Introduction
Patients with subarachnoid hemorrhage (SAH) often
experience life-threatening complications (1). Aneurysmal SAH (aSAH) is a neurological
condition with high mortality (>25%) and significant morbidity
(>50%) rates amongst survivors (2).
Cerebral vasospasm (CVS) is the leading cause of
morbidity and mortality following aSAH (3). Vasospasm occurs in 50–70% of patients
with SAH and 50% of these patients experience neurological symptoms
in a condition known as symptomatic cerebral vasospasm (SCVS)
(1). Cerebral infarction occurs in
half of all patients with SCVS and is fatal in 30% of patients
(4). It is important to study CVS
vasospasm in order to develop effective treatments and reduce the
morbidity rate of patients with this condition.
Current treatments for SCVS following SAH include,
triple-H therapy, prophylactic hyperdynamic postoperative fluid
therapy and drug therapy (5–7). However, they are limited as the
mechanism of action underlying the condition remains poorly
understood. Establishing an effective model of SCVS has therefore
been a focus of neurological research since 1961 (8). Several different animal models have
been developed, including rat, rabbit, dogs, monkey and pig models
(9–13). There are ~50 different animal models
of CVS; however, the majority have limited utility as animals are
typically asymptomatic and only a few, including rabbits and
monkeys, exhibit symptomatic neurological deficits (14). The monkey model is expensive and not
readily available (14), therefore
it is important to establish a reliable symptomatic SCVS model
using rabbits.
Researchers have measured the diameter of the
basilar artery (BA) using computed tomographic angiography (CTA) to
evaluate CVS (15). CTA is an
effective method of assessing the occurrence and extent of BA
spasms; however, it requires repeated venopuncture of the ear vein
and high doses of contrast agents, which may damage the ear vein
and adversely affect renal function (16,17).
Bilateral carotid artery ligation is required to induce SCVS in the
rabbit model and in studies conducting CTA, the decision to include
a particular rabbit is based on its neurological score following
bilateral carotid artery ligation (18). This evaluation tends to be subjective
and may affect the result of the experiment (18).
Delayed infarction is the most important modifiable
factor that affects quality of life following SAH (19). CTA is not useful for evaluating
infarctions in brain tissue, whereas magnetic resonance
angiography/magnetic resonance imaging (MRA/MRI) are highly
sensitive and specific (20). MRA is
widely used in clinical settings as no contrast agent is required
to measure the BA diameter and the ischemic area may easily be
observed (21,22). However, to the best of our knowledge,
the use of MRA in a rabbit SCVS model has not been previously
reported.
The aim of the present study was to evaluate the
feasibility of using MRA to assess a modified rabbit SCVS model by
measuring the BA diameter and ischemic area following CVS. These
measurements were then compared with those obtained by direct
pathological examination.
Materials and methods
Ethical approval
The protocol followed in the current study was
approved by the Special Committee on Animal Welfare of Wenzhou
Medical University (Wenzhou, China). All animals were treated
humanely in accordance with the guidelines for the Care and Use of
Laboratory Animals published by the U.S. National Institutions of
Health (NIH Publication No. 85-23, revised 1996).
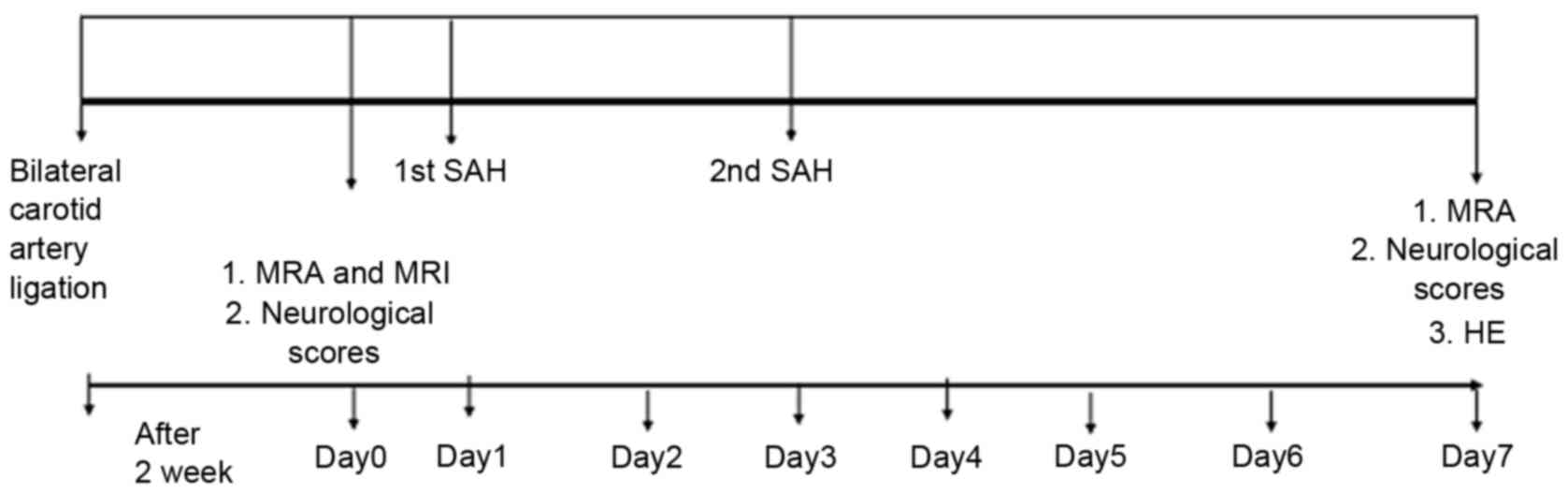
CVS model. A total of 24 male Japanese white rabbits
(2–3 months; weight 2.5–3.0 kg) were purchased from the Wenzhou
Experimental Animal Center (Wenzhou, China). The animals had free
access to standard chow and tap water in a temperature-controlled
chamber at 24°C with a 12 h light/dark cycle. Rabbits were randomly
assigned to one of two groups (n=12 each): A sham group and a SAH
model group. The sham group received a 1.0 ml/kg saline injection
into the cistern and the SAH model group received a 1.0 ml/kg
autologous blood injection into the subarachnoid spaces. These
injections were perfomed twice, with an interval of 48 h between
them.
CVS was induced following SAH, as previously
reported (15). In brief, rabbits
were anesthetized with intramuscularly injected ketamine (25 mg/kg;
cat. no. 1507294; Fujian Gutian Pharmaceutical Co. Ltd., Ningde,
China) and promethazine (12.5 mg/kg; cat. no. 13160301; Shanghai
Hefeng Pharmaceutical Co. Ltd., Shanghai, China), and bilateral
carotid artery ligation was subsequently performed. Following 2
weeks, the rabbits were evaluated using MRA/MRI to measure the
basilar artery (BA) diameter and evaluate whether brain infarction
had occurred. If brain infarction, severe neurological symptoms or
mortality were observed in any of the rabbits at 2 weeks they were
replaced to ensure that each group contained 12 rabbits (Table I).
 | Table I.Rabbits excluded from the present
study. |
Table I.
Rabbits excluded from the present
study.
| Group | Mortalities | Severe neurological
symptoms | Brain infarction
identified by MRI | Total excluded
rabbits |
|---|
| Sham | 2 | 2 | 2 | 6 |
| Subarachnoid
hemorrhage | 2 | 2 | 3 | 7 |
Following MRA, rabbits in the SAH group were
extended in a lateral position during spontaneous breathing. The
atlanto-occipital membrane was pierced with a 25-gauge needle
inserted into the cisterna magna, an attached syringe was
subsequently used to remove the cerebrospinal fluid. The needle
pierced the atlanto-occipital membrane and 1.0 ml/kg cerebrospinal
fluid was extracted. An equal volume of fresh non-heparinized
autologous arterial blood was obtained from the ear artery
following the extraction of the cerebrospinal fluid. This was
injected into the cisterna magna within 2 min. Arterial blood was
analyzed using an ABL90 FLEX blood gas analyzer (Radiometer Medical
ApS, Copenhagen, Denmark), which measured the PO2 and
PCO2. The cisterna magna was re-punctured 48 h later and
autologous arterial blood injection was repeated.
Neurological testing was performed every day
following the establishment of CVS and SAH, as previously reported
(23). Neurological deficits were
graded using a four-point system by observing the rabbits on a flat
surface, with lower scores indicating better neurological function.
Rabbits were assessed by two blinded independent investigators.
MRA/MRI evaluation
BA diameters were measured using an SIGNA HDx MRI
3.0 machine (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) 1 day
prior to the injection of blood (day 0) and 7 days following SAH.
The rabbits were anesthetized with intramuscularly injected
ketamine (25 mg/kg) and promethazine (12.5 mg/kg), and maintained
in the left lateral position. The T2 sequence of brain scans used
the following acquisition parameters: Repetition time (TR) 2,500
msec; echo time (TE) 96.9 msec; field of view (FOV), 7×7
cm2. Three-dimensional time-of-flight (3D-TOF) MRA was
used with the following acquisition parameters: TR, 31 msec; TE,
7.7 msec; FOV, 10×10 cm2. Images were transferred to a
workstation and processed using the image-processing software (both
GE Healthcare Bio-Sciences). The BA diameter of each rabbit was
measured by two experienced radiologists. The BA diameter was
measured in three individual segments: Proximal, middle and distal
(24). Images were evaluated for
abnormal signals indicating brain lesions in the hippocampal region
and the number of abnormal signals was recorded.
Histological evaluation
The rabbits were sacrificed at 7 days following SAH
and the BA and hippocampus were subsequently harvested by
perfusion-fixation. The thorax was opened and a cannula was
introduced into the left ventricle. The descending thoracic aorta
was clamped and the right atrium was opened. Perfusion was
initiated with 500 ml physiological PBS (pH 7.4) at 37°C for 10
min, followed by 500 ml 10% buffered formaldehyde at 37°C under a
perfusion pressure of 120 cm water for 10 min. The hippocampus and
BA were fixed in 10% buffered formaldehyde for 24 h at room
temperature, embedded in paraffin and sliced into 4-µm sections
with a microtome. The formalin-fixed, paraffin-embedded BA and
hippocampus sections were subsequently deparaffinized, hydrated,
washed and stained with hematoxylin and eosin (H&E) for 1 min
at room temperature. Micrographs of the BAs were observed through a
light microscope (Olympus Corporation, Tokyo, Japan) and scanned
into the computer (magnification, ×400). The cross-sectional areas
of blood vessels were measured using a high-definition medical
image analysis program (HMIAP-2000, Tongji Medical University,
Hubei, China). For each vessel, three sequential sections (the
midpoint of the proximal, middle and distal BA) were measured and
the mean was calculated Each hippocampus was evaluated by blinded
pathologists to identify the presence of karyopyknosis, cytoplasmic
staining and smaller cell bodies in the hippocampal CA1 zone and
the incidence of ischemia in all rabbits was recorded. The
experimental protocol is presented in Fig. 1.
Statistical analysis
Statistical analyses were performed using SPSS
(version 13.0; SPSS, Inc., Chicago, IL, USA). Differences in
neurological scores between the two groups were assessed using the
Wilcoxon rank sum test. A Student's t test was used to compare BA
diameters at day 7, the arterial blood gas analyses and differences
in neurological scores between the two groups. The BA diameters in
the model group were compared at each time point using the paired t
test. Pearson correlation was used to compare the methods of
evaluation. The incidence of brain damage as measured by MRA and
H&E staining was analyzed using the χ2 square test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CVS model
A total of 13 rabbits that underwent carotid
ligation surgery were excluded from the present study as they
succumbed, or developed severe neurological symptoms or brain
infarction following carotid ligation, presumably due to the lack
of collateral blood flow (Table I).
The baseline physiological parameters of the two groups are
summarized in Table II and no
significant differences were observed between the groups at
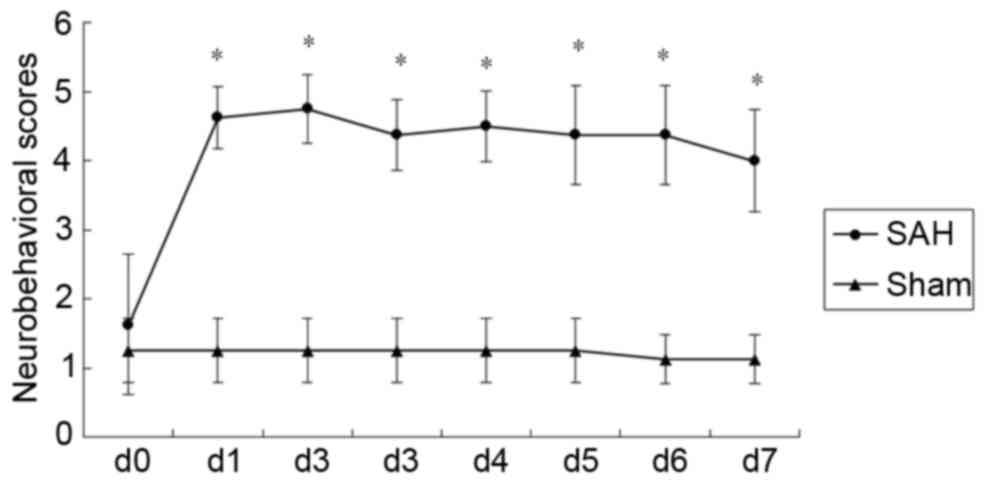
baseline. The mean neurological scores for the groups are presented
in Fig. 2. Neurological impairment
scores were significantly higher in the SAH group compared with the
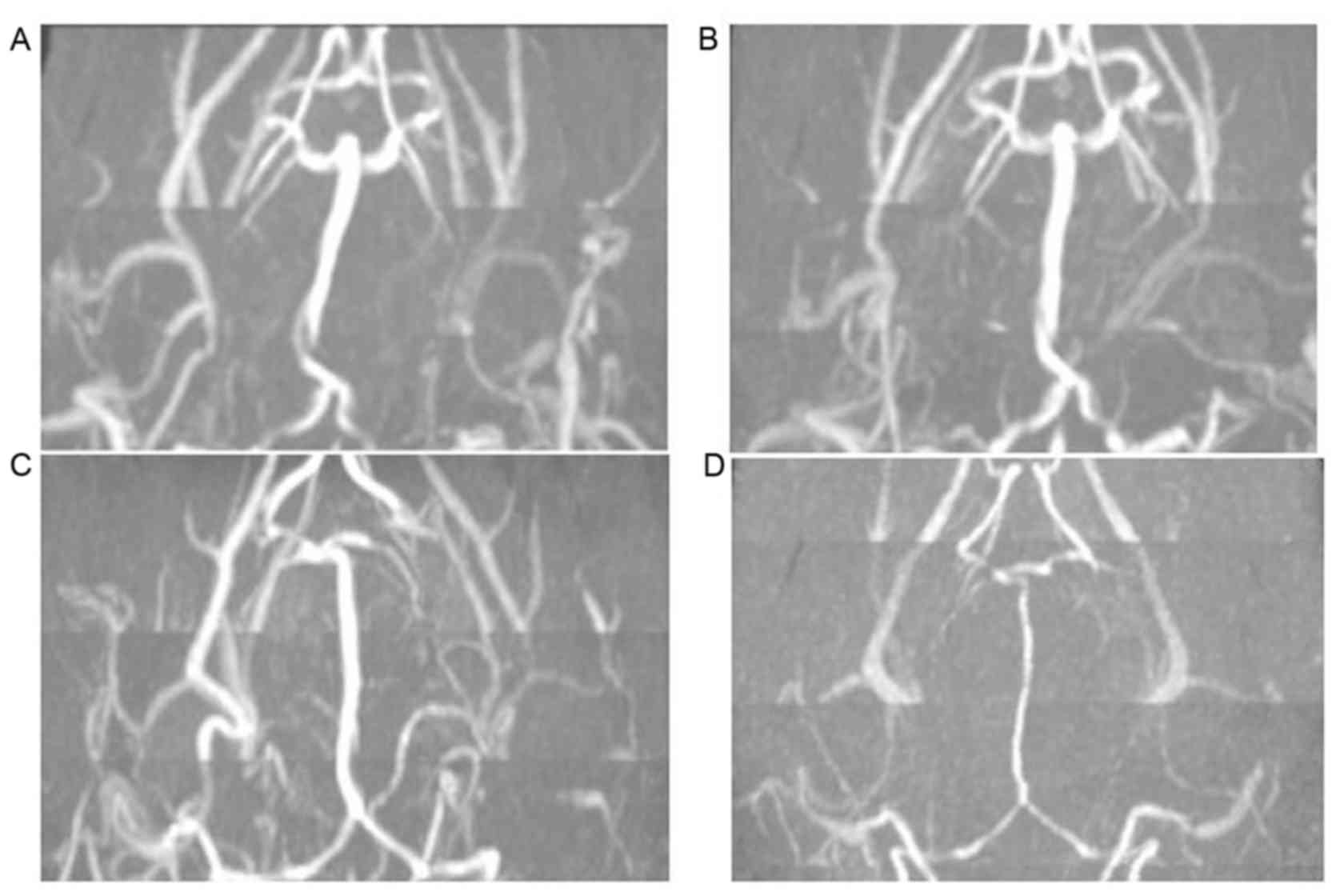
sham group at all time points post-surgery (P<0.05; Fig. 2). Representative MRA images of BA
diameters are presented in Fig. 3.
No evident differences in BA diameter in the Sham group were
observed between days 0 and 7 (Fig. 3A
and B). However, in the SAH group, the BA diameter was markedly
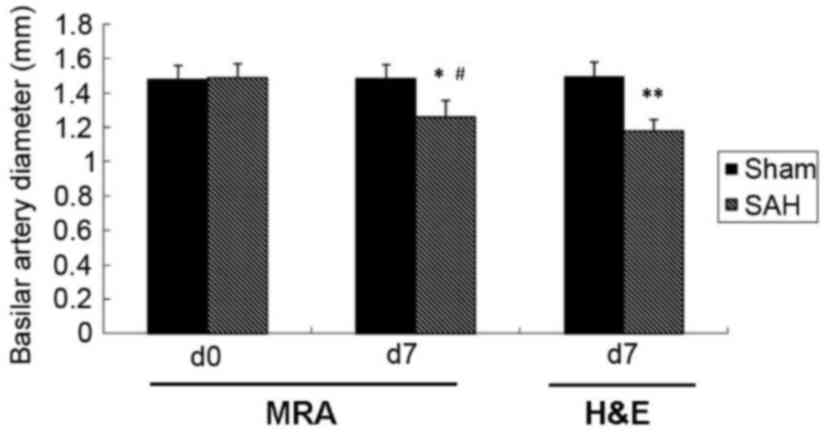
decreased at day 7 compared with day 0 (Fig. 3C and D). Quantitative analysis of MRA
results determined that the BA diameter was significantly decreased
at day 7 compared with day 0 in the SAH group (1.48±0.08 and
1.26±0.09 mm, respectively; P<0.05; Fig. 4).
 | Table II.Summary of physiological parameters
of the groups at baseline. |
Table II.
Summary of physiological parameters
of the groups at baseline.
| Group | N | pH | PO2 (mm
Hg) | PCO2 (mm
Hg) |
|---|
| Reference
range |
| 7.28–7.52 | 55–91 | 24–39 |
| Sham | 12 | 7.36±0.06 |
90.4±11.65 | 34.05±6.26 |
| Subarachnoid
hemorrhage | 12 | 7.38±0.09 | 89.63±10.47 | 32.54±4.51 |
Histological evaluation
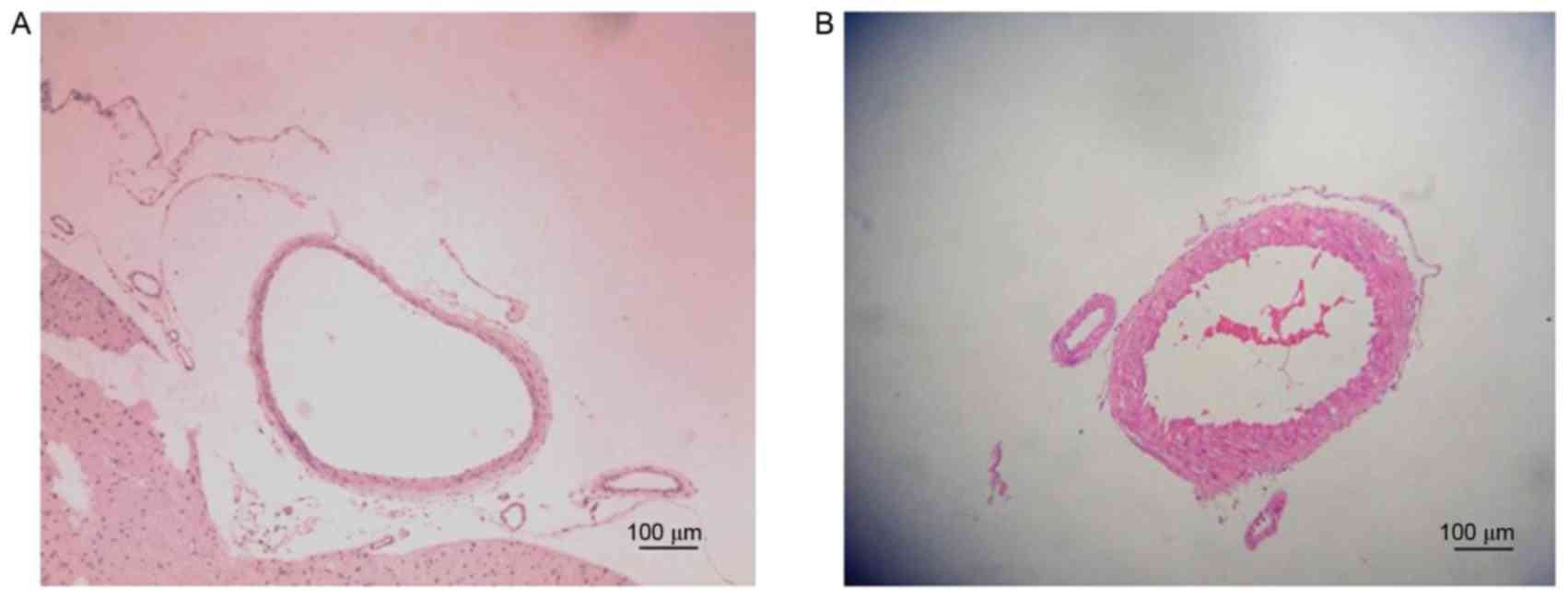
Representative histological images of the BA from
the Sham and SAH groups are presented in Fig. 5. The BA diameter on day 7 as measured
using H&E was significantly lower in the SAH group compared
with the Sham group (1.17±0.06 and 1.49±0.09 mm, respectively;
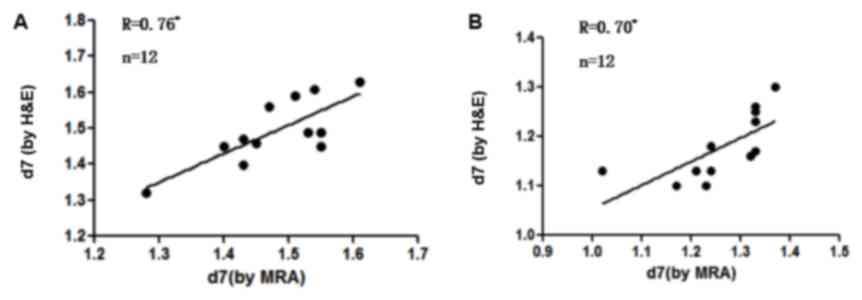
P<0.05; Fig. 4). The measurements
obtained using MRA and H&E staining were compared and a
significant positive correlation was detected between the results
of H&E and MRA in the sham group (r=0.76, P=0.004; Fig. 6A) and SAH group (r=0.70, P=0.011;
Fig. 6B) on day 7.
MRA/MRI
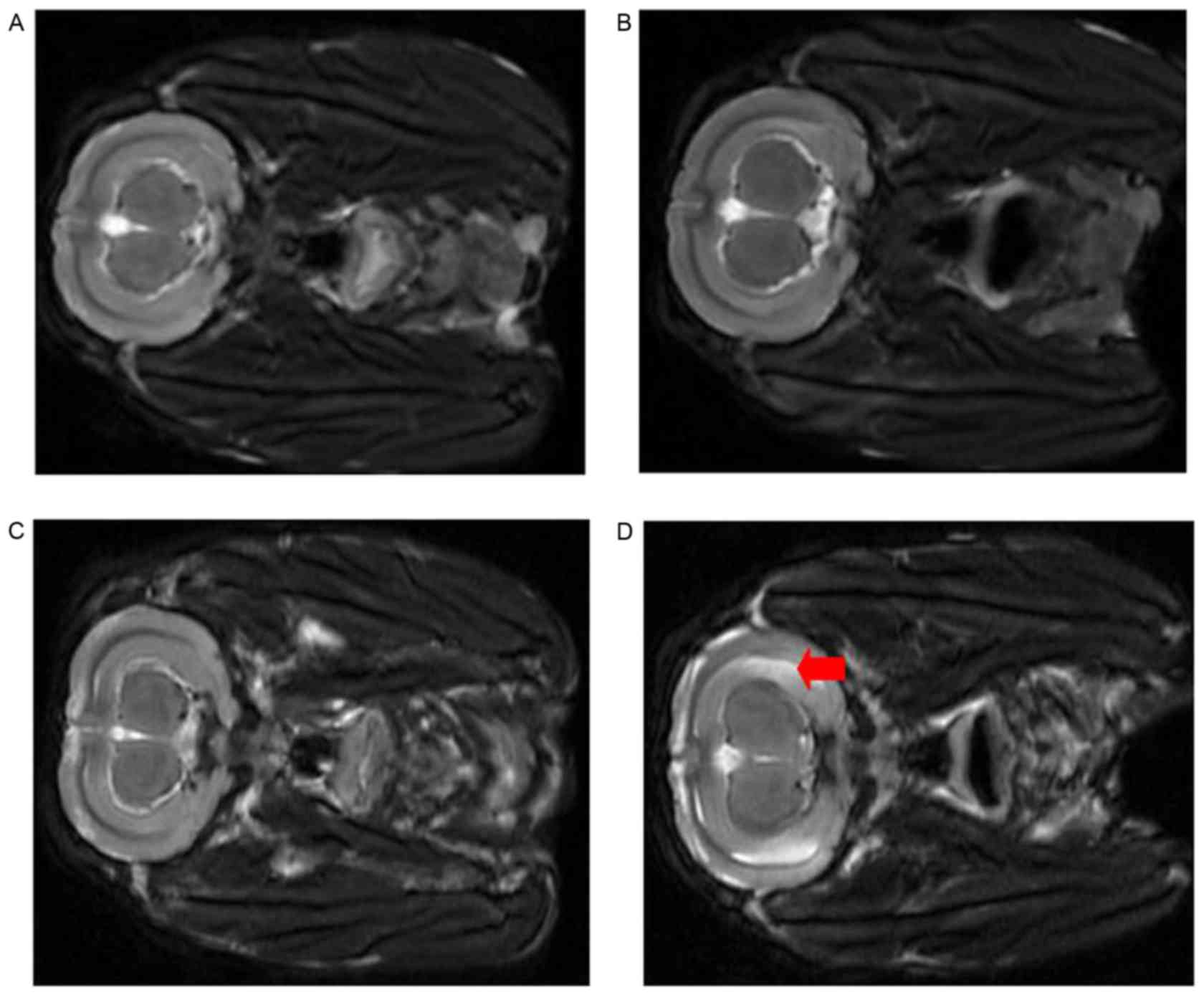
Brain MRA did not reveal ischemic changes in either
group following bilateral carotid artery ligation at 2 weeks
(Fig. 7). No significant differences
were observed prior to and following saline injection in the Sham
group (Fig. 7A and B); however,
large flake and patchy high density of the hippocampus was observed
in the SAH group 7 days post injection (Fig. 7D) compared with pre-injection
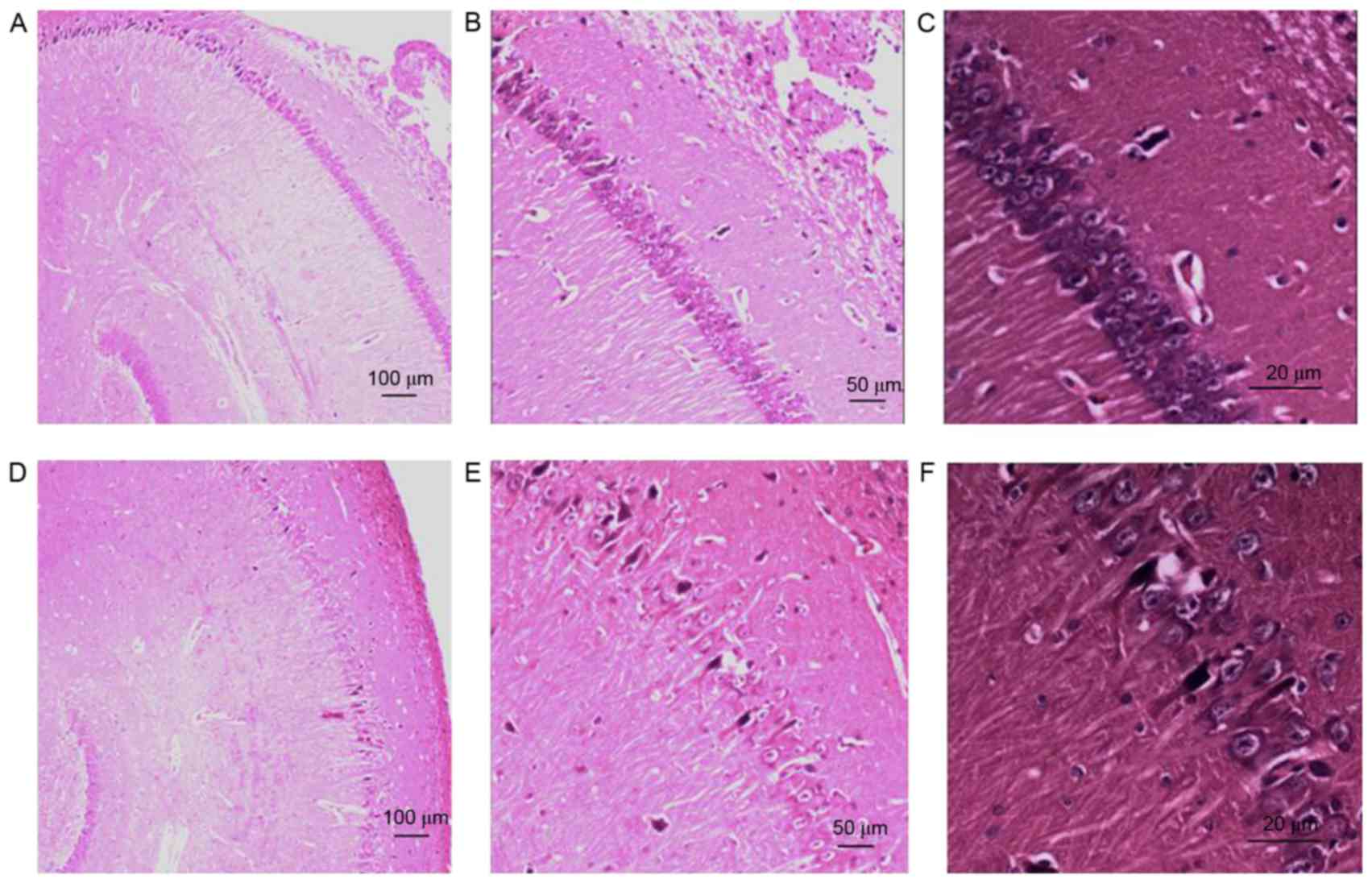
(Fig. 7C). Hippocampal H&E
staining revealed normal cells and cellular arrangements in the
Sham group at day 7 (Fig. 8A-C). In
the SAH group, H&E staining revealed karyopyknosis, cytoplasmic
staining and smaller cell bodies in the hippocampal CA1 zone 7 days
post-injection, indicating the presence of hippocampal ischemia
(Fig. 8D-F). No significant
difference was observed in the incidence of hippocampal ischemia
identified by MRA and H&E staining in the SAH group (Table III).
 | Table III.Incidence of hippocampal ischemia
assessed by MRA and H&E. |
Table III.
Incidence of hippocampal ischemia
assessed by MRA and H&E.
| Group | Hippocampus
ischemia identified by MRA | Hippocampus
ischemia identified by H&E |
|---|
| Sham | 0/12 | 0/12 |
| Subarachnoid
hemorrhage | 10/12 | 9/12 |
Discussion
In the present study, a model of CVS in rabbits was
successfully established and it was demonstrated that MRA may be
used to accurately evaluate the degree of CVS. There was a strong
correlation between H&E staining of the BA and MRA for
measuring the degree of vascular spasm. MRA revealed ischemia of
the hippocampus 7 days following SAH and postoperative pathological
examination indicated ischemia, indicating that MRA accurately
identifies ischemia in the hippocampus.
SAH is a common acute and severe cerebral vascular
disease and has the third highest incidence of all cerebrovascular
diseases (25). SAH comprises only
5% of all strokes; however its mortality rate is high (40%) due to
delayed cerebral ischemia (DCI) and neurological deterioration
occurring days following the hemorrhage (26). The severity of cerebral infarction
and ischemia that occurs is associated with the severity of
vasospasm observed on angiography (8). Delayed CVS may develop in patients with
aneurysmal SAH (8). The severity and
duration of vasospasm is associated with the thickness, density and
persistence of the blood in the ventricle (1,4). Delayed
CVS with neurological dysfunction due to DCI is the primary cause
of mortality following SAH (27).
Furthermore, the primary cause of poor prognosis in patients with
SAH is the insufficient treatment of CVS (28). To better understand the mechanism of
delayed CVS with DCI and help identify a suitable therapy, it is
necessary to establish a reliable animal model of symptomatic
delayed CVS.
The majority of animal models of CVS establish only
CVS without cerebral ischemia following SAH. Although SCVS may be
simulated in primates, their utility in research is limited due to
the high costs associated with keeping these animals. In rabbits,
cerebral angiography may be repeated and the procedure for
establishing a model of SAH with SCVS is relatively simple and
inexpensive (12). Therefore,
establishing a rabbit model of this condition is helpful to
researchers. Using this model, the degree of CVS and cerebral
ischemia may be determined by MRA/MRI. The rabbit model of CVS is
considered to be superior to that of other animals (12). Compared with dogs or rats, the
cerebral vascular system of rabbits is more similar to the human
system, as it has a limited cerebral vascular supply following
ligation of the carotid arteries (12). MRI has previously been used to
determine the occurrence of cerebral ischemia in a dog CVS model;
however, this model was unable to accurately replicate SCVS
(29).
Endo et al (23) established that SAH causes secondary
symptomatic CVS in a rabbit model using New Zealand rabbits. A
bilateral carotid artery ligation was performed to block the
anterior circulation, followed by injection of blood into the
brain. However, neurological function was evaluated based on
neurological scores and the subjectivity of this score may have
affected the results (23). In their
initial study, fresh non-heparinized autologous arterial blood was
injected into the cisterna magna twice, 2 weeks following bilateral
carotid artery ligation and the degree of cerebral vascular spasm
was assessed by digital subtraction angiography (DSA) (23). The femoral artery was ligated for the
angiographic procedure, resulting in insufficient blood supply to
the lower limbs, which may have affected neurological scores.
In the present study, rabbits were evaluated
following bilateral carotid artery ligation using neurological
scores and MRA to assess the extent of brain infarction. In a pilot
study, the Japanese white rabbit was used to study the same model
and it was identified that the mortality rate of rabbits was very
high when autologous blood was used (data not shown). Based on
this, a rabbit model of SCVS with an improved method for
calculating the volume of injected blood was used in the present
study. If MRA revealed cerebral ischemia following carotid artery
ligation after 2 weeks, the rabbits were excluded from the study.
Therefore, MRA was used as a criterion for excluding rabbits with
cerebral ischemia. The twice blood injection model typically
involves making a subocciptal incision to expose the craniospinal
junction; however, the skin and muscle are cut using this method,
which increases susceptibility to infection (12). In the present study, apparatus that
induced suction was used to establish the model without exposing
the craniospinal junction.
DSA is the gold standard for determining CVS
severity and the effectiveness of treatment in humans (30). However, there are several problems
with the application of this imaging technique in the rabbit model
(14). DSA leads to significant
damage due to femoral artery ligation and does not allow dynamic
observation of CVS in the BA (31).
In previous studies, CTA demonstrated CVS with measurements of BA
diameters (32). However, CTA
requires injection of a contrast agent that may result in renal
dysfunction, a complication that has been widely verified in
clinical practice, in addition, it can be difficult to gain venous
access multiple times (33). MRA has
previously been applied to clinical and animal experiments and may
be performed without the need for contrast injection, as
demonstrated in the present study (21).
The 3D-TOF MRA sequence allows for dynamic
observation of the BA to evaluate arterial spasticity as measured
by its quantitative imaging system (21). Based on our previous study (15), it was determined that BA vasospasm
occurs 7 days following SAH, suggesting that spasticity was most
marked in the first 7 days following SAH. Therefore, as a method of
evaluation, MRA is helpful for detecting the incidence of vasospasm
in rabbits and for effectively assessing the degree of
spasticity.
The results of the present study demonstrate that
MRA findings are consistent with the pathological changes that
occur in the BA, indicating that MRA may be used to accurately
measure the BA diameter. MRA is a noninvasive imaging technique
often used to determine the extent of brain damage (34) and previous studies have reported that
the extent of brain damage following SAH may be accurately
evaluated using MRA (35–37). The hippocampus is the region of the
brain most sensitive to ischemia and hypoxia (38). The present study demonstrated that
the T2 sequence may be used to identify the extent of brain damage
by identifying whether there was a significant increase in the
cerebral signal 7 days following surgery-results which are
consistent with ischemia in the brain tissue.
There were a number of limitations of the present
study. Firstly, although H&E staining and MRA correctly
identified the BA diameter and neurological damage following CVS,
errors in measuring the BA diameter may occur without correlative
DSA. The diameter of the BA measured by MRA was not compared with
the BA diameter measured by DSA, as the duration of anesthesia
would have been significantly longer with DSA. Similarly, there may
be inconsistencies in the determination of neurological function
scores due to inter-observer variability.
To the best of our knowledge, the current study is
the first to report the establishment of an SCVS model in Japanese
white rabbits using MRA to measure spasticity and brain damage, and
to compare the results of MRA with those from pathological
examinations. The results of the present study indicate that MRA
may be an effective method of evaluating BA vasospasm and
hippocampus ischemic change in a rabbit model of SCVS.
Acknowledgements
The present study was supported by the National
Foundation of Natural Science of China (grant nos. 81603685,
81273923 and 81573742) and the Wenzhou Science and Technology
Project (grant no. Y20150229).
References
|
1
|
van Gijn J, Kerr RS and Rinkel GJ:
Subarachnoid haemorrhage. Lancet. 369:306–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Connolly ES Jr, Rabinstein AA, Carhuapoma
JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech
AM, Ogilvy CS, et al: Guidelines for the management of aneurysmal
subarachnoid hemorrhage: A guideline for healthcare professionals
from the American Heart Association/american Stroke Association.
Stroke. 43:1711–1737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Przybycien-Szymanska MM and Ashley WW Jr:
Biomarker discovery in cerebral vasospasm after aneurysmal
subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 24:1453–1464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steiner T, Juvela S, Unterberg A, Jung C,
Forsting M and Rinkel G; European Stroke Organization, : European
stroke organization guidelines for the management of intracranial
aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 35:93–112.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sen J, Belli A, Albon H, Morgan L, Petzold
A and Kitchen N: Triple-H therapy in the management of aneurysmal
subarachnoid haemorrhage. Lancet Neurol. 2:614–621. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choudhari K: Prophylactic hyperdynamic
postoperative fluid therapy after aneurysmal subarachnoid
hemorrhage: A clinical, prospective, randomized, controlled study.
Neurosurgery. 50:1170–1172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muroi C, Seule M, Mishima K and Keller E:
Novel treatments for vasospasm after subarachnoid hemorrhage. Curr
Opin Crit Care. 18:119–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimoda M, Takeuchi M, Tominaga J, Oda S,
Kumasaka A and Tsugane R: Asymptomatic versus symptomatic infarcts
from vasospasm in patients with subarachnoid hemorrhage: Serial
magnetic resonance imaging. Neurosurgery. 49:1341–1350. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Udoetuk JD, Stiefel MF, Hurst RW, Weigele
JB and LeRoux PD: Admission angiographic cerebral circulation time
may predict subsequent angiographic vasospasm after aneurysmal
subarachnoid hemorrhage. Neurosurgery. 61:1152–1161. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lougheed WM and Tom M: A method of
introducing blood into the subarachnoid space in the region of the
circle of Willis in dogs. Can J Surg. 4:329–337. 1961.PubMed/NCBI
|
|
11
|
Prunell GF, Mathiesen T and Svendgaard NA:
A new experimental model in rats for study of the pathophysiology
of subarachnoid hemorrhage. Neuroreport. 13:2553–2556. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen S, Klebe KD, Vakhmyanin A, Fujii M
and Zhang JH: SAH models: Review, new modification, and
prospective. Springer; New York, NY: pp. 255–267. 2014
|
|
13
|
Otsuji T, Endo S, Hirashima Y, Nishijima M
and Takaku A: An experimental model of symptomatic vasospasm
induced by oxyhemoglobin in rabbits. Stroke. 25:657–662. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Titova E, Ostrowski RP, Zhang JH and Tang
J: Experimental models of subarachnoid hemorrhage for studies of
cerebral vasospasm. Neurol Res. 31:568–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yunchang M, Qinxue D, Binbin J, Xin H,
Lili Y, Linbi C, Wujun G, Pengbo Z and Junlu W: Human tissue
kallikrein ameliorates cerebral vasospasm in a rabbit model of
subarachnoid hemorrhage. Neurol Res. 37:1082–1095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stacul F, van der Molen AJ, Reimer P, Webb
JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement
O, et al: Contrast induced nephropathy: Updated ESUR contrast media
safety committee guidelines. Eur Radiol. 21:2527–2541. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seeliger E, Sendeski M, Rihal CS and
Persson PB: Contrast-induced kidney injury: Mechanisms, risk
factors, and prevention. Eur Heart J. 33:2007–2015. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kruk M: Measuring behaviour into the
twenty-first century. Trends Neurosci. 20:187–189. 1997. View Article : Google Scholar
|
|
19
|
Taufique Z, May T, Meyers E, Falo C, Mayer
SA, Agarwal S, Park S, Connolly ES, Claassen J and Schmidt JM:
Predictors of poor quality of life 1 year after subarachnoid
hemorrhage. Neurosurgery. 78:256–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang JP, Liu HJ and Liu RC: A modified
rabbit model of stroke: Evaluation using clinical MRI scanner.
Neurol Res. 31:1092–1096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Majoie CB, Sprengers ME, van Rooij WJ,
Lavini C, Sluzewski M, van Rijn JC and den Heeten GJ: MR
angiography at 3T versus digital subtraction angiography in the
follow-up of intracranial aneurysms treated with detachable coils.
AJNR Am J Neuroradiol. 26:1349–1356. 2005.PubMed/NCBI
|
|
22
|
van Amerongen MJ, Boogaarts HD, de Vries
J, Verbeek AL, Meijer FJ, Prokop M and Bartels RH: MRA versus DSA
for follow-up of coiled intracranial aneurysms: A meta-analysis.
AJNR Am J Neuroradiol. 35:1655–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Endo S, Branson PJ and Alksne JF:
Experimental model of symptomatic vasospasm in rabbits. Stroke.
19:1420–1425. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kertmen H, Gürer B, Yilmaz ER, Arikok AT,
Kanat MA, Ergüder BI and Sekerci Z: The comparative effects of
recombinant human erythropoietin and darbepoetin-alpha on cerebral
vasospasm following experimental subarachnoid hemorrhage in the
rabbit. Acta Neurochir (Wien). 156:951–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bederson JB, Connolly ES Jr, Batjer HH,
Dacey RG, Dion JE, Diringer MN, Duldner JE Jr, Harbaugh RE, Patel
AB and Rosenwasser RH; American Heart Association, : Guidelines for
the management of aneurysmal subarachnoid hemorrhage: A statement
for healthcare professionals from a special writing group of the
Stroke Council, American Heart Association. Stroke. 40:994–1025.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Venti M: Subarachnoid and intraventricular
hemorrhage. Front Neurol Neurosci. 30:149–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harrod CG, Bendok BR and Batjer HH:
Prediction of cerebral vasospasm in patients presenting with
aneurysmal subarachnoid hemorrhage: A review. Neurosurgery.
56:633–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Crowley RW, Medel R, Dumont AS, Ilodigwe
D, Kassell NF, Mayer SA, Ruefenacht D, Schmiedek P, Weidauer S,
Pasqualin A and Macdonald RL: Angiographic vasospasm is strongly
correlated with cerebral infarction after subarachnoid hemorrhage.
Stroke. 42:919–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jadhav V, Sugawara T, Zhang J, Jacobson P
and Obenaus A: Magnetic resonance imaging detects and predicts
early brain injury after subarachnoid hemorrhage in a canine
experimental model. J Neurotrauma. 25:1099–1106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar A, Kato Y, Hayakawa M, Junpei O,
Watabe T, Imizu S, Oguri D and Hirose Y: Recent advances in
diagnostic approaches for sub-arachnoid hemorrhage. Asian J
Neurosurg. 6:94–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pryor JC, Setton A, Nelson PK and
Berenstein A: Complications of diagnostic cerebral angiography and
tips on avoidance. Neuroimaging Clin N Am. 6:751–758.
1996.PubMed/NCBI
|
|
32
|
Laslo AM, Eastwood JD, Chen FX and Lee TY:
Dynamic CT perfusion imaging in subarachnoid hemorrhage-related
vasospasm. AJNR Am J Neuroradiol. 27:624–631. 2006.PubMed/NCBI
|
|
33
|
Hotta K, Sorimachi T, Osada T, Baba T,
Inoue G, Atsumi H, Ishizaka H, Matsuda M, Hayashi N and Matsumae M:
Risks and benefits of CT angiography in spontaneous intracerebral
hemorrhage. Acta Neurochir (Wien). 156:911–917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pierot L, Portefaix C, Rodriguez-Régent C,
Gallas S, Meder JF and Oppenheim C: Role of MRA in the detection of
intracranial aneurysm in the acute phase of subarachnoid
hemorrhage. J Neuroradiol. 40:204–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frontera JA, Ahmed W, Zach V, Jovine M,
Tanenbaum L, Sehba F, Patel A, Bederson JB and Gordon E: Acute
ischaemia after subarachnoid haemorrhage, relationship with early
brain injury and impact on outcome: A prospective quantitative MRI
study. J Neurol Neurosurg Psychiatry. 86:71–78. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Griffiths PD, Wilkinson ID, Mitchell P,
Patel MC, Paley MN, Romanowski CA, Powell T, Hodgson TJ, Hoggard N
and Jellinek D: Multimodality MR imaging depiction of hemodynamic
changes and cerebral ischemia in subarachnoid hemorrhage. AJNR Am J
Neuroradiol. 22:1690–1677. 2001.PubMed/NCBI
|
|
37
|
Wani AA, Phadke R, Behari S, Sahu R,
Jaiswal A and Jain V: Role of diffusion-weighted MRG in predicting
outcome in subarachnoid hemorrhage due to anterior communicating
artery aneurysms. Turk Neurosurg. 18:10–16. 2008.PubMed/NCBI
|
|
38
|
Huang CC, Lai CJ, Tsai MH, Wu YC, Chen KT,
Jou MJ, Fu PI, Wu CH and Wei IH: Effects of melatonin on the nitric
oxide system and protein nitration in the hypobaric hypoxic rat
hippocampus. BMC Neurosci. 16:612015. View Article : Google Scholar : PubMed/NCBI
|