Introduction
Systemic lupus erythematosus (SLE) is a systemic
autoimmune disease with multi-organ involvement in which
autoantibodies induce tissue damage (1). Immunosuppressive therapy with
pharmacological agents, including mycophenolate mofetil (2) and cyclophosphamide (3), is widely used to improve the clinical
outcomes of SLE. However, nonspecific suppression of the immune
system and the associated side effects reduce patient quality of
life (4). Several studies have aimed
to induce specific tolerance to enhance the therapeutic effect of
organ transplantation via utilizing immune regulatory cells, in
particular antigen-presenting cells (APCs) (5,6) and
regulatory T cells (Tregs) (7).
However, few reports have focused on the use of tolerogenic APCs as
a therapeutic strategy for the treatment of SLE.
Dendritic cells (DCs) are highly efficient APCs that
have been studied in rodents and humans (8,9). DCs may
be identified at immature and mature stages based on their
phenotypic and functional characteristics (10). Mature DCs, which express high levels
of major histocompatibility complex II (MHC II), cluster of
differentiation (CD)80 and CD86 (11), secrete the T helper (Th) 1
cell-driving cytokine interleukin (IL)-12 (12). These molecules are essential for the
T cell immunological response, which demonstrates that mature DCs
serve an important role in the initiation of immune responses
(13). Immature DCs express low
levels of MHC II and co-stimulatory molecules, including CD40, CD80
and D86 (14), and have low T cell
stimulatory ability. These cells are associated with T cell energy
induction and Treg cell generation (15). In clinical practice, infusion with
immature DCs, either alone or in combination with a co-stimulation
blockade, regulates host T cell responses and effectively prolongs
allograft survival (16–18). However, the maturity status of DCs is
not stable as immature DCs differentiate into mature DCs when
exposed to antigens and stimulation factors (19). Therefore, maintaining the status of
immature DCs is key for the successful application of immature DCs
in immunotherapy.
It has previously been reported that IL-10 is able
to inhibit DC maturation (20),
causing more IL-10 to be secreted by immature DCs; this feedback
loop amplifies the tolerogenic effect of immature DCs. IL-10
therefore serves a pivotal role in the prevention of DC maturation
and the induction of immune tolerance (21) during the progression of autoimmune
disease or transplant rejection (22). In culture, DCs transduced with IL-10
have been demonstrated to have reduced levels of co-stimulatory
molecules (CD80/CD86) and, furthermore, do not produce the potent
allo-stimulatory cytokine IL-12 (23). A previous in vivo study
revealed that animals which received IL-10-overexpressing DCs had a
reduced incidence of skin graft rejection compared with animals
that received DCs modified with a control virus, suggesting that
there was reduced mononuclear cell infiltration and less
dermo-epidermal junction destruction (24). In addition, IL-10-treated DCs inhibit
antigen-specific immune responses in pre-activated immunocytes and
these effects persist following repeated antigen restimulation
(25). Immature DCs have been
introduced as a therapy for SLE (26), and it has been reported that
cytotoxic T lymphocyte-associated antigen 4-immunoglobulin
(CTLA4-Ig) is able to induce immune suppression in autoimmune
diseases (27) and organ
transplantation (28). It was
therefore hypothesized that the combination of immature DCs and
CTLA4-Ig may effectively induce immune tolerance.
The aim of the present study was to explore the
effect of DC-induced immune tolerance in SLE. Immature DCs, which
were prevented from maturing using IL-10, were injected into the
caudal vein of lupus-prone B6.MRL-Faslpr/J mice. The
mice were also treated with CTLA4, which may serve to prevent the
transmission of co-stimulatory signals and induce T cells to
undergo apoptosis, become inactivated or anergic, thus inducing
immune tolerance.
Materials and methods
Materials
Recombinant mouse granulocyte-macrophage
colony-stimulating factor (rmGM-CSF), rmIL-10, and rmIL-4 were
purchased from PeproTech, Inc. (Rocky Hill, NJ, USA). Mouse
antibodies directed against CD40 (cat no. 11-0402-82), CD80 (cat
no. 15-0801-82), CD86 (cat no. 12-0862-82), MHC II (cat no.
12-5321-82), IL-17A (cat no. 12-7177-81), IgG2a (cat no.
12-4321-80), CD4 (cat no. 11-0042-82) and forkhead box protein P3
(Foxp3; cat no. 12-4774-42) were purchased from eBioscience (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). rmCTLA4-Ig was
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
RPMI-1640 medium and fetal bovine serum (FBS) was purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). IL-17A
(cat no. kt21287), anti-nuclear antibody (ANA; cat no. kt40119),
and double-stranded (ds)DNA (cat no. kt21274) ELISA kits were
purchased from MSK Biological Technology, Ltd. (Wuhan, Hubei,
China).
B6.MRL-Faslpr/J lupus mice were purchased
from the Model Animal Research Center of Nanjing University
(Nanjing, China). C57Bl/6J mice were purchased from Hunan SJA
Laboratory Animals Co., Ltd. (Changsha, Hunan, China).
Immature DC culture
All experiments used in the present study were
approved by the Ethical Review Committee of the First Affiliated
Hospital of Guangxi Medical University (Nanning, China), and all
experimental procedures were conducted in conformity with the
institutional guidelines for the care and use of laboratory
animals. All surgeries were performed under sodium pentobarbital
(Merck KGaA, Darmstadt, Germany) anesthesia.
All mice were housed in an SPF level lab under
controlled a temperature of 20–24°C and a relative humidity of
50–60% with a 12/12 h light-dark cycle. All of them had free access
to formula feed and water.
A total of 10 female B6.MRL-Faslpr/J
lupus mice (2-months-old; weighing 18–20 g) were sacrificed,
following which femurs and tibias were carefully harvested under
aseptic conditions. Mouse bone marrow cells were collected by
flushing the medullary cavity gently using RPMI-1640 medium
supplemented with 10% FBS. The cells (1×106 cells/ml)
were transferred into 6-well plates (2 ml/well) and incubated at
37°C in an humidified atmosphere containing 5% CO2 for
4–6 h. Non-adherent cells were removed and 2 ml RPMI-1640 with 10%
FBS, 20 ng/ml rmGM-CSF and 10 ng/ml rmIL-4 was added to each well.
The medium was replaced every other day. At 5 days, IL-10 (10
ng/ml) was added to the medium, and cells were cultured for an
additional 1 and 4 days until day 6 (6-IL-10-DC group) or day 9
(9-IL-10-DC group), respectively. Non-treated cells were also
cultured for 6 days (D6-DC group) or 9 days (D9-DC group). Changes
in the morphology and number of cells in the four groups were
observed using an inverted microscope. A total of 10 high power
fields of vision (magnification, ×200) from each group were chosen
randomly to count the cell numbers. The expression of CD80, CD86,
MHC II and CD40 was examined using flow cytometry to identify the
purity and maturity of the cells. Prior to detection, cells
(1×107 cells/ml) were washed with PBS 3 times (5 min
each time). Fc receptor-blocking pharmacon (1:50; eBioscience; cat
no. MFCR00-4; Thermo Fisher Scientific, Inc) was added into the
reaction tube and incubated for 15 min at 4°C. All primary
antibodies, including CD80 (cat no. 15-0801-82), CD86 (cat no.
12-0862-82), MHC II (cat no. 12-5321-82) and CD40 (cat no.
11-0402-82; all Thermo Fisher Scientific, Inc.) were directly
labeled with phycoerythrin or fluorescein isothiocyanate. Each
antibody was incubated for 30 min at room temperature. Samples with
the added antibody (CD80, 1:50; CD86, 1:50; MHC II, 1:200 and CD40,
1:200) were incubated at room temperature in the dark for 30 min.
The cells were washed with PBS 3 times, cells were fixed in
paraformaldehyde (1:50) at 4°C and analyzed using a FACS Calibur
flow cytometer within 24 h. Data were quantified using FCS software
(version no. 4.0; BD Biosciences, Franklin Lakes, NJ, USA).
SLE mouse treatment
A total of 24 4-month-old female B6.
MRL-Faslpr/J lupus mice weighing 18–20 g were randomly
divided into the following groups (n=6/group): IL-10-DC group;
CTLA4-Ig group; IL-10-DCs + CTLA4-Ig group; and PBS group. Mice
were administered with 0.1 ml IL-10-treated DCs (1×108
cells/ml), 0.1 ml CTLA4-Ig (100 µg/ml), 0.1 ml mixture of
IL-10-treated DCs and CTLA4-Ig, or 0.1 ml PBS (1 M/l) via the tail
vein. The normal group included 6 female C57Bl/6J mice weighing
24–27 g with no interventions. All interventions were repeated at
0, 2, 4 and 6 weeks. Housing conditions were as described
above.
Biochemical analysis
Samples of urine and serum from SLE and normal mice
were collected 2 days prior to the first intervention and 2 weeks
following the last intervention. Mice were fasted for 12 h and
transferred to metabolic cages prior to urine collection. During
the 24 h collection period, urine was collected every 6 h via
collecting tubes, the samples were centrifuged at 108 × g for 5 min
at room temperature to remove impurities and the supernatant was
collected and stored at 4°C for further experiments. Urine proteins
(Shanghai Enzyme-linked Biotechnology Co., Ltd.; cat no. 1037585,
Shanghai, China) were detected using an automatic biochemical
analyzer according to the manufacturer's protocol. The
concentrations of IL-17A (MSK, cat no. kt21287, Wuhan, Hubei,
China), ANA (MSK, cat no. kt40119, Wuhan, Hubei, China) and dsDNA
(MSK, cat no. kt21274, Wuhan, Hubei, China) antibodies in the serum
were detected using ELISA kits according to the manufacturer's
protocol.
Lymphocyte separation
Mice in each group were sacrificed at 2 weeks
following the last intervention. Spleens were harvested and
macerated in RPMI-1640 with 4% FBS. Spleen cells were filtered and
lymphocytes were collected using density gradient centrifugation.
Spleen cells suspension was added to the lymphocyte separation
medium at a ratio of 1:1. The cell mixture was divided into 4
layers following centrifugation at 672 × g for 20 min at room
temperature. The second layer solution was extracted (counting from
top to bottom) by washing twice with PBS at 1:6 ratio and
centrifuged at 168 × g for 10 min at room temperature. After
discarding the PBS, spleen lymphocytes were collected and
resuspended in RPMI-1640 at a density of 2×106
cells/ml.
Th17 cell detection
The proportion of Th17 cells out of the lymphocytes
derived from SLE mouse spleens was quantified. Briefly, cell
suspensions (2×106 cells/ml) were added into centrifuge
tubes (0.5 ml) with phorbol 12-myristate 13-acetate (1:40)
(Shanghai Yisheng Biotechnology Co. Ltd, cat no. 50601ES02,
Shanghai, China), ionomycin (1:50) and brefeldin A (1:50), and
incubated for 4 h at 37°C. Cells were washed with PBS and
centrifuged at 168 × g for 5 min at room temperature. Prior to
incubation with antibodies, Fc receptor-blocking pharmacon (1:50)
(eBioscience, cat no. MFCR00-4, Thermo Fisher Scientific, Inc.) was
added and incubated for 15 min at 4°C. CD4 antibodies (1:200;
eBioscience, cat no. 12-0041-82, Thermo Fisher Scientific, Inc.)
were subsequently added to the cell suspensions and incubated at
4°C in the dark for 30 min. Cells were washed with permeabilization
buffer (eBioscience, cat no. 00-8333-56, Thermo Fisher Scientific,
Inc.) and centrifuged at 168 × g for 5 min at 4°C. Subsequently,
fixation/permeabilization solution (1:100; eBioscience, cat no.
00-5123-43, Thermo Fisher Scientific, Inc.) was added and the
suspension was incubated for 45 min at 4°C in the dark. Cells were
washed with permeabilization buffer (eBioscience, cat no
00-8333-56, Thermo Fisher Scientific, Inc.) and centrifuged at 168
× g for 5 min. Fc receptor-blocking pharmacon (1:50) was added
secondly and incubated for 15 min. IL-17A (1:100; eBioscience, cat
no. 12-7177-81, Thermo Fisher Scientific, Inc.) antibodies or an
isotype control IgG2a (1:100; eBioscience, cat no. 12-4321-80,
Thermo Fisher Scientific, Inc.) antibodies were added to each tube,
which were incubated for 15 min at 4°C. Cells were subsequently
washed with PBS and centrifuged at 168 × g for 5 min at 4°C. The
supernatant was discarded, and the cells were resuspended with 0.2
ml paraformaldehyde (1:50) at 4°C. The cells were analyzed by flow
cytometry within 24 h. Data were quantified using FCS software
(version no. 4.0). CD4+IL-17A+ cells were
identified as the Th17 cell subset.
Treg cell examination
A low proportion of Th17 cells and high proportion
of Treg cells is an indicator of immune tolerance induced in
vivo (29). Therefore, the
proportion of Treg cells out of the lymphocytes derived from the
mouse spleens was examined and the Th17/Treg cell ratio was
analyzed to investigate the tolerogenic effect of combined
treatment with IL-10-treated DCs and CTLA4-Ig in SLE. All
preparation was controlled at 4°C. Briefly, cell suspensions
(2×106 cells/ml) were added to tubes (1×106
cells/tube) and then Fc receptor-blocking pharmacon (1:50) (cat no.
MFCR00-4; Thermo Fisher Scientific, Inc) and CD4 (1:200) antibodies
(eBioscience, cat no. 11-0042-82, Thermo Fisher Scientific, Inc.)
were added to the reaction tubes and incubated for 30 min at 4°C.
Cells were washed with permeabilization buffer (eBioscience, cat
no. 00-8333-56, Thermo Fisher Scientific, Inc.) and centrifuged at
168 × g for 5 min at 4°C. After fixation and permeabilization with
fixation/permeabilization solution (1:100; eBioscience, cat no.
00-5123-43, Thermo Fisher Scientific, Inc.) for 45 min, cells were
washed with permeabilization buffer (cat no. 00-8333-56;
eBioscience; Thermo Fisher Scientific, Inc.) and centrifuged at 168
× g for 5 min at 4°C. Fc receptor-blocking pharmacon was then added
and incubated for 15 min at 4°C. Foxp3 (1:100) (eBioscience, cat
no. 12-4774-42, Thermo Fisher Scientific, Inc.) or lgG2a (1:100)
(eBioscience, cat no. 12-4321-80, Thermo Fisher Scientific, Inc.)
were added and incubated for 45 min at 4°C. Cells were washed with
permeabilization buffer and centrifuged at 168 × g for 5 min twice
at 4°C. The cells were fixed with 0.2 ml paraformaldehyde (1:50) at
4°C and analyzed by flow cytometer within 24 h.
CD4+Foxp3+ cells were identified as the Treg
cell subset, and IgG2a was used as an isotype control.
Statistical analysis
All experiments were performed a minimum of three
times with six replicate samples for each group. All data are
presented as the mean ± standard deviation. The homogeneity of
variances in the data was calculated using Levene's test. One-way
ANOVA was used to analyse the data. Multiple comparisons were
conducted using Games-Howell and Student-Newman-Keuls tests for
unequal and equal variances, respectively. P<0.05 was considered
to indicate a statistically significant difference.
Results
Immature DCs are induced by IL-10
To identify the maturation state of DCs that were
cultured with or without IL-10 in vitro, the DC maturation
state markers MHC II, CD40, CD86 and CD80 were detected using flow
cytometry. The results revealed that the expression of MHC II,
CD40, CD86, and CD80 was significantly lower in IL-10-treated DCs
compared with the non-treated DC groups (P<0.05; Fig. 1). Furthermore, the expression of MHC
II (P<0.05), CD80 (P<0.05) and CD86 (P<0.001) was
significantly lower in D6-DCs compared with D9-DCs (Fig. 1), indicating that, even with exposure
to IL-10, DCs are able to differentiate into mature DCs during
long-term in vitro culture. Based on these results, D6-DCs
were used for the in vivo study.
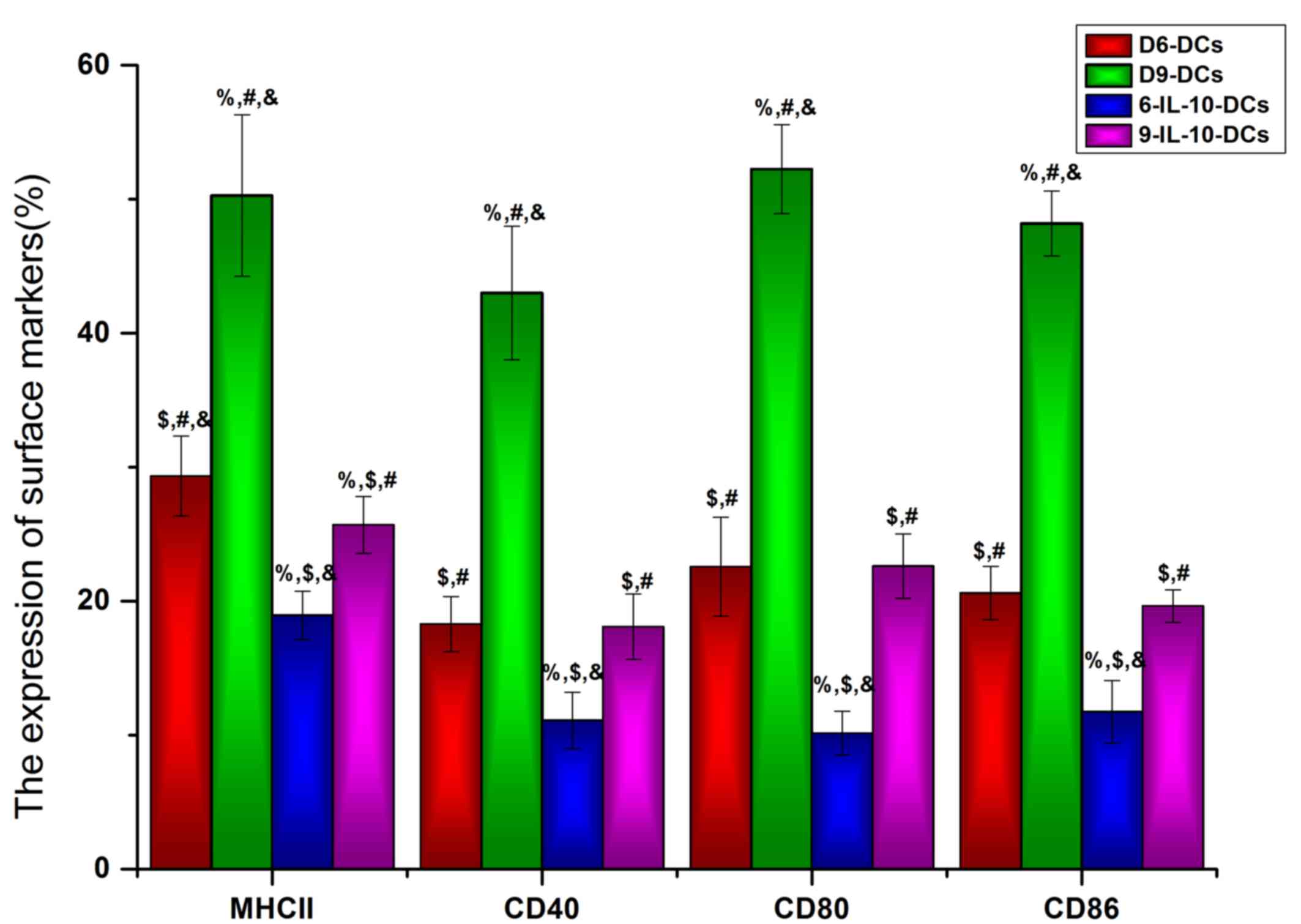 | Figure 1.Immature DCs are induced by IL-10.
The DC maturation state markers MHC II, CD40, CD86 and CD80 were
detected using flow cytometry. DCs, dendritic cells; IL,
interleukin; MHC II, major histocompatibility complex II; CD,
cluster of differentiation; D6-DCs, DCs cultured until day 6;
D9-DCs, DCs cultured until day 9; 6-IL-10-DCs; D6-DCs; treated with
IL-10; 9-IL-10-DCs, D9-DCs treated with IL-10.
%P<0.05 vs. the D6-DCs group, $P<0.05
vs. the D9-DCs group, #P<0.05 vs. the 6-IL-10-DCs
group, &P<0.05 vs. the 9-IL-10-DCs group. |
Immune tolerance is induced by
IL-10-DCs and CTLA4-Ig
Urine protein levels of the mice were assessed using
an automatic biochemical analyzer, whereas IL-17A, ANA and
anti-dsDNA levels were analyzed using ELISA kits. No significant
differences were observed in urine protein levels among the SLE
groups prior to treatment (Fig. 2A).
However, urine protein levels were significantly higher in the
IL-10-DCs, CTLA4-Ig, IL-10-DCs + CTLA4-Ig and PBS groups compared
with the normal group (P<0.05; Fig.
2A). Following treatment, urine protein levels in the
IL-10-DCs, CTLA4-Ig and IL-10-DCs + CTLA4-Ig groups were
significantly lower compared with pre-treatment values (P<0.05)
and the PBS group (P<0.05; Fig.
2A). Post-intervention urine protein levels were significantly
lower in the IL-10-DCs + CTLA4-Ig group compared with the IL-10-DCs
and CTLA4-Ig groups (P<0.001), and were close to those in the
normal group (P>0.05; Fig.
2A).
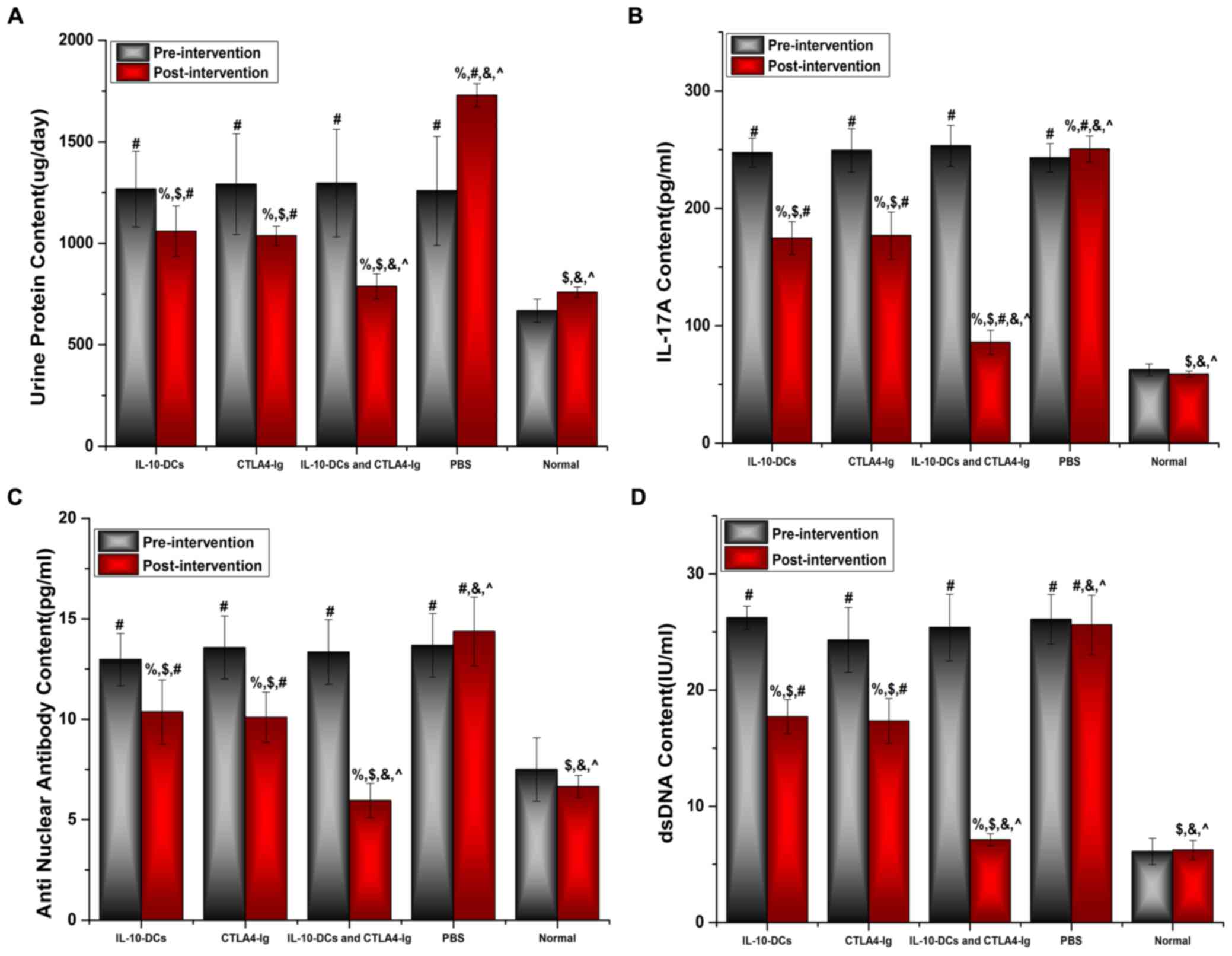 | Figure 2.Immune tolerance is induced by
IL-10-DCs and CTLA4-Ig. (A) Urine protein, (B) IL-17A, (C) ANA and
(D) dsDNA levels in mice pre- and post-intervention. IL,
interleukin; DCs, dendritic cells; CTLA4-Ig, cytotoxic T lymphocyte
antigen 4-immunoglobulin; ANA, anti-nuclear antibody; ds, double
stranded; Normal, untreated C57Bl/6J mice. %P<0.05
vs. the same group pre-intervention, $P<0.05 vs. the
PBS group post-intervention, #P<0.05 vs. the normal
group, &P<0.05 vs. the IL-10-DCs group,
^P<0.05 vs. the CTLA4-Ig group. |
No significant difference was observed in IL-17A,
ANA or dsDNA levels among the SLE groups prior to treatment
(Fig. 2B-D); however, these levels
were significantly higher in the IL-10-DCs, CTLA4-Ig, IL-10-DCs +
CTLA4-Ig and PBS groups compared with the normal mouse group
(Fig. 2B-D). Levels of IL-17A, ANA
and dsDNA were significantly lower following treatment compared
with pre-treatment in the IL-10-DC, CTLA4-Ig and IL-10-DCs +
CTLA4-Ig groups (%P<0.05; Fig. 2B-D). Post-intervention the relative
levels of IL-17A, ANA and dsDNA were significantly lower in the
IL-10-DCs + CTLA4-Ig group compared with the other treatment groups
(Fig. 2B-D). These results indicate
that SLE activity was most reduced in the IL-10-DCs + CTLA4-Ig
group and that immune tolerance was induced.
T cell responses
The proportions of Th17 and Treg cells, identified
by IL-17A and Foxp3 expression, respectively, were analyzed using
flow cytometry (Fig. 3). The results
revealed that the proportion of Th17 cells was significantly lower
in the IL-10-DCs, CTLA4-Ig and IL-10-DCs + CTLA4-Ig groups compared
with the PBS group (P<0.05), whereas it was significantly higher
in the treatment groups compared with the normal group (P<0.05;
Fig. 3B). Following treatment, the
proportion of Th17 cells in the IL-10-DCs + CTLA4-Ig group was
significantly lower compared with the IL-10-DC and CTLA4-Ig groups
(P<0.05; Fig. 3B).
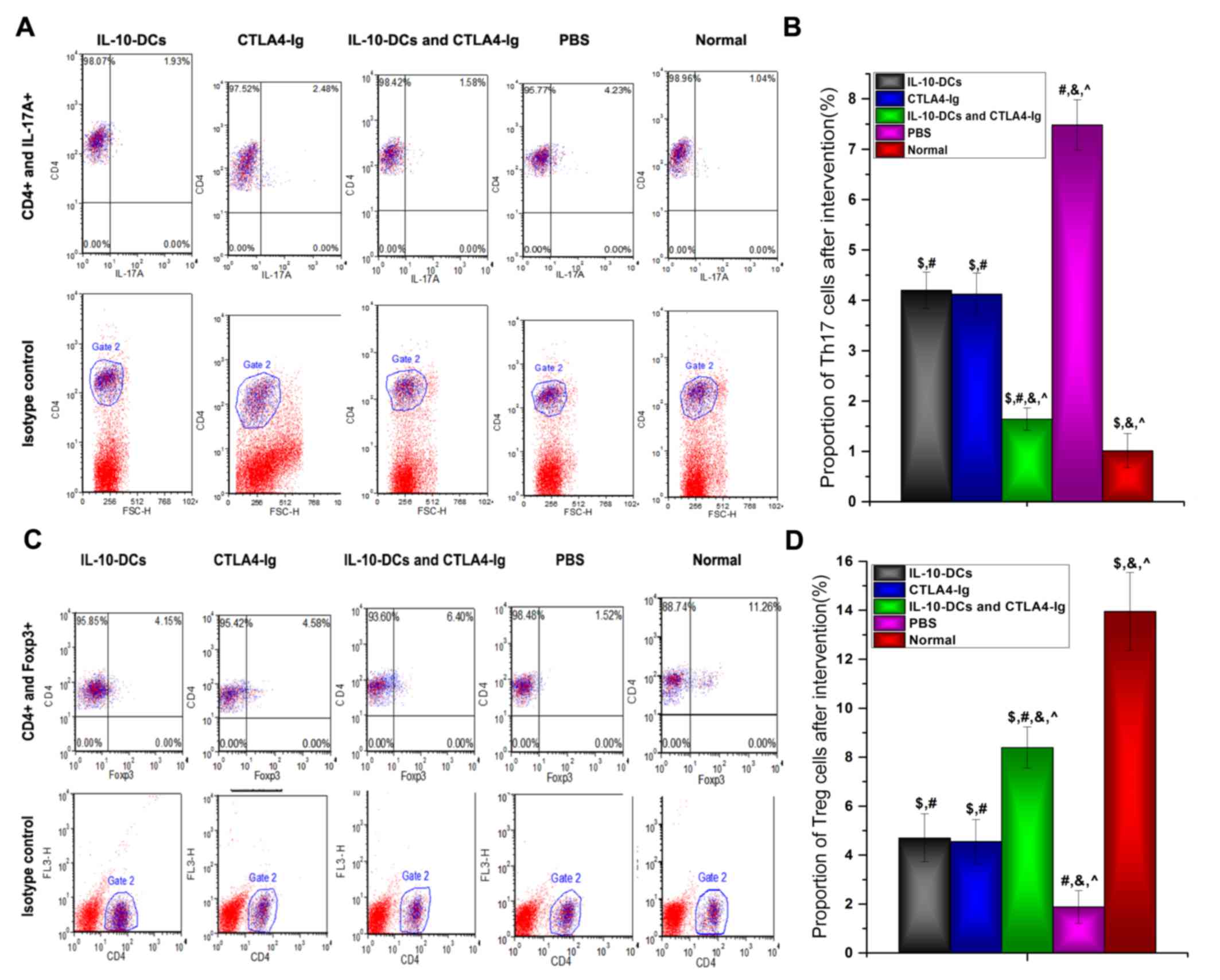 | Figure 3.Proportion of Th17 and Treg cells as
analyzed by flow cytometry. (A) CD4+IL-17A+
cells were identified as the Th17 cell subset and (B) analyzed. (C)
CD4+Foxp3+ cells were identified as the Treg
cell subset and (D) analyzed. Th, T helper cells; Treg, regulatory
T cells; CD, cluster of differentiation; IL, interleukin; Foxp3,
forkhead box protein P3; DCs, dendritic cells; CTLA4-Ig, cytotoxic
T lymphocyte antigen 4-immunoglobulin; PE, phycoerythrin; Normal,
untreated C57Bl/6J mice. $P<0.05 vs. the PBS group
post-intervention, #P<0.05 vs. the normal group,
&P<0.05 vs. the IL-10-DCs group,
^P<0.05 vs. the CTLA4-Ig group. |
In contrast, the proportion of Treg cells was
significantly higher in the IL-10-DCs, CTLA4-Ig and IL-10-DCs +
CTLA4-Ig groups compared with the PBS group (P<0.05) and
significantly lower compared with the normal group (P<0.05;,
Fig. 3D). Following treatment, Treg
cell numbers in the IL-10-DCs + CTLA4-Ig group were significantly
higher compared with the IL-10-DCs and CTLA4-Ig groups (Fig. 3D).
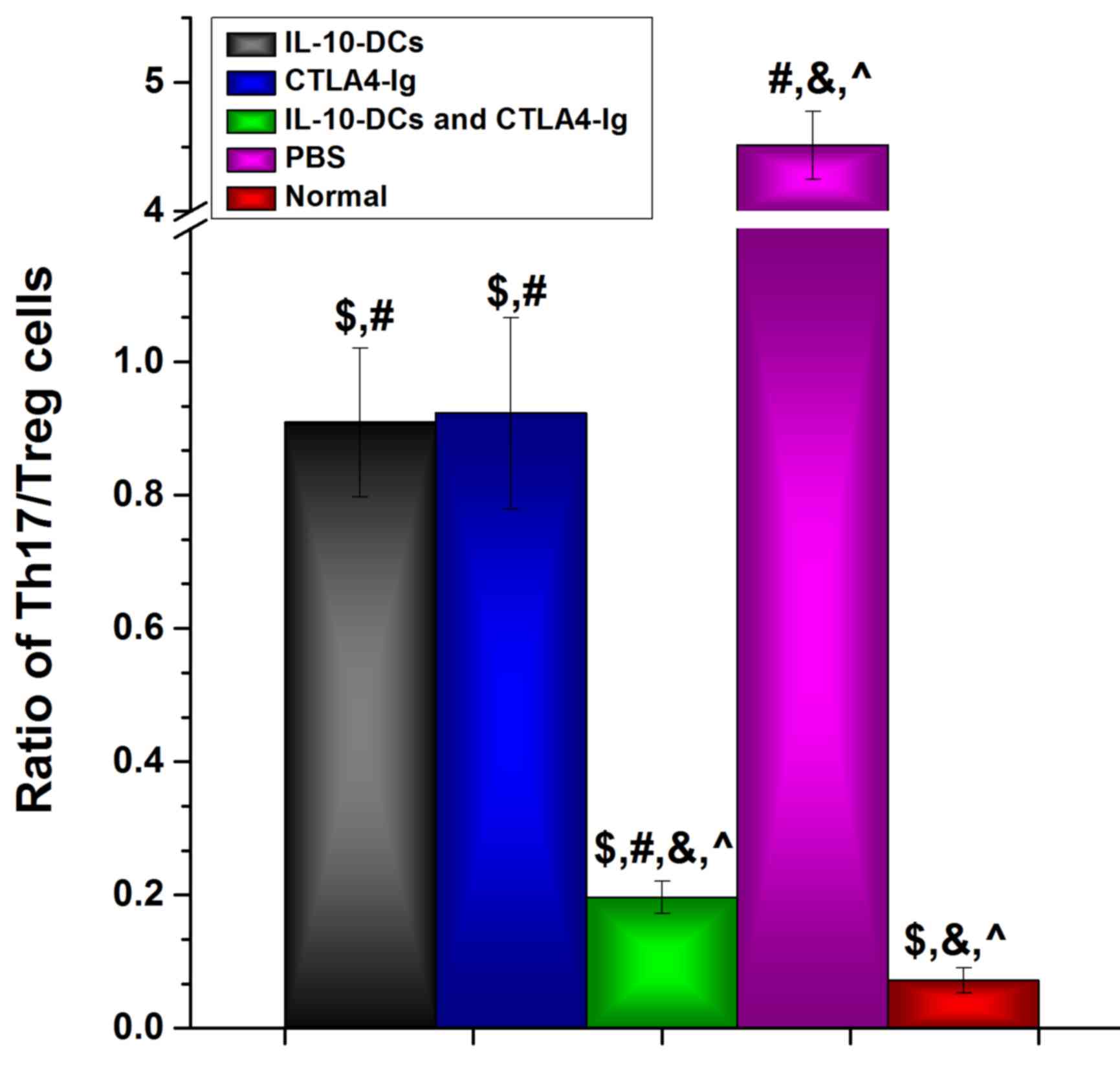
Following intervention, the Th17/Treg cell ratio in
the IL-10-DC, CTLA4-Ig and IL-10-DCs + CTLA4-Ig groups was
significantly lower compared with the PBS group (P<0.05) and
significantly higher compared with the normal group (P<0.05;
Fig. 4). The Th17/Treg cell ratio in
the IL-10-DCs + CTLA4-Ig group was much lower compared with the
IL-10-DC and CTLA4-Ig groups (Fig.
4). These results suggest that immune tolerance was induced by
the administration of IL-10-DCs and CTLA4-Ig in mice with SLE.
Discussion
The present study aimed to investigate DC-induced
immune tolerance in mice with SLE, an effect that was amplified by
the administration of CTLA4-Ig. In vitro, lower levels of
MHC II, CD40, CD80, and CD86 were expressed by IL-10-treated DCs
compared with non-treated cells, indicating that the process of
maturation was prevented. In the in vivo study,
administering mice with SLE with IL-10-treated DCs reduced the
levels of urine protein, ANA, dsDNA and IL-17A. Furthermore,
treatment with IL-10-treated DCs decreased the proportion of Th17
cells and increased the proportion of
CD4+Foxp3+ Treg cells, suggesting that immune
tolerance was induced by immature DCs in mice with SLE.
Coadministration with IL-10-DCs and CTLA4-Ig resulted in a greater
tolerance-inducing effect compared with IL-10-DCs or CTLA4-Ig
treatment alone.
To prevent DCs from maturing, IL-10 was added into
the culture medium in vitro. DCs are APCs that are
specialized to regulate T cell immunity, including activation of T
cells and maintenance of peripheral tolerance (30), and the function of DCs is dependent
on their state of activation and differentiation (31). Mature DCs are able to induce the
development of T effector cells (32), whereas immature DCs are associated
with the maintenance of immunological tolerance (33). In an in vitro culture system,
FBS acts as an antigen to facilitate DC maturation (34). For the D6-DCs group, the expression
of surface markers, including the immune response molecules MHC II,
CD40, CD86 and CD80, was evident in 25–30% of mature DCs. However,
in the D9-DCs group this expression increased to 40% under the same
conditions, indicating that DCs differentiate into mature cells
when cultured for an extended period. As an immunomodulatory
cytokine that inhibits DC function, IL-10 is a major factor that
prevents the differentiation of DCs from monocytes (35–37).
Furthermore, IL-10 is able to inhibit receptor-mediated
macropinocytosis and endocytosis following exposure to a soluble
immunogen (36), and serves an
important role in immune tolerance (38). In addition to exogenous IL-10,
autocrine IL-10 prevents spontaneous DC maturation in vitro,
limits lipopolysaccharide- and CD40-mediated maturation, and
increases IL-10 production by DCs (39). IL-10 secretion therefore assists in
maintaining the immature state of DCs.
The capacity of immature DCs and CTLA4-Ig to induce
immune tolerance was further explored in mice with SLE. The results
demonstrated that immune tolerance was strongly induced, as
evidenced by lower expression of urine protein, ANA, dsDNA and
IL-17A, as well as a decrease in Th17 cells and an increase in
CD4+Foxp3+ Treg cells. Immature DCs are
considered to be prototypic tolerogenic DCs due to their poor T
cell stimulatory capacity (40).
Immature DCs have therefore been utilized to induce
immunosuppression in mammals with specific malignancies or
autoimmune disease, as well as following transplantation (41–43).
Several methods, including co-culture with marrow stromal cells
(42), anti-vascular endothelial
growth factor antibody (44) and
IL-10 (45), have been used to
maintain the immature state of DCs. IL-10-treated DCs induce
alloantigen-specific T cell hyporesponsiveness and are also able to
inhibit antigen-specific immunological responses (46,47),
thereby prolonging liver allograft survival and enhancing immune
tolerance (48). Furthermore, in the
present study, CTLA4-Ig amplified the immune tolerance of
IL-10-treated DCs in vivo. The interaction of CD80 and CD86
molecules expressed on DCs with CD28 molecules expressed on T cells
is crucial for inducing T cell immune responses (49). CTLA4-Ig blocks CD28-mediated
co-stimulatory signaling to T cells to induce tolerance (50) and maintain the tolerogenic state of
DCs (51). It has previously been
demonstrated that the
CD4+CD25+Foxp3+ Treg population in
mouse joints and spleen is increased in CTLA4-Ig-treated
collagen-induced arthritis model mice and that DCs are modified to
become tolerogenic (52). However,
there are few reports on the effects of co-injection with immature
DCs and CTLA4-Ig in SLE. In the present study, the combination of
immature DCs and CTLA4-Ig effectively induced immune tolerance in
mice with SLE.
In summary, the results of the present study
demonstrate that combined treatment with IL-10-treated immature DCs
and CTLA4-Ig induces immune tolerance in mice with SLE.
Co-injection of DCs and CTLA4-Ig may therefore have potential as a
therapeutic strategy to promote immune tolerance, alleviate
multiple organ dysfunction and improve the quality of life of
patients with SLE.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460475), Guangxi
Natural Science Foundation (grant nos. 2013GXNSFDA253001 and
2010GXNSFA013186) and the Guangxi Health Department Key Project
(grant no. Guiweizhong 200829).
Glossary
Abbreviations
Abbreviations:
|
ANA
|
anti-nuclear antibody
|
|
APCs
|
antigen-presenting cells
|
|
DCs
|
dendritic cells
|
|
dsDNA
|
double-stranded DNA
|
|
IL
|
interleukin
|
|
MHC II
|
major histocompatibility complex
II
|
|
PE
|
phycoerythrin
|
|
CTLA4-Ig
|
cytotoxic T lymphocyte antigen
4-immunoglobulin
|
|
rmGM-CSF
|
recombinant mouse
granulocyte-macrophage colony-stimulating factor
|
|
SLE
|
systemic lupus erythematosus
|
|
Th
|
T helper cells
|
|
Treg
|
regulatory T cells
|
References
|
1
|
Tsokos GC and Kammer GM: Molecular
aberrations in human systemic lupus erythematosus. Mol Med Today.
6:418–424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fassbinder T, Saunders U, Mickholz E, Jung
E, Becker H, Schluter B and Jacobi AM: Differential effects of
cyclophosphamide and mycophenolate mofetil on cellular and
serological parameters in patients with systemic lupus
erythematosus. Arthritis Res Ther. 17:922015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiffer L, Sinha J, Wang X, Huang W, von
Gersdorff G, Schiffer M, Madaio MP and Davidson A: Short term
administration of costimulatory blockade and cyclophosphamide
induces remission of systemic lupus erythematosus nephritis in
NZB/W F1 mice by a mechanism downstream of renal immune complex
deposition. J Immunol. 171:489–497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griffiths B, Emery P, Ryan V, Isenberg D,
Akil M, Thompson R, Maddison P, Griffiths ID, Lorenzi A, Miles S,
et al: The BILAG multi-centre open randomized controlled trial
comparing ciclosporin vs azathioprine in patients with severe SLE.
Rheumatology (Oxford). 49:723–732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shen C, He Y, Cheng K, Zhang D, Miao S,
Zhang A, Meng F, Miao F and Zhang J: Killer artificial
antigen-presenting cells deplete alloantigen-specific T cells in a
murine model of alloskin transplantation. Immunol Lett.
138:144–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mozaffarian N, Wiedeman AE and Stevens AM:
Active systemic lupus erythematosus is associated with failure of
antigen-presenting cells to express programmed death ligand-1.
Rheumatology (Oxford). 47:1335–1341. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sawla P, Hossain A, Hahn BH and Singh RP:
Regulatory T cells in systemic lupus erythematosus (SLE); role of
peptide tolerance. Autoimmun Rev. 11:611–614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smyth LA, Ratnasothy K, Moreau A, Alcock
S, Sagoo P, Meader L, Tanriver Y, Buckland M, Lechler R and
Lombardi G: Tolerogenic donor-derived dendritic cells risk
sensitization in vivo owing to processing and presentation by
recipient APCs. J Immunol. 190:4848–4860. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spisek R, Bretaudeau L, Barbieux I, Meflah
K and Gregoire M: Standardized generation of fully mature p70 IL-12
secreting monocyte-derived dendritic cells for clinical use. Cancer
Immunol Immunother. 50:417–427. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Real E, Kaiser A, Raposo G, Amara A,
Nardin A, Trautmann A and Donnadieu E: Immature dendritic cells
(DCs) use chemokines and intercellular adhesion molecule (ICAM)-1,
but not DC-specific ICAM-3-grabbing nonintegrin, to stimulate CD4+
T cells in the absence of exogenous antigen. J Immunol. 173:50–60.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang LL, Zhang Z, Zheng JS, Sheng JF and
Liu KZ: Phenotypic and functional characteristics of dendritic
cells derived from human peripheral blood monocytes. J Zhejiang
Univ Sci B. 6:1176–1181. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kalinski P, Schuitemaker JH, Hilkens CM,
Wierenga EA and Kapsenberg ML: Final maturation of dendritic cells
is associated with impaired responsiveness to IFN-gamma and to
bacterial IL-12 inducers: Decreased ability of mature dendritic
cells to produce IL-12 during the interaction with Th cells. J
Immunol. 162:3231–3236. 1999.PubMed/NCBI
|
|
13
|
Gombos I, Detre C, Vámosi G and Matkó J:
Rafting MHC-II domains in the APC (presynaptic) plasma membrane and
the thresholds for T-cell activation and immunological synapse
formation. Immunol Lett. 92:117–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cochand L, Isler P, Songeon F and Nicod
LP: Human lung dendritic cells have an immature phenotype with
efficient mannose receptors. Am J Respir Cell Mol Biol. 21:547–554.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Janikashvili N, Bonnotte B, Katsanis E and
Larmonier N: The dendritic cell-regulatory T lymphocyte crosstalk
contributes to tumor-induced tolerance. Clin Dev Immunol.
2011:4303942011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi KH, Choi BH, Park SR, Kim BJ and Min
BH: The chondrogenic differentiation of mesenchymal stem cells on
an extracellular matrix scaffold derived from porcine chondrocytes.
Biomaterials. 31:5355–5365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lutz MB, Suri RM, Niimi M, Ogilvie AL,
Kukutsch NA, Rössner S, Schuler G and Austyn JM: Immature dendritic
cells generated with low doses of GM-CSF in the absence of IL-4 are
maturation resistant and prolong allograft survival in vivo. Eur J
Immunol. 30:1813–1822. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lott DG, Dan O, Lu L and Strome M: Decoy
NF-kappaB fortified immature dendritic cells maintain laryngeal
allograft integrity and provide enhancement of regulatory T cells.
Laryngoscope. 120:44–52. 2010.PubMed/NCBI
|
|
19
|
Liu QL, Wang YS and Wang JX: Effect of
growth hormone on the immune function of dendritic cells. Chin Med
J (Engl). 123:1078–1083. 2010.PubMed/NCBI
|
|
20
|
Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin
L, Zhang MM and Yu B: Novel mechanism of inhibition of dendritic
cells maturation by mesenchymal stem cells via interleukin-10 and
the JAK1/STAT3 signaling pathway. PLoS One. 8:e554872013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Commeren DL, Van Soest PL, Karimi K,
Löwenberg B, Cornelissen JJ and Braakman E: Paradoxical effects of
interleukin-10 on the maturation of murine myeloid dendritic cells.
Immunology. 110:188–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee WC, Qiani S, Wan Y, Li W, Xing Z,
Gauldie J, Fung JJ, Thomson AW and Lu L: Contrasting effects of
myeloid dendritic cells transduced with an adenoviral vector
encoding interleukin-10 on organ allograft and tumour rejection.
Immunology. 101:233–241. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oberholzer A, Oberholzer C, Efron PA,
Scumpia PO, Uchida T, Bahjat K, Ungaro R, Tannahill CL, Murday M,
Bahjat FR, et al: Functional modification of dendritic cells with
recombinant adenovirus encoding interleukin 10 for the treatment of
sepsis. Shock. 23:507–515. 2005.PubMed/NCBI
|
|
24
|
Coates PT, Krishnan R, Kireta S, Johnston
J and Russ GR: Human myeloid dendritic cells transduced with an
adenoviral interleukin-10 gene construct inhibit human skin graft
rejection in humanized NOD-scid chimeric mice. Gene Ther.
8:1224–1233. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Müller G, Müller A, Tüting T, Steinbrink
K, Saloga J, Szalma C, Knop J and Enk AH: Interleukin-10-treated
dendritic cells modulate immune responses of naive and sensitized T
cells in vivo. J Invest Dermatol. 119:836–841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mok MY: Tolerogenic dendritic cells: Role
and therapeutic implications in systemic lupus erythematosus. Int J
Rheum Dis. 18:250–259. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oracki SA, Tsantikos E, Quilici C, Light
A, Schmidt T, Lew AM, Martin JE, Smith KG, Hibbs ML and Tarlinton
DM: CTLA4Ig alters the course of autoimmune disease development in
Lyn−/− mice. J Immunol. 184:757–763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Badell IR, Russell MC, Cardona K, Shaffer
VO, Turner AP, Avila JG, Cano JA, Leopardi FV, Song M, Strobert EA,
et al: CTLA4Ig prevents alloantibody formation following nonhuman
primate islet transplantation using the CD40-specific antibody 3A8.
Am J Transplant. 12:1918–1923. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou Y, Leng X, Luo S, Su Z, Luo X, Guo H,
Mo C, Zou Q, Liu Y and Wang Y: Tolerogenic dendritic cells
generated with tofacitinib ameliorate experimental autoimmune
encephalomyelitis through modulation of Th17/Treg balance. J
Immunol Res. 2016:50215372016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ureta G, Osorio F, Morales J, Rosemblatt
M, Bono MR and Fierro JA: Generation of dendritic cells with
regulatory properties. Transplant Proc. 39:pp. 633–637. 2007;
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mahnke K, Schmitt E, Bonifaz L, Enk AH and
Jonuleit H: Immature, but not inactive: The tolerogenic function of
immature dendritic cells. Immunol Cell Biol. 80:477–483. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laborde EA, Vanzulli S, Beigier-Bompadre
M, Isturiz MA, Ruggiero RA, Fourcade MG, Catalan Pellet AC, Sozzani
S and Vulcano M: Immune complexes inhibit differentiation,
maturation, and function of human monocyte-derived dendritic cells.
J Immunol. 179:673–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Griffiths KL and O'Neill HC: Dendritic
cells as immune regulators: The mouse model. J Cell Mol Med.
12:1909–1914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Röner S, Zinser E, Menges M, Wiethe C,
Littmann L, Hänig J, Steinkasserer A and Lutz MB: Minor role of
bystander tolerance to fetal calf serum in a peptide-specific
dendritic cell vaccine model against autoimmunity: Comparison with
serum-free cultures. J Immunother. 31:656–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allavena P, Piemonti L, Longoni D,
Bernasconi S, Stoppacciaro A, Ruco L and Mantovani A: IL-10
prevents the differentiation of monocytes to dendritic cells but
promotes their maturation to macrophages. Eur J Immunol.
28:359–369. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morel AS, Quaratino S, Douek DC and Londei
M: Split activity of interleukin-10 on antigen capture and antigen
presentation by human dendritic cells: Definition of a maturative
step. Eur J Immunol. 27:26–34. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Buelens C, Verhasselt V, De Groote D,
Thielemans K, Goldman M and Willems F: Interleukin-10 prevents the
generation of dendritic cells from human peripheral blood
mononuclear cells cultured with interleukin-4 and
granulocyte/macrophage-colony-stimulating factor. Eur J Immunol.
27:756–762. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akdis CA and Akdis M: Mechanisms of immune
tolerance to allergens: Role of IL-10 and Tregs. J Clin Invest.
124:4678–4680. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Corinti S, Albanesi C, la Sala A, Pastore
S and Girolomoni G: Regulatory activity of autocrine IL-10 on
dendritic cell functions. J Immunol. 166:4312–4318. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stoop JN, Harry RA, von Delwig A, Isaacs
JD, Robinson JH and Hilkens CM: Therapeutic effect of tolerogenic
dendritic cells in established collagen-induced arthritis is
associated with a reduction in Th17 responses. Arthritis Rheum.
62:3656–3665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oh BC, Lee HM, Lim DP, Cho JJ, Lee G, Lee
DS and Lee JR: Effect of immature dendritic cell injection before
heterotropic cardiac allograft. Transplant Proc. 38:pp. 3189–3192.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yin W, Ouyang S, Li Y, Xiao B and Yang H:
Immature dendritic cell-derived exosomes: A promise subcellular
vaccine for autoimmunity. Inflammation. 36:232–240. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bloy N, Pol J, Aranda F, Eggermont A,
Cremer I, Fridman WH, Fučíková J, Galon J, Tartour E, Spisek R, et
al: Trial watch: Dendritic cell-based anticancer therapy.
Oncoimmunology. 3:e9634242014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Osada T, Chong G, Tansik R, Hong T,
Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, et al:
The effect of anti-VEGF therapy on immature myeloid cell and
dendritic cells in cancer patients. Cancer Immunol Immunother.
57:1115–1124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ma ZH, Lu H, Lu Q, Yao ZF and Han Y: CD1d
blockade suppresses the capacity of immature dendritic cells to
prime allogeneic T cell response. J Surg Res. 183:894–899. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Enk AH, Angeloni VL, Udey MC and Katz SI:
Inhibition of Langerhans cell antigen-presenting function by IL-10.
A role for IL-10 in induction of tolerance. J Immunol.
151:2390–2398. 1993.PubMed/NCBI
|
|
47
|
Steinbrink K, Jonuleit H, Müller G,
Schuler G, Knop J and Enk AH: Interleukin-10-treated human
dendritic cells induce a melanoma-antigen-specific anergy in CD8(+)
T cells resulting in a failure to lyse tumor cells. Blood.
93:1634–1642. 1999.PubMed/NCBI
|
|
48
|
Chen L, Zheng L, He W, Qiu M, Gao L, Liu J
and Huang A: Cotransfection with IL-10 and TGF-β1 into immature
dendritic cells enhances immune tolerance in a rat liver
transplantation model. Am J Physiol Gastrointest Liver Physiol.
306:G575–G581. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bakdash G, Sittig SP, van Dijk T, Figdor
CG and de Vries IJ: The nature of activatory and tolerogenic
dendritic cell-derived signal II. Front Immunol. 4:532013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Linsley PS and Nadler SG: The clinical
utility of inhibiting CD28-mediated costimulation. Immunol Rev.
229:307–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tian M, Lv Y, Zhai C, Zhu H, Yu L and Wang
B: Alternative immunomodulatory strategies for xenotransplantation:
CD80/CD86-CTLA4 pathway-modified immature dendritic cells promote
xenograft survival. PLoS One. 8:e696402013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ko HJ, Cho ML, Lee SY, Oh HJ, Heo YJ, Moon
YM, Kang CM, Kwok SK, Ju JH, Park SH, et al: CTLA4-Ig modifies
dendritic cells from mice with collagen-induced arthritis to
increase the CD4+CD25+Foxp3+ regulatory T cell population. J
Autoimmun. 34:111–120. 2010. View Article : Google Scholar : PubMed/NCBI
|