Introduction
Ovarian cancer is one of the three malignant tumors
of female reproductive system. Statistics revealed that in 2014,
there were ~21,980 new cases of ovarian cancer and ~14,270 patients
succumbed to the disease in the United States (1). Due to the lack of specific symptoms in
early stage of the disease and the lack of effective screening
strategies, the majority of cases progress to an advanced stage at
the time of primary diagnosis (1).
As treatment is for ovarian cancer is long, painful and decreases
patients' quality of life, and the recurrence rate is high, it was
reported that 60–80% patients with advanced ovarian cancer relapse
2–3 years after the initial treatment (2) Therefore, ovarian cancer is regarded as
the leading cause of mortality in female patients with a
reproductive system malignant tumor (1). The most effective treatment for ovarian
cancer is surgical cytoreduction and platinum-based chemotherapy
(3). Although these treatments have
improved the prognosis of patients to some extent, the 5-year
survival rate of ovarian cancer remains at approximately 30–40%
(4).
Resistance to platinum-based agents is one of the
biggest factors affecting the therapeutic efficacy of
pharmacological agents for ovarian cancer (5); therefore, identifying a highly
efficient and low-toxic anti-cancer treatment that is able to
attenuate resistance to platinum-based agents in ovarian cancer
cells is of great importance. The Chinese medicine naringin, which
is a natural flavonoid drug, has been demonstrated to have
anti-cancer potential, with properties including inhibiting tumor
cell proliferation, promoting tumor cell apoptosis and interfering
in tumor cell signal transduction (6). A previous study by our group revealed
that 20–40 µmol/l naringin was able to significantly inhibit the
proliferation of ovarian cancer cells resistant to platinum-based
agents in vitro (7); however,
the underlying mechanisms by which naringin reverses this
resistance remain unclear. A previous study indicated that the
nuclear factor (NF)-κB signaling pathway serves a role in the
development and progression of ovarian cancer (8). The aim of the present study is to
investigate the mechanism of action by which naringin inhibits the
expression of P-glycoprotein (P-gp) in a cisplatin-resistant human
epithelial ovarian cancer cell line (SKOV3/CDDP) from the
perspective of NF-κB signal transduction, thereby providing an
experimental rationale for the development and application of
naringin as a treatment for ovarian cancer.
Materials and methods
Cells and reagents
The cisplatin-resistant ovarian cancer cell line
SKOV3/CDDP was purchased from the Chinese Academy of Sciences
(Beijing, China). Naringin was provided by the Institute of
Pharmacology at Nanchang University (Nanchang, China), and
cisplatin was purchased from Qilu Pharmaceutical Co., Ltd.
(Shandong, China). RPMI-1640 culture medium and fetal bovine serum
(FBS) were purchased from Beijing Soledad Lite-On Technology Co.,
Ltd. (Beijing, China). Antibodies directed against NF-κB (cat. no.
ab16502) and P-gp (cat. no. ab103477) were purchased from Abcam
(Cambridge, UK), horseradish peroxidase-labeled (HRP) goat
anti-rabbit IgG (cat. no. TA130015) was purchased from OriGene
Technologies, Inc. (Beijing, China). TRIzol was purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Primers for polymerase chain reaction (PCR) were synthesized by
GenScript (Piscataway, NJ, USA). The NF-κB small interfering
(si)RNA (sense, 5-GGA GUA CCC UGA GGC UAU ATT-3′ and anti-sense,
5-UAU AGC CUC AGG GUA CUC CTT-3), the negative control siRNA
(sense, 5-UUC UCC GAA CGU GUC ACG UdT dT-3 and anti-sense,
5-ACGUGACACGUUCGGAGAAdTdT-3) and plasmid construction was performed
by Guangzhou Ruibo Biological Technology Co., Ltd. (Guangzhou,
China).
Experimental methods
Cell culture
SKOV3/CDDP cells were cultured in RPMI-l640 complete
culture medium containing 10% FBS, 100 U/ml penicillin and 100 U/ml
streptomycin at 37°C in an atmosphere containing 5% CO2,
with the medium replaced every other day and passage performed
every 2–3 days. Cells were cultured to the logarithmic phase and
were then randomly assigned to either the normal control group
(SKOV3/CDDP cells cultured in RPMI-l640 with 10% FBS, 100 U/ml
penicillin and 100 U/ml streptomycin at 37°C and 5% CO2)
or the naringin treatment group (treated with 10, 20 or 40 µmol/l
naringin). Cells were cultured for 48 h at 37°C with or without
naringin according to their group, with 5 duplicates performed for
each group. All experiments were performed in triplicate.
Preparation of naringin
Naringin stock solution (7 mmol/l) was prepared by
dissolving 4 mg naringin in 1 ml dimethylsulfoxide. Naringin
solution (200 µmol/l) was prepared by adding, 200 µl naringin
solution to the RPMI-1640 culture medium (6.8 ml). Different
concentrations of naringin solution (40, 20 and 10 µmol/l) were
prepared by diluting this solution with RPMI-1640 to different
ratios.
Semi-quantitative reverse
transcription (RT) PCR analysis
Total RNA was extracted from cells in the above
groups using TRIzol according to the manufacturer's protocol, and
RNA was converted into cDNA using a reverse transcription kit (cat.
no. DRR037A; Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol. RT was performed using a
thermocycler at 37°C for 15 min and at 85°C for 5 sec. Primer
sequences for PCR are presented in Table
I. Amplification was performed using
2×EasyTaq® PCR SuperMix (Beijing Transgen Biotech
Co., Ltd., Beijing, China) and a thermocycler as follows:
Pre-denaturation at 4°C for 5 min, then 29 cycles of denaturation
at 94°C for 30 sec and annealing at 55°C for 30 sec, followed by
extension at 72°C for 30 sec and 72°C for 10 min. The PCR products
separated on a 2% agarose gel with ethidium bromide and detected
using a chemiluminescent gel imaging system (ChemiDoc™
XRS+; Bio-Rad, Hercules, CA, USA), and the Gel-Pro software
(version 4.0; Media Cybernetics, Inc., Rockville, MD, USA) was used
to analyze the integrated optical density (IOD) values of the
target gene bands.
 | Table I.Primer sequences and product size. |
Table I.
Primer sequences and product size.
| Gene name | Primer sequence
(5′-3′) | Product size
(bp) |
|---|
| Nuclear
factor-κB | F:
CAAGGAAGTCCCAGACCAAACC | 121 |
|
| R:
CTTCCTGCCCTACAGAGGTC |
|
| P-glycoprotein | F:
TCAGTCAAGTTCAGAGTCTTCA | 187 |
|
| R: CAAGGCAGTCAG
TTACAGTCC |
|
| β-actin | F: CGGGAAATCGTG
CGTGAC | 480 |
|
| R: GGACTCGTCATA
CTCCTGCT |
|
Western blotting assay
Cells were collected and lysed with protein lysate
on ice for 30 min at 4°C, followed by centrifugation 12,000 × g at
4°C for 15 min. The supernatant was collected and protein
concentrations were determined using the bicinchoninic acid assay
method, and each sample was diluted to a final concentration of 2
µg/µl. A total of 8 µg protein samples were loaded per lane and
separated by a 4% (stacking) and 10% (resolving) SDS-PAGE. The
proteins were transferred to a nitrocellulose membrane, which was
blocked with 5% skimmed milk for 2 h at room temperature. The
membrane was then incubated with NF-κB, P-gp and anti-tubulin (cat.
no. T3526; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) primary
antibodies overnight at 4°C, and incubated with the HRP-conjugated
secondary antibodies for 2 h at room temperature. The
nitrocellulose membrane was removed for visualization using
Pierce™ ECL Western Blotting Substrate (Thermo Fisher
Scientific, Inc.); anti-tubulin was used as the internal control.
IOD values of the bands were analyzed using Image Pro Plus software
(version 6.0; Media Cybernetics, Inc.) and the relatively
quantitative method [IOD (target protein)/IOD (reference protein)
ratio] was used to detect the expression level of the target
protein.
Cell transfection and
intervention
When the SKOV3/CDDP cells reached 90–95% confluence,
they were harvested and seeded in 6-well plates at a density of
2×105 cells per well. Cells were cultured in an
incubator at 37°C in an atmosphere containing 5% CO2,
until cells covered 70–80% of the plates (~24 h). Cells were
subsequently transfected with 100 pmol plasmids or 100 pmol siRNA
according to their group, using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according the manufacturer's
protocol. The cells were divided into the following groups: Blank
control group (no plasmids or siRNA), empty plasmid control group
(plasmids without a gene), NF-κB overexpression group (NF-κB
plasmids), siRNA control group (negative control siRNA) and the
NF-κB siRNA group (NF-κB siRNA). The expression of P-gp mRNA was
detected by semi-quantitative RT-PCR as previously mentioned at 48
h following transfection of NF-κB siRNA or plasmid overexpression,
and intervention with naringin was performed in SKOV3/CDDP cells
transfected with NF-κB overexpression plasmids. A previous study by
our group demonstrated that, the growth rate of drug resistant
cells was significantly decreased with a naringin dosage of 20
µmol/l (7). Therefore, a working
concentration of 20 µmol/l naringin was used in the present study,
and the groups used were as follows: Naringin (20 µmol/l) + NF-κB
overexpression group and the empty plasmid control group. A total
of 48 h after cell culturing, the expression of P-gp mRNA was
detected by semi-quantitative RT-PCR as previously mentioned.
Statistical analysis
Statistical analysis was performed using SPSS
(version 17.0; SPSS, Inc., Chicago, IL, USA). Student's t-test was
used for comparisons of the mean values between the two groups.
Intergroup comparisons were made using one-way analysis of
variance, and the Fisher's least significant differences t-test was
further adopted for pairwise comparison. P<0.05 was considered
to indicate a statistically significant difference.
Results
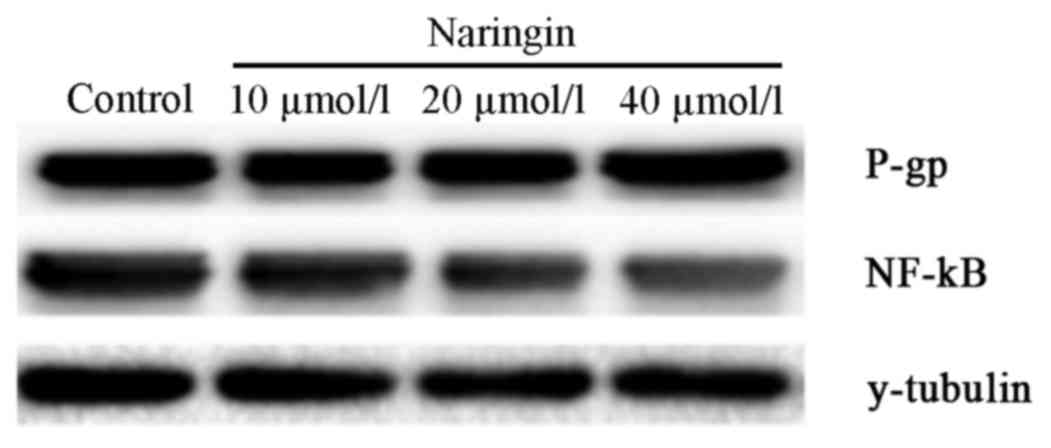
Effects of naringin on the expression
of NF-κB and P-gp at both mRNA and protein levels in SKOV3/CDDP
cell lines
Semi-quantitative RT-PCR and western blotting
results revealed that naringin significantly decreased the
expression of NF-κB and P-gp in a dose-dependent manner (P<0.05;
Tables II and III; Fig.
1). Significant differences in NF-κB and P-gp levels were
observed between the 40 µmol/l naringin and 10 µmol/l naringin
groups, and between the 20 µmol/l naringin and 10 µmol/l naringin
groups (both P<0.05; Tables II
and III; Fig. 1). However, there was no significant
difference between the 40 µmol/l naringin and 20 µmol/l naringin
groups.
 | Table II.Effects of different concentrations of
naringin on the expression of nuclear factor-κB and P-glycoprotein
mRNA in the SKOV3/CDDP cell line. |
Table II.
Effects of different concentrations of
naringin on the expression of nuclear factor-κB and P-glycoprotein
mRNA in the SKOV3/CDDP cell line.
| Group | Nuclear
factor-κB | P-glycoprotein |
|---|
| Control |
1.227±0.065 |
1.253±0.103 |
| Naringin (10
µmol/l) |
0.803±0.067a |
0.907±0.031a |
| Naringin (20
µmol/l) |
0.640±0.060a,b |
0.720±0.053a,b |
| Naringin (40
µmol/l) |
0.607±0.102a,b |
0.660±0.060a,b |
 | Table III.Relative amount of nuclear factor-κB
and P-glycoprotein proteins in the SKOV3/CDDP cell line with
different concentrations of naringin. |
Table III.
Relative amount of nuclear factor-κB
and P-glycoprotein proteins in the SKOV3/CDDP cell line with
different concentrations of naringin.
| Group | Nuclear
factor-κB | P-glycoprotein |
|---|
| Control group |
4.267±0.172 |
3.323±0.097 |
| Naringin (10
µmol/l) |
3.297±0.207a |
2.200±0.125a |
| Naringin (20
µmol/l) |
2.890±0.120a,b |
1.847±0.093a,b |
| Naringin (40
µmol/l) |
2.733±0.097a,b |
1.703±0.147a,b |
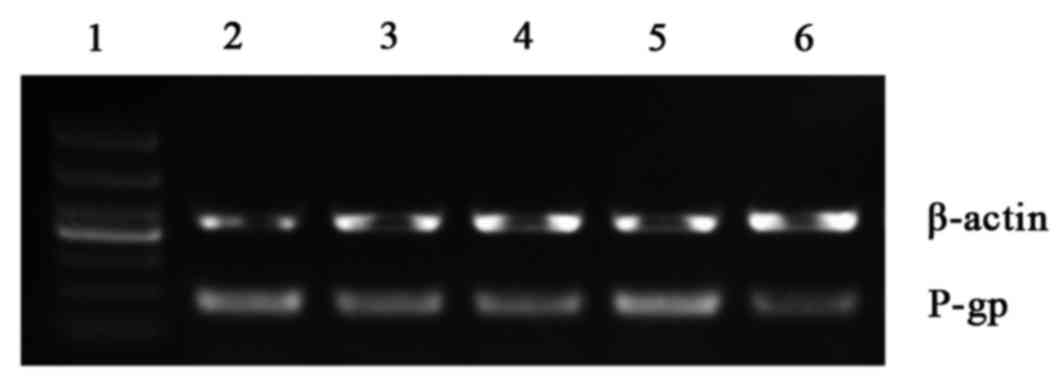
Effects of overexpression and
silencing of NF-κB on P-gp mRNA levels
RT-qPCR revealed that the expression of P-gp mRNA in
the NF-κB overexpression group was significantly upregulated
compared with the blank control and empty plasmid control groups
(both P<0.05; Table IV; Fig. 2). However, P-gp expression was
significantly downregulated in the siRNA NF-κB group compared with
the blank control and siRNA control groups (P<0.05; Table IV; Fig.
2).
 | Table IV.Relative expression levels of
P-glycoprotein mRNA following overexpression and silencing of
NF-κB. |
Table IV.
Relative expression levels of
P-glycoprotein mRNA following overexpression and silencing of
NF-κB.
| Group | P-glycoprotein |
|---|
| Blank control |
0.990±0.026 |
| Empty plasmid
control |
1.056±0.085 |
| siRNA control |
1.030±0.020 |
| NF-κB
overexpression |
1.810±0.070a,b |
| NF-κB siRNA |
0.437±0.095a,c |
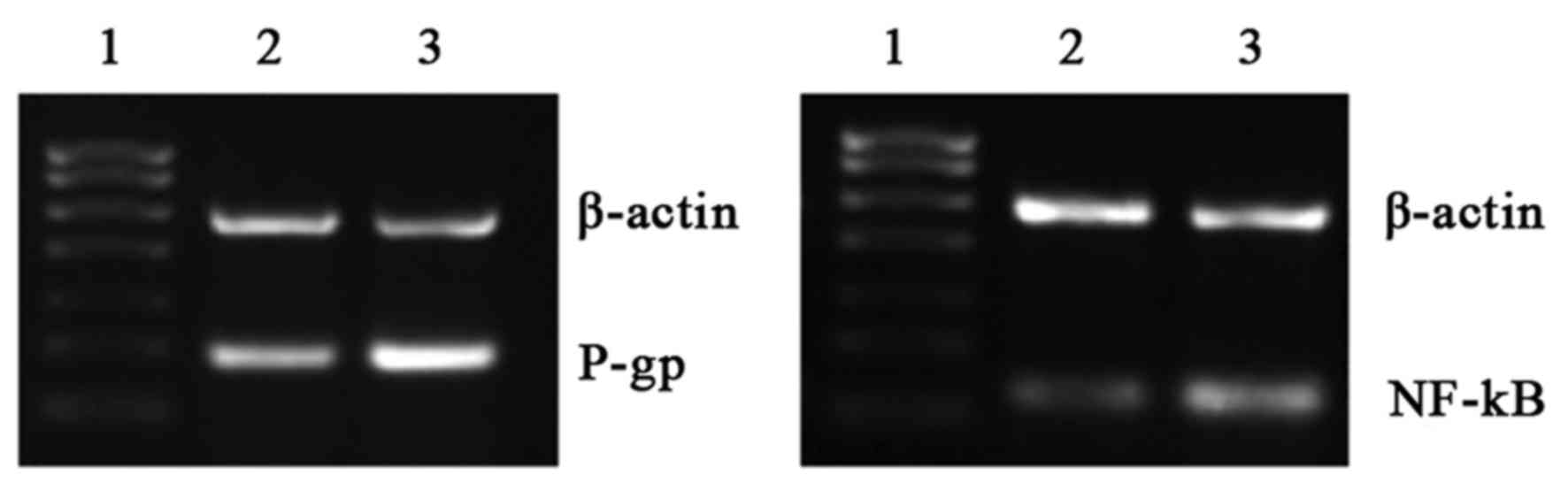
Effects of overexpression and
silencing of NF-κB and subsequent intervention with naringin
RT-qPCR results demonstrated that P-gp mRNA levels
were significantly decreased in the NF-κB overexpression group
compared with the control group following intervention with
naringin (P<0.05; Table V;
Fig. 3).
 | Table V.Effects of NF-κB over-expression and
intervention with naringin on the expression of mRNA of P-gp. |
Table V.
Effects of NF-κB over-expression and
intervention with naringin on the expression of mRNA of P-gp.
| Group | P-gp | NF-κB | T-value | P-value |
|---|
| Naringin (20
µmol/l) + NF-κB overexpression group |
1.077±0.065a |
0.610±0.089a |
−8.496 | 0.001 |
| Empty plasmid
control group |
1.753±0.080 |
1.797±0.064 | −12.266 | 0.000 |
Discussion
Ovarian cancer is the primary cause of reproductive
cancer-related mortality in women in the United States (1). Typically, symptoms do not present in
the early stages of ovarian cancer, which renders it difficult to
diagnose, and the majority of cases are at an advanced stage when
diagnosed (9). At present, the
suggested treatment methods of ovarian cancer are surgery and
chemotherapy (10–13), and platinum-based agents and
paclitaxel have become the first-line chemotherapeutic agents for
the treatment of ovarian cancer (14,15).
However, the emergence of multi-drug resistant ovarian cancer
cells, on which chemotherapeutic agents are ineffective, may reduce
the efficacy of chemotherapy (16).
Various traditional Chinese medicines have demonstrated potential
as anti-cancer agents, with advantages including no toxic reactions
and the ability to reverse resistance to chemotherapeutic agents
(17,18).
Naringin is a natural flavonoid compound that
primarily exists in the peels of pomelo, grapefruit, lime and
similar fruits (19). A previous
study reported that Naringin exhibits anti-inflammatory,
anti-oxidative stress, hypoglycemic, myocardial protective and
anti-tumor effects (20). Naringin
achieves its anti-tumor effects primarily via the inhibition of
tumor cell proliferation, promoting tumor cell apoptosis and
interfering in tumor cell signal transduction (21). The results of our previous study
suggested that naringin is able to significantly inhibit the
proliferation of SKOV3 and SKOV3/CDDP cells in vitro in a
time- and dose-dependent manner (22). However, the mechanism by which
naringin reverses resistance to platinum-based agents remains
unclear. The results of the present study suggest that naringin is
able to inhibit the expression of NF-κB and P-gp proteins in the
drug resistant ovarian cancer SKOV3/CDDP cell line in vitro
in a concentration-dependent manner.
One important factor leading to chemotherapy
resistance is drug efflux from cells. P-gp is an energy-dependent
drug pump encoded by the multidrug resistance 1 (MDR1) gene, and
its activation and expression are associated with a variety of
signaling pathways, such as the phosphoinositide 3-kinase/protein
kinase B, mitogen-activated protein kinase (MAPK), NF-κB,
Wnt/β-catenin Ras-Raf-MAPK kinase-extracellular signal-regulated
kinase and prostaglandin E2-cAMP-protein kinase C-NF-κB pathways
(23). P-gp discharges multiple
cytoxic drugs using ATP within the cells, which increases the
resistance of tumor cells to pharmacological agents, thereby
causing the resistance of tumor cells (24). MDR1/P-gp is expressed in almost all
human tumor cells to different degrees, and its expression in
tumors insensitive to chemotherapy or with poor efficacy is often
high (25). Previous studies have
reported that the sensitivity of some cancer patients to
chemotherapy was increased following the administration of P-gp
inhibitors (24,26–28). In
the present study, downregulation of P-gp protein by naringin was
also observed, and therefore it was inferred that the ameliorating
effect of naringin on cisplatin resistance of ovarian cancer cells
was associated with the downregulation of P-gp.
The results of the present study indicate that
naringin inhibits the expression of NF-κB in SKOV3/CDDP cells.
Studies have reported that NF-κB is associated with the
development, progression and metastasis of tumors, and many
proteins it encodes promote tumor growth (29–31). One
study demonstrated that NF-κB was persistently highly expressed in
cisplatin-resistant ovarian cancer cells, and that it served an
important role in drug resistance of ovarian cancer cells (5). NF-κB exerts anti-apoptotic effects via
regulating downstream target proteins B-cell lymphoma (Bcl)-xL,
Bcl-2, Fas/FasL, X-linked inhibitor of apoptosis protein, survivin,
cellular inhibitor of apoptosis protein 1/2, cyclin-dependent
kinase2, vascular endothelial growth factor and cyclooxygenase-2,
whereby the viability of tumor cells is increased, resulting in
chemotherapy resistance (8).
The NF-κB signaling pathway has been demonstrated by
a number of studies to be associated with the development and
progression of tumors (32,33). To further investigate the association
between NF-κB and P-gp, which is an important gene contributing to
drug resistance in ovarian cancer, the expression of P-gp mRNA was
assessed before and after overexpression or silencing of NF-κB. The
present results indicated that the expression of P-gp mRNA in the
NF-κB overexpression group was increased, whereas it was decreased
in the siRNA NF-κB group. This indicates that P-gp is subject to
regulation by the NF-κB signaling pathway. In a later experiment,
naringin was added as an intervention condition, and the expression
of P-gp mRNA in the NF-κB overexpression group was decreased. These
results suggest that the naringin-induced reversal of ovarian
cancer resistance to platinum-based agents may be associated with
the regulation of P-gp via the NF-κB signaling pathway in the
SKOV3/CDDP cell line.
In conclusion, naringin is able to inhibit the
expression of NF-κB and P-gp in SKOV3/CDDP cells. Such an
inhibitory effect may be dose-dependent, and is associated with the
blockade of the NF-κB signaling pathway.
Acknowledgements
The present study was supported by the Science And
Technology Plan Projects of Jiangxi Province (grant no.
20152ACG70022).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eoh KJ, Lee JY, Yoon JW, Nam EJ, Kim S,
Kim SW and Kim YT: Role of systematic lymphadenectomy as part of
primary debulking surgery for optimally cytoreduced advanced
ovarian cancer: Reappraisal in the era of radical surgery.
Oncotarget. 8:37807–37816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jessmon P, Boulanger T, Zhou W and
Patwardhan P: Epidemiology and treatment patterns of epithelial
ovarian cancer. Expert Rev Anticancer Ther. 17:427–437. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poveda Velasco A, Casado Herráez A,
Cervantes Ruipérez A, Gallardo Rincón D, García García E, González
Martín A, López García G, Mendiola Fernández C and Ojeda González
B; GEICO Group, : Treatment guidelines in ovarian cancer. Clin
Transl Oncol. 9:308–316. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi M, Fuller CD, Thomas CR Jr and Wang
SJ: Conditional survival in ovarian cancer: Results from the SEER
dataset 1988–2001. Gynecol Oncol. 109:203–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Birt DF, Hendrieh S and Wang W: Dietary
agents in cancer prevention: Flavonoids and isoflavonoids.
Pharmacol Thera. 90:157–177. 2001. View Article : Google Scholar
|
|
7
|
Song SH, Hu X, Xiong YQ and Cai LP:
Effects of Naringin on expression of COX-2 mRNA and protein in
human ovarian cancer cell line SKOV3. Chin J Clin Pharmacol Ther.
18:271–276. 2013.(In Chinese).
|
|
8
|
Godwin P, Baird AM, Heavey S, Barr MP,
O'Byrne KJ and Gately K: Targeting nuclear factor kappaB to
overcome resistance to chemotherapy. Front Oncol. 3:1202013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goff B: Symptoms associated with ovarian
cancer. Clin Obstet Gynecol. 55:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mucciolk M and Benencia F: Toll-like
receptors in ovarian cancer as targets for immunotherapies. Front
Immunol. 5:3412014.PubMed/NCBI
|
|
11
|
Bookman MA: First 1ine chemotherapy in
epithelial ovarian cancer. Clin Obstet Gynecol. 55:96–113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raja FA, Counsell N, Colombo N, Pfisterer
J, du Bois A, Parmar MK, Vergote IB, Gonzalez-Martin A, Alberts DS,
Plante M, et al: Platinum versus platinum, Combination chemotherapy
in platinum-sensitive recurrent ovarian cancer: A meta-analysis
using individual patient data. Ann Onco1. 24:3028–3034. 2013.
View Article : Google Scholar
|
|
13
|
Schwab CL, English DP, Roque DM and Santin
AD: Taxanes: Their impact on gynecologic malignancy. Anticancer
Drugs. 25:522–535. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pellicciotta I, Yang CP, Venditti CA,
Goldberg GL and Shahabi S: Response to microtubμle-interacting
agents in primary epithelial ovarian cancer cells. Cancer Cell Int.
13:332013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sorbe B, Graflund M, Nygren L and Horvath
G: A study of docetaxel weekly or every three weeks in combination
with carboplatin as first line chemotherapy in epithelial ovarian
cancer: Hematological and non-hematological toxicity proftes. Oncol
Lett. 5:1140–1148. 2014. View Article : Google Scholar
|
|
16
|
Colombo PE, Fabbro M, Theillet C, Bibeau
F, Rouanet P and Ray-Coquard I: Sensitivity and resistance to
treatment in the primary management of epithelial ovarian cancer.
Crit Rev Oncol Hematol. 89:207–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Han A, Chen E, Singh RK,
Chichester CO, Moore RG, Singh AP and Vorsa N: The cranberry
flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and
cell cycle arrest and increase cisplatin sensitivity in ovarian
cancer cells. Int J Oncol. 46:1924–1934. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li J, Wang Y, Lei JC, Hao Y, Yang Y, Yang
CX and Yu JQ: Sensitisation of ovarian cancer cells to cisplatin by
flavonoids from Scutellaria barbata. Nat Prod Res. 28:683–689.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salmah Y, Hasanah MG and Gan SK: Naringin
content in local citrus fruits. Food Chem. 37:113–121. 1990.
View Article : Google Scholar
|
|
20
|
You Q and Wu K: Naringin cardiovascular
pharmacological effects. Guangdong Med J. 31:3006–3008. 2010.
|
|
21
|
Meiyanto E, Hermawan A and Anindyajati:
Natural products for cancer-targeted therapy: Citrus flavonoids as
potent chemopreventive agents. Asian Pac J Cancer Prev. 13:427–436.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang L and Cai LP: Reversal of drug
resistance and reversal mechanism of human ovarian cancer resistant
SKOV3/DDP cells by naringin. J Clin Oncol. 21:598–602. 2016.
|
|
23
|
Dharmapuri G, Doneti R, Philip GH and
Kalle AM: Celecoxib sensitize simatinib-resistant K562 cell
stoimatinib by inhibiting MRP1-5, ABCA2 and ABCG2 transporters via
Wnt and Ras signaling pathways. Leuk Res. 39:696–701. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Januchowski R, Wojtowicz K,
Sujka-Kordowska P, Andrzejewska M and Zabel M: MDR gene expression
analysis of six drug-resistant ovarian cancer cell lines. Biomed
Res Int. 2013:2417632013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Januchowski R, Sterzyńska K, Zaorska K,
Sosińska P, Klejewski A, Brązert M, Nowicki M and Zabel M: Analysis
of MDR genes expression and cross-resistance in eight drug
resistant ovarian cancer cell lines. J Ovarian Res. 9:652016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abraham J, Salama NN and Azab AK: The role
of P-glycoprotein in drug resistance in multiple myeloma. Leuk
Lymphoma. 156:26–33. 2015. View Article : Google Scholar
|
|
27
|
Tomiyasu H, Watanabe M, Sugita K,
Goto-Koshino Y, Fujino Y, Ohno K, Sugano S and Tsujimoto H:
Regμlations of ABCB1 and ABCG2 expression through MAPK pathways in
acute lymphoblastic leukemia cell lines. Anticancer Res.
33:5317–5323. 2013.PubMed/NCBI
|
|
28
|
Vasconcelos FC, Silva KL, Souza PS, Silva
LF, Moellmann-Coelho A, Klumb CE and Maia RC: Variation of MDR
proteins expression and activity levels according to clinical
status and evolution of CML patients. Cytometry B Clin Cytom.
80:158–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Allen CT, Ricker JL, Chen Z and Van Waes
C: Role of activated nuclear factor-kappaB in the pathogenesis and
therapy of squamous cell carcinoma of the head and neck. Head Neck.
29:959–971. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weichert W, Boehm M, Gekeler V, Bahra M,
Langrehr J, Neuhaus P, Denkert C, Imre G, Weller C, Hofmann HP, et
al: High expression of Rel A/P65is associated with activation of
nuclear factor-kappa B-dependent signaling in pancreatic cancer and
marks a patient population with poor prognosis. Br J Cancer.
97:523–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bivona TG, Hieronymus H, Parker J, Chang
K, Taron M, Rosell R, Moonsamy P, Dahlman K, Miller VA, Costa C, et
al: FAS and NF-κB signaling modulate dependence of lung cancers on
mutant EGFR. Nature. 471:523–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cho HH, Song JS, Yu JM, Yu SS, Choi SJ,
Kim DH and Jung JS: Differential effect of NF-kappaB activity on
beta-catenin/Tcf pathway in various cancer cells. FEBS Lett.
582:616–622. 2008. View Article : Google Scholar : PubMed/NCBI
|