/l) was significantly
higher compared with that in the healthy controls (0.47±0.29 µg/l)
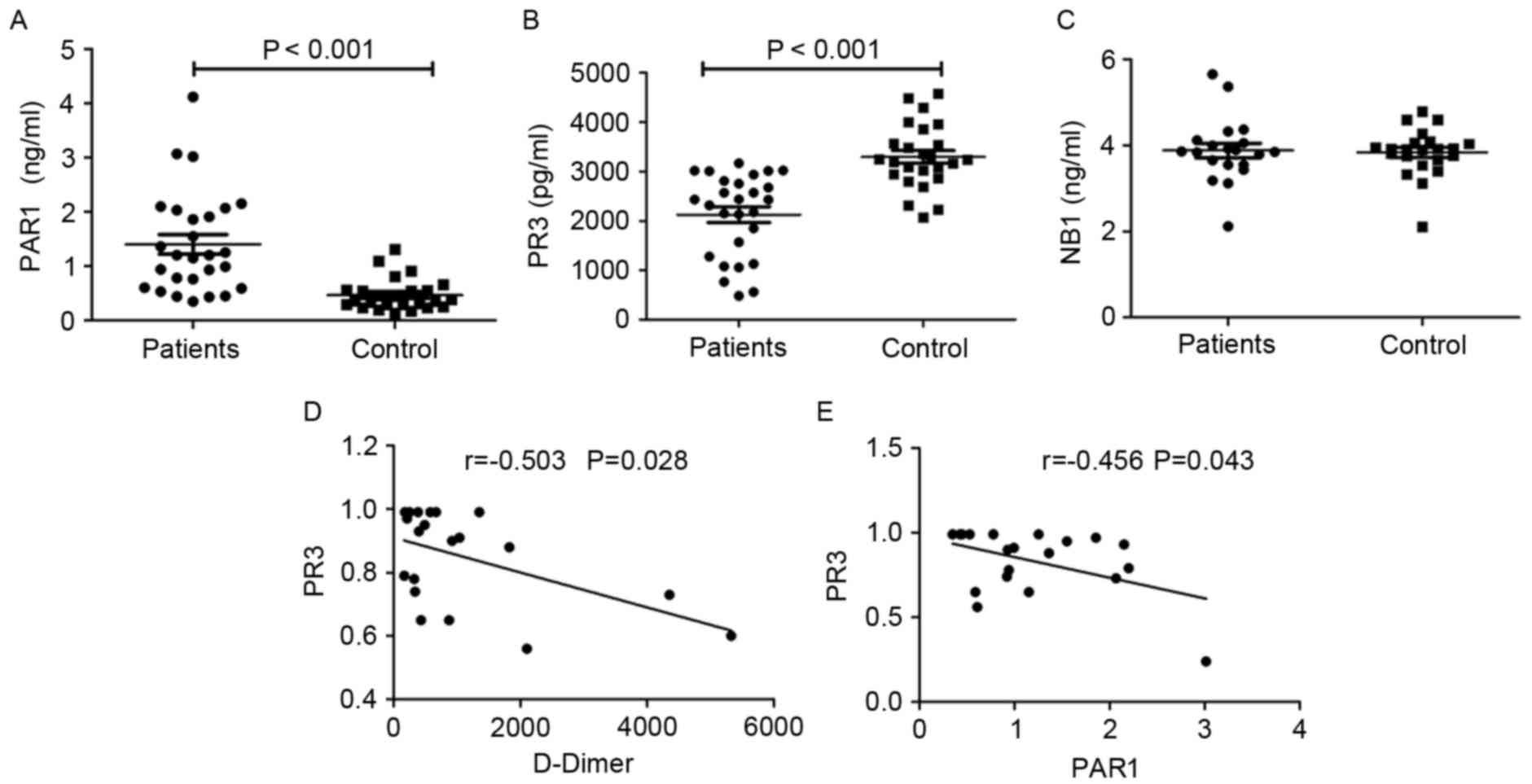
(P<0.001; Fig. 2A). The serum
level of PR3 in patients with PNH/PNH-AA (2,262.72±802.80 pg/ml)
was significantly lower compared with that in the healthy controls
(3,292.92±651.68 pg/ml) (P<0.0001; Fig. 2B). However, the serum level of NB1 in
patients with PNH/PNH-AA (3.881±0.1663 ng/ml) demonstrated no
significant difference compared with that in the healthy controls
(3.840±0.1188 ng/ml) (P=0.2007l; Fig.
2C).
The median level of D-Dimer in patients with
PNH/PNH-AA (511 ng/dl) was significantly higher compared with the
healthy controls (343 ng/dl) (P=0.04; data not shown). Furthermore,
the level of D-Dimer between the patients with thrombosis (Table II) and without thrombosis was
compared. The results revealed that the median level of D-Dimer in
the 5 patients with thrombosis (2,104 ng/dl) was significantly
increased compared with those without thrombosis (226 ng/dl)
(P=0.001; data not shown). In addition, the levels of D-Dimer
(r=−0.503; P=0.028; Fig. 2D) and
PAR1 (r=−0.456; P=0.043; Fig. 2E)
were significantly negatively correlated with the level of PR3.
 | Table II.PR3, NB1 and D-Dimer serum levels in
patients with paroxysmal nocturnal hemoglobinuria combined with
thrombosis. |
Table II.
PR3, NB1 and D-Dimer serum levels in
patients with paroxysmal nocturnal hemoglobinuria combined with
thrombosis.
| Patient no. | G, CD59−
(%) | D-Dimer level
(ng/dl) |
CD59−PR3+/CD59−
(%) |
CD59+PR3+/CD59−
(%) |
CD59−NB1+/CD59−
(%) |
CD59+NB1+/CD59−
(%) |
|---|
| 8 | 85.46 |
830 | 24 | 25 | 11 | 81 |
| 10 | 97.98 | 2,104 | 56 | 91 | 16 | 80 |
| 11 | 86.75 | 1,355 | 79 | 86 | 1 | 13 |
| 15 | 92.57 | 5,324 | 99 | 99 |
0.9 | 93 |
| 17 | 59.41 | 5,835 | 96 | 98 |
0.5 | 69 |
PNH clones exhibit no significant
difference in mRNA and protein levels of PR3 and NB1 compared with
neutrophils from the healthy controls
CD59− neutrophils were sorted by MACS
following their isolation from the neutrophils of the patients. The
purity of CD59− cells, sorted by MACS (Fig. 3A, B), was >85%. The expression of
PR3 and NB1 in CD59− cells in the patients and controls
(CD59+ cells) was then analyzed by RT-qPCR and western
blot analyses.
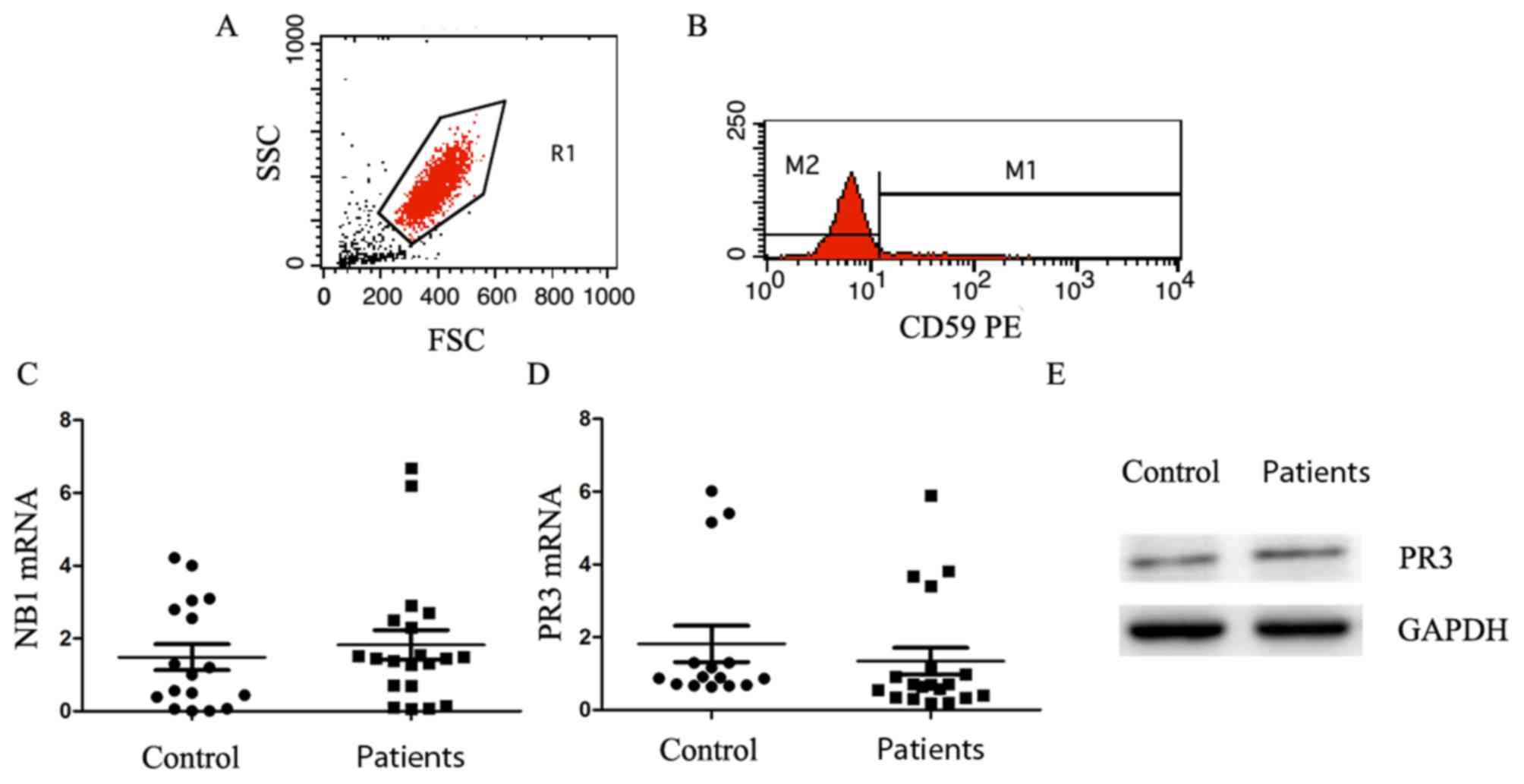 | Figure 3.PNH clones exhibit no significant
difference in mRNA and protein levels of PR3 and NB1 compared with
neutrophils from the controls. (A) The neutrophils were isolated
from the blood of patients with PNH/PNH-AA; the purity, detected by
flow cytometry, was >85%. (B) CD59− neutrophils were
sorted by magnetic-activated cell sorting and the purity was
determined to be >90%. No significant differences in the mRNA
expression of (C) PR3 or (D) NB1 were identified between patients
with PNH/PNH-AA and the healthy controls (presented as the mean ±
standard error of the mean). (E) No notable differences in PR3
protein expression were identified between patients with PNH/PNH-AA
and the healthy controls. SSC, side-scattered light; FSC,
forward-scattered light; CD, cluster of differentiation; NB1, CD177
antigen; PE, phycoerythrin; PR3, proteinase 3; PNH, paroxysmal
nocturnal hemoglobinuria; AA, aplastic anemia. |
The mRNA level of PR3 in patients with PNH/PNH-AA
(1.344±0.3679) demonstrated that there was no significant
difference compared with the controls (1.815±0.5005) (P=0.4439;
Fig. 3C). Similarly, the mRNA level
of NB1 in the patients with PNH/PNH-AA (1.826±0.4010) and the
healthy controls (1.485±0.3563) demonstrated no significant
difference (P=0.5359; Fig. 3D). The
western blotting results revealed that there was no marked
difference in PR3 protein levels between patients with PNH/PNH-AA
and the healthy control group (Fig.
3E).
Discussion
Thromboembolism is the primary cause of mortality in
patients with PNH and usually occurs in the hepatic veins, which
leads to Budd-Chiari syndrome, the cerebral veins and sinuses. Thus
far, the mechanism of thrombosis in PNH has been unclear. NO
synthesis in endothelial cells maintains normal flow of blood and
inhibits platelet aggregation. In patients with PNH, extensive
intravascular hemolysis results in the production of large amounts
of free hemoglobin in plasma. The free hemoglobin serves a role as
a NO effective scavenger in combination with NO, and NO is
depleted, resulting in platelet aggregation and activation
(24,25). Another factor associated with
thrombosis is urokinase-type plasminogen activator receptor (uPAR),
a GPI-linked protein expressed on neutrophils that mediates
endogenous thrombolysis through a urokinase-dependent mechanism
(26–28). Sloand et al (29) demonstrated that in patients with PNH,
membrane GPI-anchored uPAR is decreased or absent on granulocytes
and platelets, while soluble uPAR (suPAR) levels are increased in
patients' plasma. Increased levels of su-PAR compete with urokinase
receptors on the cell membrane, reducing plasmin production,
thereby reducing fibrinolytic activity and promoting thrombosis and
stabilization. A previous study demonstrated that the adhesion and
aggregation of platelets was compensatively decreased in patients
with PNH, particularly in CD59+ platelets (30).
The present study aimed to explore the expression of
PR3 and its effect on thrombosis in patients with PNH. Several
studies have suggested that there is an association between NB1 and
PR3, which are co-expressed on the plasma membrane of the same
subset of neutrophils; these studies indicated that NB1 is a
receptor of PR3 (16,31–33).
However, Hu et al (34,35)
demonstrated that neutrophils from NB1 negative individuals
expressed low levels of PR3 following priming with tumor necrosis
factor αumor necrosis factor low leis not an exclusive binding
partner of PR3. The flow cytometry results in the present study
demonstrated that PR3 and NB1 expression decreased on
CD59− neutrophils due to a lack of GPI-linked proteins;
however, there was no correlation between PR3 and NB1 expression.
In addition, the IF results demonstrated that PR3 was partially
expressed on the CD59− neutrophils of patients with PNH,
while there was no NB1 expression. A hypothesis for the low
expression of PR3 on CD59− neutrophils from patients
with PNH compared with CD59+ neutrophils from patients
with PNH and normal controls may be that PR3 binds to other
receptor(s) to exert its function.
Furthermore, the level of PR3 in serum was
identified to be significantly decreased in patients with
PNH/PNH-AA, and negatively correlated with PAR1 and D-Dimer levels.
PR3 can degrade PAR1, causing inhibition of active thrombin and
regulating platelet activation (21). PAR1 combines with thrombin to induce
thrombosis. Thrombin binding to PAR1 on platelets induces platelet
activation and strengthens platelet adhesion in order to promote
the aggregation of platelets and thus cause thrombosis (36,37).
Another study demonstrated that PR3 induced platelets to change
shape via the Rho/Rho kinase and Ca2+ signaling pathways
(38). The results of the present
study indicated that lower PR3 level in serum of patients with
PNH/PNH-AA resulted in increased PAR1 level, and thus an increased
concentration of activated platelets. To investigate the hypothesis
of NB1 regulation of PR3 expression, RT-qPCR analysis was
performed. No significant difference in PR3 and NB1 mRNA levels was
identified between the patients with PNH/PNH-AA and the control
group. Thus, this hypothesis was not validated.
In conclusion, the expression of PR3 on the plasma
membrane of neutrophils decreased in patients with PNH/PNH-AA, but
was still partially expressed. The level of PR3 in the serum of
patients with PNH/PNH-AA also decreased, which lead to an increase
in PAR1 expression, indicating increased platelet activation.
However, the mechanism regulating PR3 expression in patients with
PNH requires further exploration in the future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81570106, 81600088,
81600093 and 81770110), the Tianjin Municipal Natural Science
Foundation (grant nos. 14JCYBJC25400 and 15JCYBJC24300), and the
Science and Technology Foundation of Tianjin Municipal Health
Bureau (grant nos. 2011kz115 and 2014kz120).
References
|
1
|
Hillmen P, Lewis SM, Bessler M, Luzzatto L
and Dacie JV: Natural history of paroxysmal nocturnal
hemoglobinuria. N Engl J Med. 333:1253–1258. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeda J, Miyata T, Kawagoe K, Iida Y,
Endo Y, Fujita T, Takahashi M, Kitani T and Kinoshita T: Deficiency
of the GPI anchor caused by a somatic mutation of the PIG-A gene in
paroxysmal nocturnal hemoglobinuria. Cell. 73:703–711. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nicholson-Weller A, Spicer DB and Austen
KF: Deficiency of the complement regulatory protein,
‘decay-accelerating factor,’ on membranes of granulocytes,
monocytes, and platelets in paroxysmal nocturnal hemoglobinuria. N
Engl J Med. 312:1091–1097. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diep DB, Nelson KL, Raja SM, Pleshak EN
and Buckley JT: Glycosylphosphatidylinositol anchors of membrane
glycoproteins are binding determinants for the channel-forming
toxin aerolysin. J Biol Chem. 273:2355–2360. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brodsky RA, Mukhina GL, Nelson KL,
Lawrence TS, Jones RJ and Buckley JT: Resistance of paroxysmal
nocturnal hemoglobinuria cells to the
glycosylphosphatidylinositol-binding toxin aerolysin. Blood.
93:1749–1756. 1999.PubMed/NCBI
|
|
6
|
Devalet B, Mullier F, Chatelain B, Dogne
JM and Chatelain C: The central role of extracellular vesicles in
the mechanisms of thrombosis in paroxysmal nocturnal
haemoglobinuria: A review. J Extracell Vesicles. 3:233042014.
View Article : Google Scholar
|
|
7
|
Ziakas PD, Poulou LS and Pomoni A:
Thrombosis in paroxysmal nocturnal hemoglobinuria at a glance: A
clinical review. Curr Vasc Pharmacol. 6:347–353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hill A, Kelly RJ and Hillmen P: Thrombosis
in paroxysmal nocturnal hemoglobinuria. Blood. 121:4985–4996; quiz
5105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stroncek DF, Caruccio L and Bettinotti M:
CD177: A member of the LY-6 gene superfamily involved with
neutrophil proliferation and polycythemia vera. J Transl Med.
2:82004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kissel K, Santoso S, Hofmann C, Stroncek D
and Bux J: Molecular basis of the neutrophil glycoprotein NB1
(CD177) involved in the pathogenesis of immune neutropenias and
transfusion reactions. Eur J Immunol. 31:1301–1309. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lalezari P, Murphy GB and Allen FH Jr:
NB1, a new neutrophil-specific antigen involved in the pathogenesis
of neonatal neutropenia. J Clin Invest. 50:1108–1115. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Toyoda T, Tsukamoto T, Yamamoto M, Ban H,
Saito N, Takasu S, Shi L, Saito A, Ito S, Yamamura Y, et al: Gene
expression analysis of a Helicobacter pylori-infected and high-salt
diet-treated mouse gastric tumor model: Identification of CD177 as
a novel prognostic factor in patients with gastric cancer. BMC
Gastroenterol. 13:1222013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teofili L, Martini M, Guidi F, Venditti D,
Leone G and Larocca ML: The PRV-1 gene expression in essential
thrombocythemia. Blood. 104:2995–2996. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Teofili L, Martini M, Luongo M, Di Mario
A, Leone G, De Stefano V and Larocca LM: Overexpression of the
polycythemia rubra vera-1 gene in essential thrombocythemia. J Clin
Oncol. 20:4249–4254. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bux J, Becker F, Seeger W, Kilpatrick D,
Chapman J and Waters A: Transfusion-related acute lung injury due
to HLA-A2-specific antibodies in recipient and NB1-specific
antibodies in donor blood. Br J Haematol. 93:707–713. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
von Vietinghoff S, Tunnemann G, Eulenberg
C, Wellner M, Cristina Cardoso M, Luft FC and Kettritz R: NB1
mediates surface expression of the ANCA antigen proteinase 3 on
human neutrophils. Blood. 109:4487–4493. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saragih H, Zilian E, Jaimes Y, Paine A,
Figueiredo C, Eiz-Vesper B, Blasczyk R, Larmann J, Theilmeier G,
Burg-Roderfeld M, et al: PECAM-1-dependent heme oxygenase-1
regulation via an Nrf2-mediated pathway in endothelial cells.
Thromb Haemost. 111:1077–1088. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sachs UJ, Andrei-Selmer CL, Maniar A,
Weiss T, Paddock C, Orlova VV, Choi EY, Newman PJ, Preissner KT,
Chavakis T and Santoso S: The neutrophil-specific antigen CD177 is
a counter-receptor for platelet endothelial cell adhesion
molecule-1 (CD31). J Biol Chem. 282:23603–23612. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rao NV, Wehner NG, Marshall BC, Gray WR,
Gray BH and Hoidal JR: Characterization of proteinase-3 (PR-3), a
neutrophil serine proteinase. Structural and functional properties.
J Biol Chem. 266:9540–9548. 1991.PubMed/NCBI
|
|
20
|
Renesto P, Si-Tahar M, Moniatte M, Balloy
V, Van Dorsselaer A, Pidard D and Chignard M: Specific inhibition
of thrombin-induced cell activation by the neutrophil proteinases
elastase, cathepsin G, and proteinase 3: Evidence for distinct
cleavage sites within the aminoterminal domain of the thrombin
receptor. Blood. 89:1944–1953. 1997.PubMed/NCBI
|
|
21
|
Mihara K, Ramachandran R, Renaux B,
Saifeddine M and Hollenberg MD: Neutrophil elastase and
proteinase-3 trigger G protein-biased signaling through
proteinase-activated receptor-1 (PAR1). J Biol Chem.
288:32979–32990. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chinese Society of Hematology, Chinese
Medical Association, . Expert consensus of diagnosis and treatment
of paroxysmal nocturnal hemoglobinuria. Zhonghua Xue Ye Xue Za Zhi.
34:276–279. 2013.(In Chinese). PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin X, Hu W, Song W, Blair P, Wu G, Hu X,
Song Y, Bauer S, Feelisch M, Leopold JA, et al: Balancing role of
nitric oxide in complement-mediated activation of platelets from
mCd59a and mCd59b double-knockout mice. Am J Hematol. 84:221–227.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pu JJ and Brodsky RA: Paroxysmal nocturnal
hemoglobinuria from bench to bedside. Clin Transl Sci. 4:219–224.
2014. View Article : Google Scholar
|
|
26
|
Rønne E, Pappot H, Grøndahl-Hansen J,
Høyer-Hansen G, Plesner T, Hansen NE and Danø K: The receptor for
urokinase plasminogen activator is present in plasma from healthy
donors and elevated in patients with paroxysmal nocturnal
haemoglobinuria. Br J Haematol. 89:576–581. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ploug M, Plesner T, Rønne E, Ellis V,
Høyer-Hansen G, Hansen NE and Danø K: The receptor for
urokinase-type plasminogen activator is deficient on peripheral
blood leukocytes in patients with paroxysmal nocturnal
hemoglobinuria. Blood. 79:1447–1455. 1992.PubMed/NCBI
|
|
28
|
Engström G, Zöller B, Svensson PJ,
Melander O and Persson M: Soluble urokinase plasminogen activator
receptor and incidence of venous thromboembolism. Thromb Haemost.
115:657–662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sloand EM, Pfannes L, Scheinberg P, More
K, Wu CO, Horne M and Young NS: Increased soluble urokinase
plasminogen activator receptor (suPAR) is associated with
thrombosis and inhibition of plasmin generation in paroxysmal
nocturnal hemoglobinuria (PNH) patients. Exp Hematol. 36:1616–1624.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fu R, Meng Y, Wang Y, Liu H, Liu Y, Li L,
Ding S, Wang G, Song J and Shao Z: The dysfunction of platelets in
paroxysmal nocturnal hemoglobinuria. Thromb Res. 148:50–55. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
von Vietinghoff S, Eulenberg C, Wellner M,
Luft FC and Kettritz R: Neutrophil surface presentation of the
anti-neutrophil cytoplasmic antibody-antigen proteinase 3 depends
on N-terminal processing. Clin Exp Immunol. 152:508–516. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bauer S, Abdgawad M, Gunnarsson L,
Segelmark M, Tapper H and Hellmark T: Proteinase 3 and CD177 are
expressed on the plasma membrane of the same subset of neutrophils.
J Leukoc Biol. 81:458–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jankowska AM, Szpurka H, Calabro M, Mohan
S, Schade AE, Clemente M, Silverstein RL and Maciejewski JP: Loss
of expression of neutrophil proteinase-3: A factor contributing to
thrombotic risk in paroxysmal nocturnal hemoglobinuria.
Haematologica. 96:954–962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu N, Westra J, Huitema MG, Bijl M,
Brouwer E, Stegeman CA, Heeringa P, Limburg PC and Kallenberg CG:
Coexpression of CD177 and membrane proteinase 3 on neutrophils in
antineutrophil cytoplasmic autoantibody-associated systemic
vasculitis: Anti-proteinase 3-mediated neutrophil activation is
independent of the role of CD177-expressing neutrophils. Arthritis
Rheum. 60:1548–1557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu N, Westra J and Kallenberg CG:
Membrane-bound proteinase 3 and its receptors: Relevance for the
pathogenesis of Wegener's Granulomatosis. Autoimmun Rev. 8:510–514.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han Y, Pasquet JM, Nurden A and Ruan CG:
Mechanism of action of protease-activated receptors 1 and 4 in
platelet activation. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
11:495–498. 2003.(In Chinese). PubMed/NCBI
|
|
37
|
Kahn ML, Nakanishi-Matsui M, Shapiro MJ,
Ishihara H and Coughlin SR: Protease-activated receptors 1 and 4
mediate activation of human platelets by thrombin. J Clin Invest.
103:879–887. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peng X, Ramström S, Kurz T, Grenegård M
and Segelmark M: The neutrophil serine protease PR3 induces shape
change of platelets via the Rho/Rho kinase and Ca(2+) signaling
pathways. Thromb Res. 134:418–425. 2014. View Article : Google Scholar : PubMed/NCBI
|