Introduction
Asthma is a complex disorder characterized by
chronic inflammation of the airways, airway hyperresponsiveness
(AHR), airflow obstruction, and airway wall remodeling (1). Eosinophilic inflammation is generally
considered to be the main feature of asthmatic airways and is
assumed to be crucial in the pathogenesis of allergic asthma
(2). However, recent studies have
profoundly altered this simplistic view of eosinophils and their
function. A group of asthmatic patients characterized by the high
levels of sputum neutrophils were found in clinic. Moreover, unlike
conventional eosinophilia asthmatics, the patients with
neutrophilia have poor response to corticosteroids which are the
cornerstone of maintenance therapy for asthma (3). This strongly suggests that there are
different underlying pathophysiological mechanisms in asthma. For
this, researchers divided asthma into four subtypes according to
the cell characteristics in the sputum in clinic: Eosinophilic
asthma, neutrophilic asthma, mixed-granulocytic asthma and
paucigranulocytic asthma (4). It is
estimated that approximately 40% asthma cases are accompanied by
characteristics of neutrophilia, in which half were accompanied by
eosinophilia simultaneously. These patients are at great risk of
dying, thus they require amounts of healthcare time and costs, due
to the unclear pathomechanism (5).
As a result of ethical and moral issues preventing
patients from mechanistic research, the development and
characterization of animal models are needed to further
understanding of asthma. Appropriate animal models which represent
different subtypes of asthma are of great significance to the
research and are beneficial to the targeted treatment of individial
asthma patients. Immunization with ovalbumin (OVA) is a classic
approach to induce eosinophilic asthma (6). However, using merely one animal model
is not sufficient to reflect all the characteristics of asthma
patients, with in-depth understanding of asthma, and considering
the classification of clinical asthma in pathological features.
Many studies have reported that asthma associated with neutrophilia
was related to bacterial endotoxin, ozone and particulate air
pullution (7–9). It has been demonstrated that domestic
endotoxin exposure could trigger exacerbation of pre-existing
asthma in children and adults (10).
These patients are often accompanied by infection in airways,
suggesting that the bacteria and virus may be the inducer of
neutrophilia in acute attack of asthma.
In the present study, we established eosinophilic
asthma through traditional OVA immunization and, on this basis,
established neutrophilic asthma and mixed-granulocytic asthma by
intratracheal administration of different doses of
lipopolysaccharide (LPS). The cell characteristics in
bronchoalveolar lavage fluid (BALF), pathological changes of lung
tissue and disease related protein levels were analyzed to verify
whether each model of asthma has been successfully established.
Furthermore, the changes of the asthma symptoms in mice with
different asthma models were identified following treatment of
dexamethasone, a type of corticosteroid medication.
Materials and methods
Mice
Female BALB/c mice (6–8 weeks of age) were purchased
from Centers for Disease Control (CDC; Hubei, China). It has been
demonstrated that female mice develop a more pronounced type of
allergic airway inflammation than male mice after OVA challenge
(11). Mice were housed in a
condition of SPF room, and allowed access to food and water ad
libitum. All the experiments in this study were performed according
to the Guidelines for Use and Experimentation as set forth by the
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China). The study was approved by the ethics
committee of Department of Immunology, School of Basic Medicine,
Tongji Medical College, Huazhong University of Science and
Technology.
Asthma models
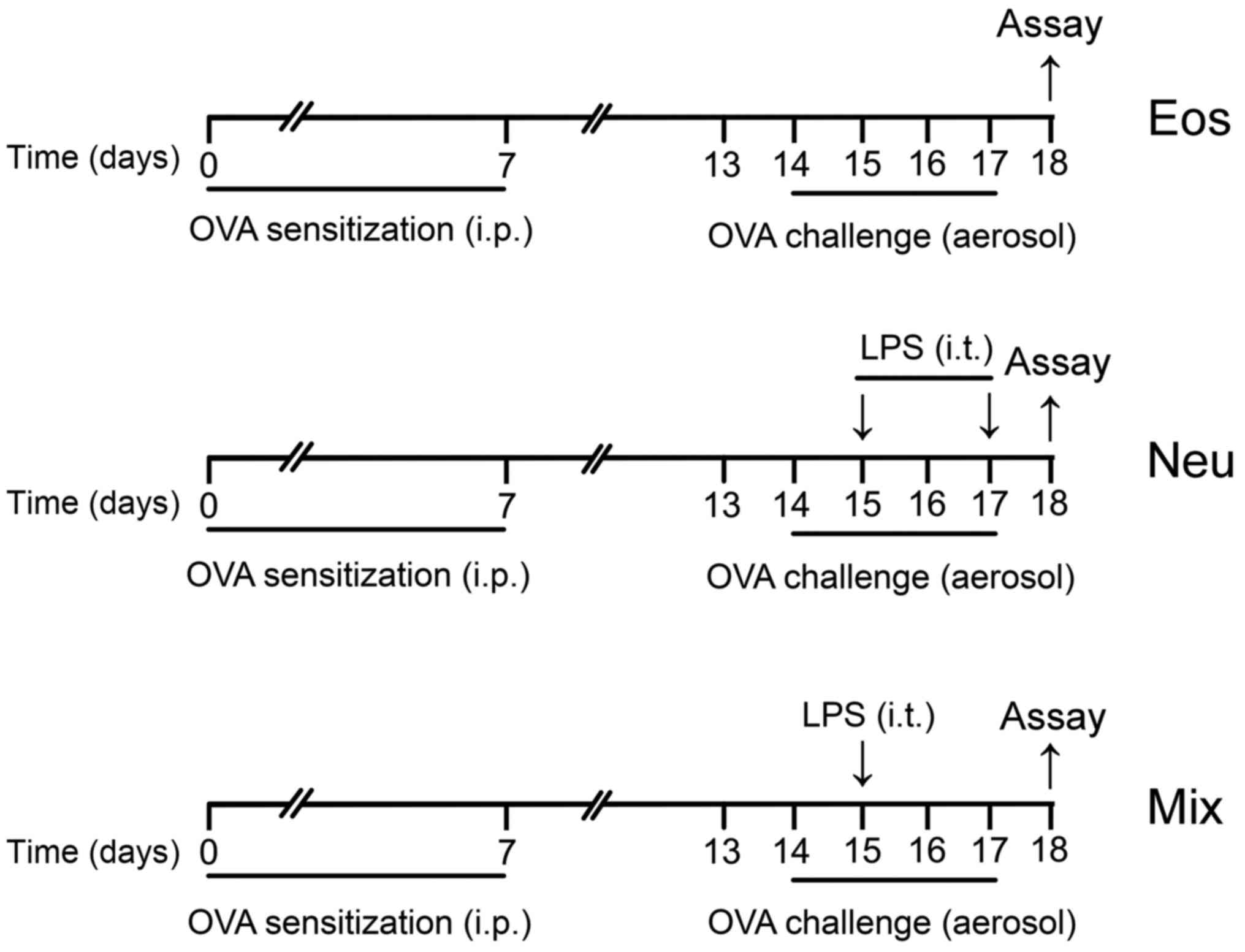
For the eosinophilic asthma group (Eos), mice were
sensitized with an intraperitoneal (i.p.) injection of 10 µg OVA
(Grade V; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) emulsified
in aluminium hydroxide gel (InvivoGen, San Diego, CA, USA) on days
0 and 7. The animals were then challenged with 6% OVA diluted in
phosphate-buffered saline (PBS) for 25 min on days 14–17. The
aerosol exposure was performed in a chamber coupled to an
ultrasonic nebulizer (Leyi Industry Co., Ltd., Shanghai, China).
For the neutrophilic asthma group (Neu), mice were sensitized and
challenged with OVA as described above, and performed transtracheal
administration of 10 µg LPS (Escherichia coli serotype
O55:B5; Sigma-Aldrich; Merck KGaA) diluted in PBS on days 15 and
17. For the mixed-granulocytic asthma group (Mix), mice were
sensitized and challenged with OVA as described above, and
performed transtracheal administration of 1 µg LPS diluted in PBS
on day 15 (Fig. 1). For
dexamethasone treatment (Con + DEX, Eos + DEX, Neu + DEX, Mix +
DEX), mice were administered 5 µg/kg dexamethasone i.p. one day
before the first challenge and lasted for five consecutive days
until sacrificed. PBS aerosol was used as a negative control (Con).
At 24 h after the last OVA challenge (day 18), all mice were
sacrificed for assay.
Measurement of AHR
Mice were anesthetized with i.p. injections of
pentobarbital sodium and a tracheostomy tube was placed. The
internal jugular vein was cannulated and connected to a
microsyringe for intravenous methacholine administration. Lung
resistance (RI) in response to increasing concentrations of
nebulized methacholine (3, 6, 12 and 24 mg/ml) were recorded using
the FinePointe data acquisition and analysis software (Buxco,
Wilmington, NC, USA). Results are expressed as a percentage of the
respective basal values in response to PBS.
Bronchoalveolar lavage and cell
count
Tracheae was cannulated and the left lung was
lavaged slowly 2 times with 0.6 ml PBS following the right lung
ligation. Each fluid was centrifuged and the supernatant was
rapidly frozen at −80°C. The cells in BALF were resuspended in PBS
and centrifuged in a cytocentrifuge. The cells were then stained
with Wright-Giemsa (Quick Wright-Giemsa Stain; Jiancheng
Bioengineering Institute, Nanjing, China) and identified as
macrophages, eosinophils, neutrophils and lymphocytes based on
cellular morphology and staining characteristics. At least 200
cells were counted under ×400 magnification.
Blood collection
Blood sample was obtained from the orbital venous
plexus, and the serum was collected for immunoglobin (Ig)E and OVA
specific IgE assay.
Lung histology
The right lung tissues were fixed with 4%
paraformaldehyde, embedded, sectioned and stained with hematoxylin
and eosin (H&E) for detection of inflammatory cells and
periodic acid-schiff (PAS) for detection of mucin in goblet cells
(mucus-secreting cells) by light microscopy.
ELISA
Quantitation of cytokines IL-4, IL-5, IL-13, IL-17A,
IL-33, IFN-γ in BALF and serum levels of IgE were measured by
sandwich ELISA using paired Abs (BD Biosciences, San Jose, CA,
USA). Serum levels of OVA-IgE were measured using a mouse OVA
specific IgE ELISA kit (Cusabio Biotech, Wuhan, China). The
sensitivity of detection were 4 pg/ml for IL-4, IL-5 IL-13, IL-17A,
15 pg/ml for IFN-γ, 25 pg/ml for IL-33 and 5 ng/ml for IgE.
Statistical analysis
Results were shown as the mean ± SEM. Statistical
significance of differences was performed with the unpaired
two-tailed Student's t-test (for comparison between two groups),
repeated-measures ANOVA with the Dunnett posttest (for AHR
dose-response curves) or one-way ANOVA with the Tukey post test
(for comparison between three or more groups). The software package
GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) was
used for data analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
AHR
AHR is a hallmark of bronchial asthma. The essential
measurement to assess AHR in mice is airway RI, which is defined as
the pressure driving respiration divided by flow (12). In comparison with control mice that
were instilled with PBS, OVA challenged mice of all three groups
showed significantly enhanced RI after treatment with methacholine.
Dexamethasone dramatically reduced RI in mice of Eos, suggesting
that immune-mediated airway pathology in vivo was modified.
However, dexamethasone did not show any inhibitory effects on AHR
in mice of Neu and Mix (Fig. 2).
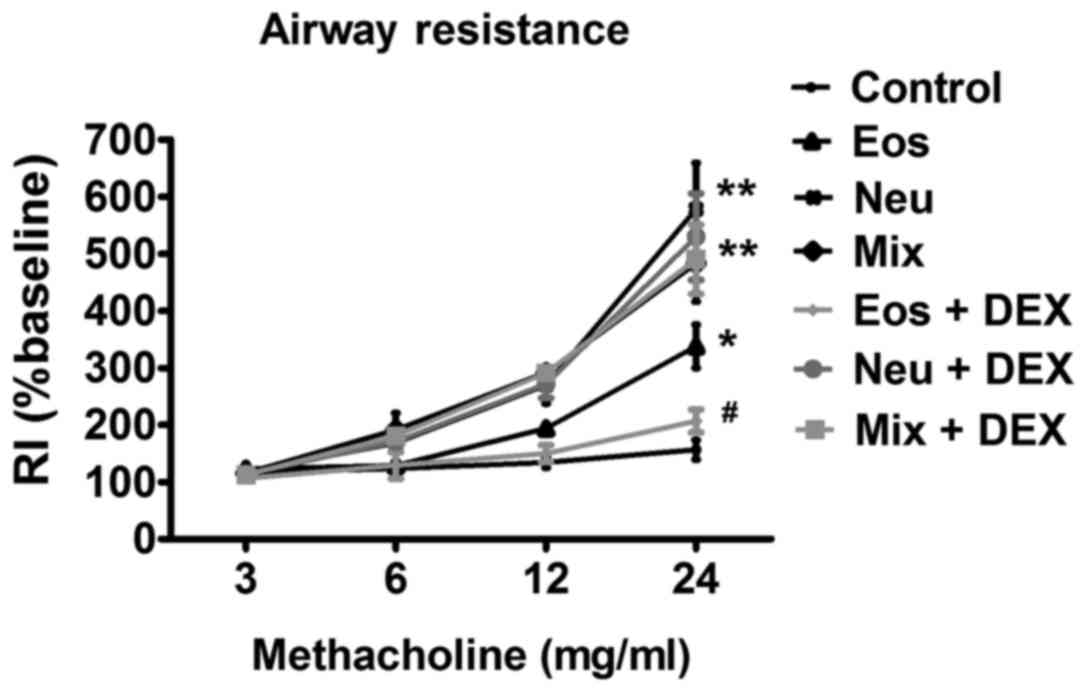 | Figure 2.The AHR of OVA-sensitized/challenged
BALB/c mice supplemented with LPS or not. Airway responsiveness of
mechanically ventilated mice in response to aerosolized
methacholine was measured 24 h after the last phosphate-buffered
saline aerosol or OVA aerosol. AHR is expressed as percentage
change from the baseline level of lung resistance (RI, n=6 mice).
Data (mean ± SEM) are representative of three
independent-experiments (n=6). *P<0.05, **P<0.01, vs. control
group. #P<0.05, Eos vs. Eos + DEX. Con, control; Eos,
Eosinophilic asthma; Neu, Neutrophilic asthma; Mix,
Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated
with dexamethasone; Neu + Dex, neutrophilic asthma treated with
dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with
dexamethasone; Dex, dexamethasone; OVA, ovalbumin; AHR, airway
hyperresponsiveness. |
Characteristics of cells in BALF in
each model of asthma
Cell accumulation in the pulmonary airways is
another hallmark feature of allergic asthma. Recent studies using
sputum induction and/or BAL techniques to measure and characterise
airways inflammation in asthmatic subjects have shown that
potentially a substantial proportion of cases have an underlying
pathology that is related to non-eosinophiic asthma (13). Analysis of BALF from mice in Eos
showed that the influx of inflammatory cells was dominated by
eosinophils (Fig. 3A, green arrow),
which constituted ~50% of total BALF cells. In Neu, huge amounts of
neutrophil, not eosinophils, was observed in BALF (Fig. 3A, red arrow), which constituted ~60%
of total BALF cells. Increased percentagesof both eosinophils and
neutrophils were shown in BALF in Mix (Fig. 3A), which constituted ~20 and ~50% of
total BALF cells, repsectively. In addition, the total number of
cells in the BALF in mice of three groups were all increased
(Fig. 3B). These results showed that
the cell characteristics in BALF in each model of asthma were
consistent with that have obsevered in corresponding subtypes of
asthma in clinic.
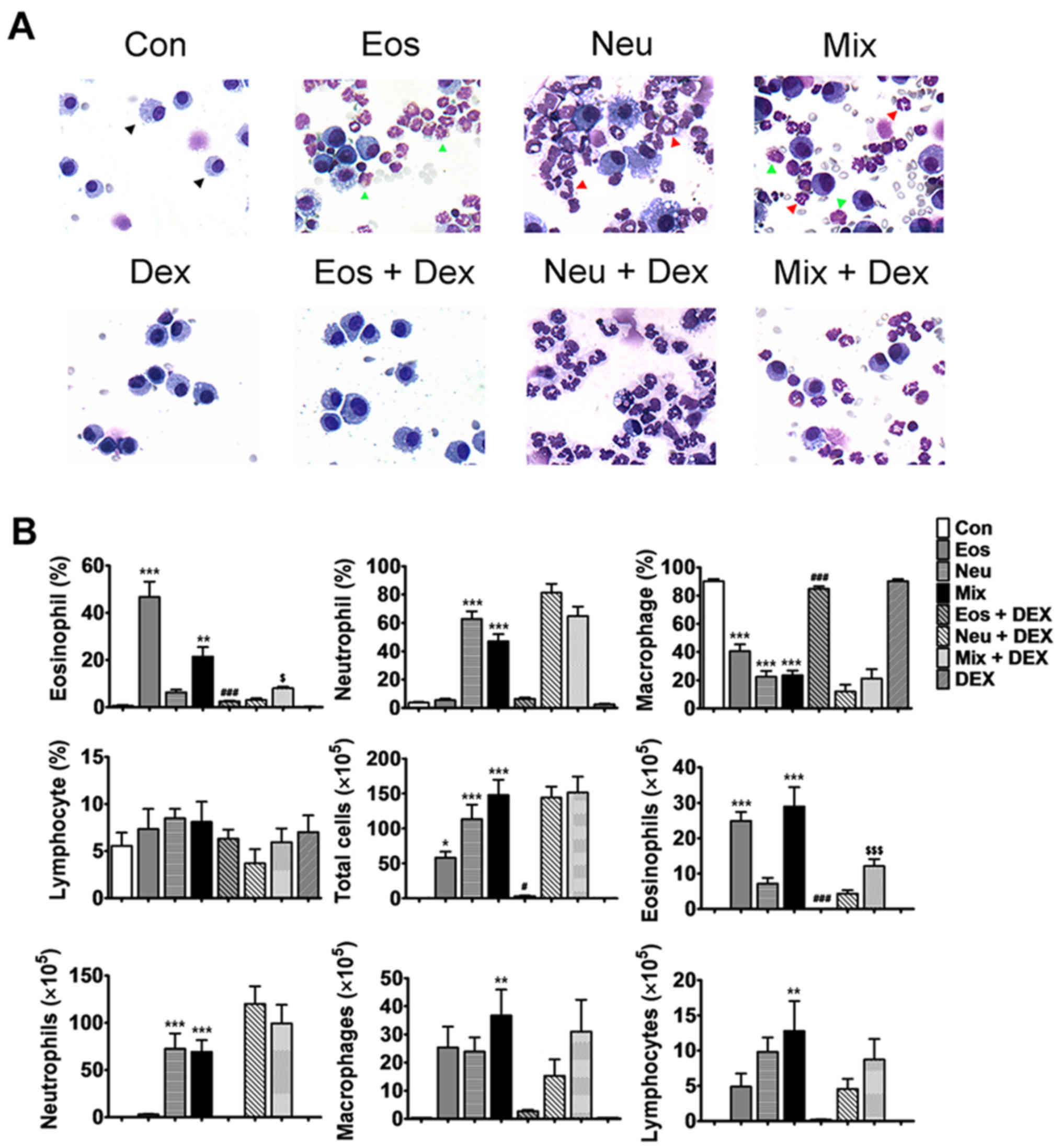 | Figure 3.Characteristics of cells in BALF of
mice. (A) Wright-Giemsa staining shows the macrophage (black
arrow), eosinophil (green arrow) and neutrophil (red arrow) under
microscopy (magnification, ×400). (B) The statistical analysis of
the numbers and proportions of the eosinophil, neutrophil,
macrophage and lymphocyte in BALF of each model of asthma with or
without dexamethasone treatment. Data (mean ± SEM) are
representative of three independent-experiments (n=6). *P<0.05,
**P<0.01, ***P<0.001, vs. control group.
#P<0.05, ###P<0.01, vs. Eos.
$P<0.05, $$$P<0.001, vs. Mix. Con,
control; Eos, Eosinophilic asthma; Neu, Neutrophilic asthma; Mix,
Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated
with dexamethasone; Neu + Dex, neutrophilic asthma treated with
dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with
dexamethasone; Dex, dexamethasone; BALF, bronchoalveolar lavage
fluid. |
After the treatment of dexmathesone, 10-fold and
5-fold decrease in the proportion of eosinophils recovered in Eos +
DEX and Mix + DEX were observed, respectively. However, the
neutrophils was not decreased in Neu + DEX nor Mix + DEX,
suggesting that dexamethasone has an inhibitory effect on
eosinophils but not neutrophils (Fig. 3A
and B).
Lung tissue inflammation and bronchial
mucus secretionin each model of asthma
To examine the histological changes of lung tissues
in each group of asthma model, H&E and PAS staining were
performed to observe the inflammation and mucus secretion in the
lung tissues. The inflammtory cells only infiltrated around the
bronchi and vessels in Eos, which represent a moderate
inflammation. The inflammatory cells infiltrated in nearly lungs in
Neu and Mix, consistent with that asthma patients accompanied by
neutrophilia have a more severe respiratory inflammation (Fig. 4A). On the other hand, OVA-challenged
mice, but not saline-challenged mice, developed marked goblet cell
hyperplasia and mucus hypersecretion within the bronchi in the lung
(Fig. 4B).
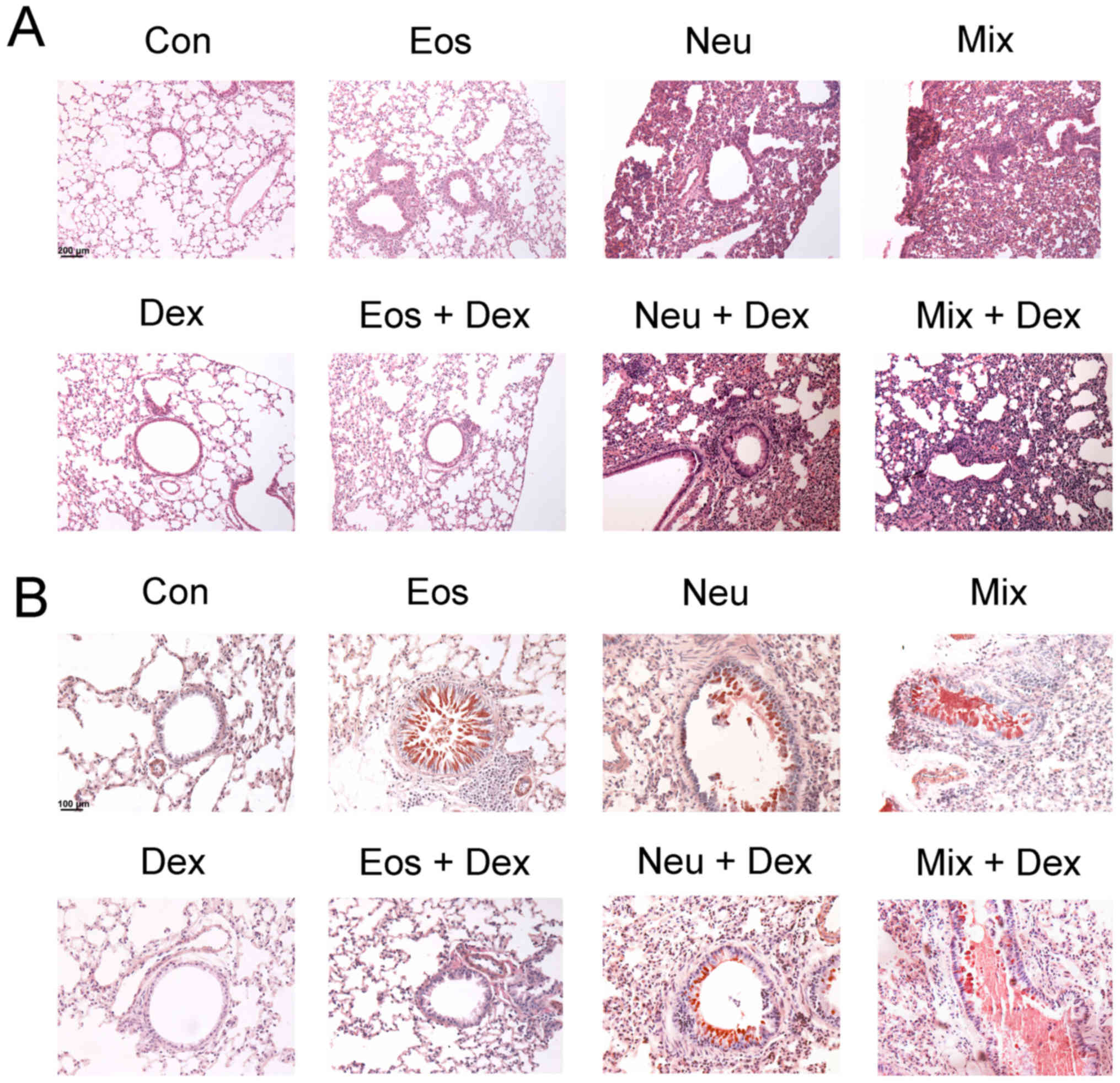 | Figure 4.Degree of lung inflammation and mucus
secretion in the bronchi of mice. The lung sections were stained
with (A) H&E or (B) PAS and examined under microscopy
(magnification, ×200). Con, control; Eos, Eosinophilic asthma; Neu,
Neutrophilic asthma; Mix, Mixed-granulocytic asthma; Eos + Dex,
eosinophilic asthma treated with dexamethasone; Neu + Dex,
neutrophilic asthma treated with dexamethasone; Mix + Dex,
mixed-granulocytic asthma treated with dexamethasone; Dex,
dexamethasone; H&E, hematoxylin and eosin; PAS, periodic
acid-schiff. |
The inhibitory effects of dexamethasone
administration were evident in Eos + DEX, as lung tissue sections
showed a reduction in inflammatory cells in airway and tissue
mucus-secreting goblet cells in the airway epithelium. However,
dexamethasone could not ameliorate the inflammation of lung tissues
or bronchial mucus secretion in Neu + DEX and Mix + DEX (Fig. 4A and B).
Cytokine levels in BALF in each model
of asthma
To probe the mechanisms of AHR and cell accumulation
in lung tissues, we assayed key mediaors of disease in each asthma
model. Eosinophilic asthma includes either allergic or nonallergic
phenotypes underlying immune responses mediated by T helper (Th)2
cell-derived cytokines, whilst neutrophilic asthma is mostly
dependent on Th1 and Th17 cell-induced mechanisms (14,15). In
the present study, our results showed that elevated levels of Th2
cytokines (IL-4, IL-5, IL-13 and IL-33) were detected in BALF in
Eos, and elevated levels of Th1 (IFN-γ) and Th17 (IL-17A)
cytokines, but not Th2 cytokines in BALF were detected in Neu.
Surprisingly, elevated levels of Th1, Th2 and Th17 cytokines were
detected in BALF in Mix. Dexamethasone abated OVA-induced elevation
of BALF Th2 cytokines in Eos + DEX, buthas no effect on Th1 and
Th17 cytokine production in Neu nor Th1, Th2 and Th17 cytokines in
Mix (Fig. 5).
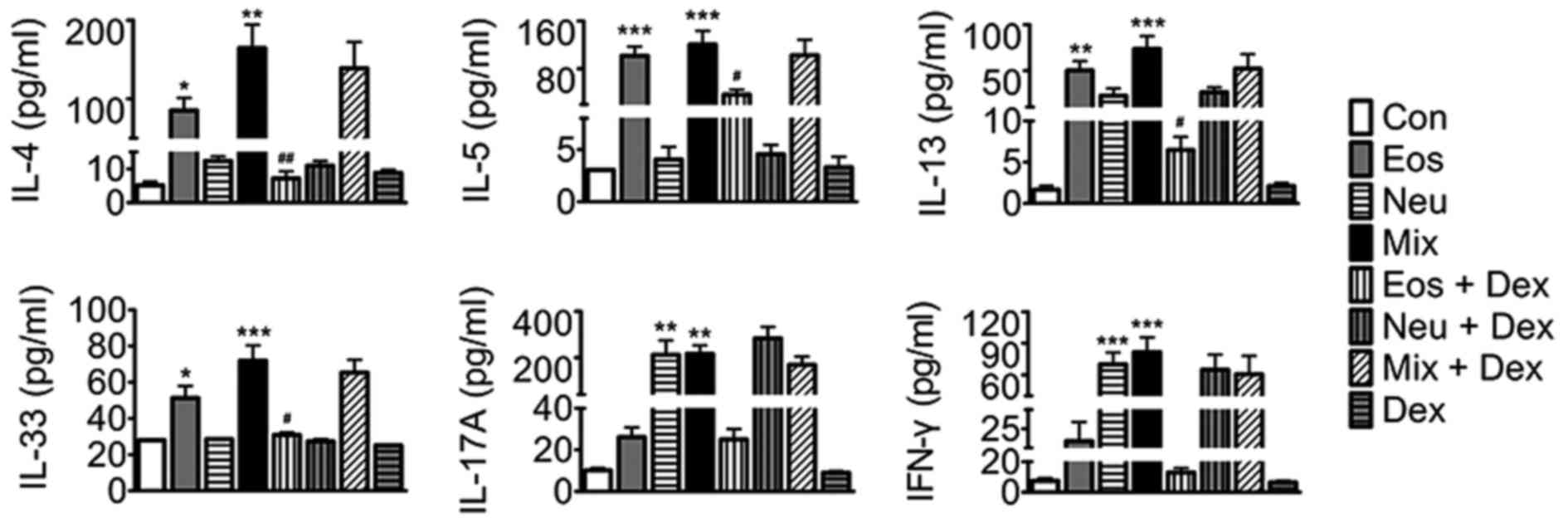 | Figure 5.The levels of IL-4, IL-5, IL-13,
IL-33, IFN-γ and IL-17A in BALF of mice. Data (mean ± SEM) are
representative of three independent-experiments (n=6). *P<0.05,
**P<0.01, ***P<0.001, vs. control group.
#P<0.05, ##P<0.01, vs. Eos. Con,
control; Eos, Eosinophilic asthma; Neu, Neutrophilic asthma; Mix,
Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated
with dexamethasone; Neu + Dex, neutrophilic asthma treated with
dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with
dexamethasone; Dex, dexamethasone; IL, interleukin; BALF,
bronchoalveolar lavage fluid. |
The levels of IgE and OVA-IgE in serum
in each model of asthma
IgE is an important mediator of allergic reactions
and has a central role in allergic asthma pathophysiology. Our
results showed that IgE and OVA-IgE levels were elevated in the
serum in all three models of asthma. Dexamethasone decreases the
levels of IgE and OVA-IgE in Eos, whereas was ineffective in Neu or
Mix, suggesting that dexamethasone exihibit a poor effect on asthma
with neutrophilia (Fig. 6).
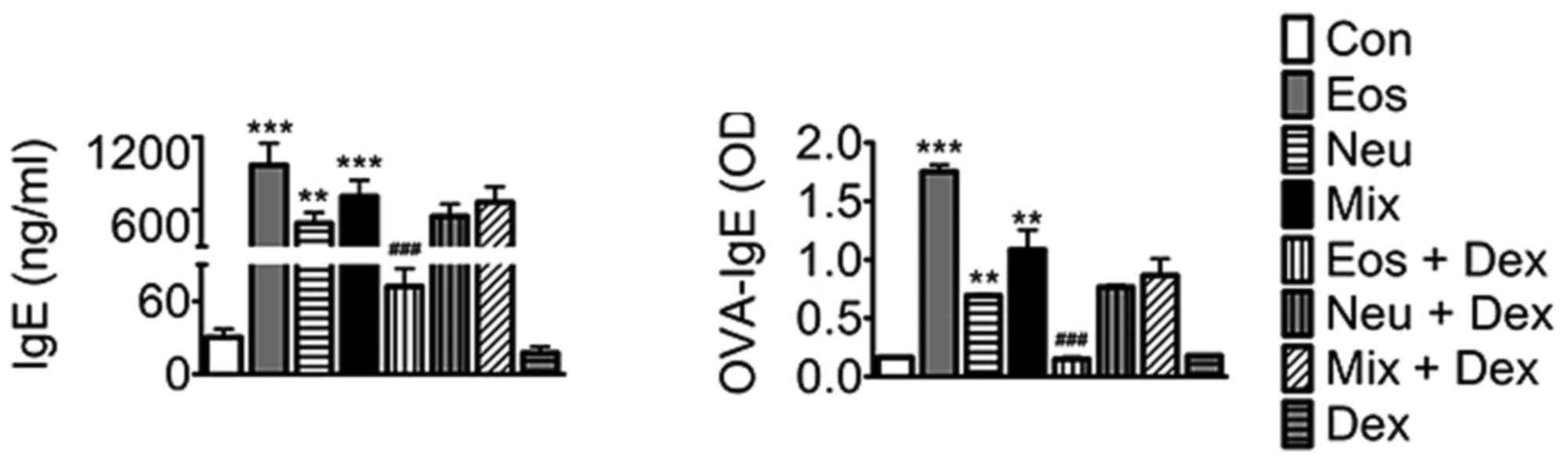 | Figure 6.The levels of IgE and OVA-IgE in serum
of mice. Data (mean ± SEM) are representative of three
independent-experiments (n=6). **P<0.01, ***P<0.001, vs.
control group. ###P<0.01, vs. Eos. Con, control; Eos,
Eosinophilic asthma; Neu, Neutrophilic asthma; Mix,
Mixed-granulocytic asthma; Eos + Dex, eosinophilic asthma treated
with dexamethasone; Neu + Dex, neutrophilic asthma treated with
dexamethasone; Mix + Dex, mixed-granulocytic asthma treated with
dexamethasone; Dex, dexamethasone; IgE, immunoglobin E; OVA,
ovalbumin. |
Discussion
Asthma is now viewed as a heterogeneous inflammatory
airways disorder which gives rise to several different clinical
phenotypes. Eosinophilic inflammation is generally considered to be
the main feature of asthmatic airways, however, recent reports
suggest that many patients had sputum evidence of neutrophilic
airway inflammation (16,17). Researchers have divided asthma into
four subtypes according to the cell characteristics in the sputum:
Eosinophilic, neutrophilic, paucigranulocytic or mixed cellularity
(4). This classification, based on
the underlying pattern of airway inflammation, has been
demonstrated to be in agreement with the findings in
bronchoalveolar lavage, airway biopsy and peripheral blood now
(18,19). Moreover, sputum induction using
nebulized hypertonic saline has been used as an alternative method
to obtain lower airway lining fluid, with evidence of good
repeatability and reproducibility.
Because of the ethical and moral issues preventing
patients from mechanistic research, the development of animal
models is of great significance to the research. Immunization with
OVA is a classic approach to induce eosinophilic asthma (20). The features in Eos, including AHR to
methacholine, inflammation of the airways (with infiltrates
containing many eosinophils), airway remodeling (increases in mucus
secretion of epithelial goblet cells), markedly increased lung
expression of Th2 cytokines and serum expression of IgE and
OVA-IgE, demonstrated that we have successfully established the
eosinophilic asthma. Corticosteroid, which could effectively
inhibit eosinophils activation and recruitment, and reduce their
survival by inducing them apoptosis, is considered to be the most
traditional approach for asthma (21,22). The
results in the study showed that the dexamethasone, a drug belong
to corticosteroid, inhibited hallmark features of eosinophilic
asthma, including AHR, eosinophilic accumulation, airway
remodeling, Th2 cytokine production and serum IgE synthesis.
There is increasing evidence that inflammatory
mechanisms other than eosinophilic inflammation may be involved in
producing the final common pathway of enhanced bronchial reactivity
and reversible airflow obstruction that characterises asthma
(23). It is supposed that a major
proportion of asthma is based on neutrophilic airway inflammation,
possibly triggered by environmental exposure to bacterial
endotoxin, particulate air pollution, and ozone, as well as viral
infections (24). As a powerful
bacterial virulence factor, we hypothesize that LPS may participate
in the pathogenesis of neutrophilic asthma. For this, we try to
establish the mouse model of neutrophilic asthma using high dose of
LPS (10 µg) in combination with OVA sensitization and chanllenge.
Despite eosinophilic asthma is considered a Th2 cell-associated
disorder, both the Th1-associated cytokine IFN-γ and
Th17-associated cytokine IL-17 have been implicated in neutrophilic
asthma. For example, transgenic mouse experiments clearly
demonstrated that high levels of IFN-γ in airways induce
neutrophilic lung inflammationand AHR (25). In addition, Th17 cytokines could
recruit neutrophils to the airway by increasing secretion of
epithelial-derived neutrophilic chemokines, and have pleotropic
effects on airway smooth muscle resulting in airway narrowing
(26). Asthma patients accomanied by
neutrophilia frequently do not respond to corticosteroid, which
attributed to that it decrease apoptosis in neutrophils and thus
prolong their survival (27). Our
results demonstrated that the asthma symptoms does not alleviated
after the treatment of dexamethasone, and what is more, the numbers
of neutrophilis increased. Together, all the results above
demonstrate that we developed a model that exhibits several
features of neutrophilic asthma in clinic, including airway
neutrophilia, AHR, serum IgE and OVA-IgE synthesis, and what is
more, resistent to the dexamethasone treatment.
Apart from neutrophilic asthma, some patients with
severe disease is sustained by mixed patterns of inflammation
including both eosinophils and neutrophils (28,29). In
a recent study, Bafadhel et al reported that approximately
15% asthma patients who found in sputum with mixture of those two
types of cells were resistant to corticosteroid (30). When establishing the mouse model of
neutrophilic asthma, we found that the high dose of LPS have an
inhibitory effect on eosinophilia. Thus, we try to establish the
mouse model of mixed-granulocytic asthma using low dose of LPS (1
µg) in combination with OVA sensitization and chanllenge, which may
partially suppress eosinophilia. The results showed that we
successfully established the mixed-granulocytic asthma as
demonstrated by AHR, inflammation of the airways (with infiltrates
containing both eosinophils and neutrophils), airway remodeling
(increases in mucus secretion of epithelial goblet cells) and serum
IgE synthesis and, moreover, the infiltration of eosinophils and
neutrophils as well as AHR has nochange after dexamethasone
treatment. Interestingly, we found that the levels of Th1, Th2 and
Th17 cytokines in BALF all elevated in this model. The explanation
for this is not clear. Although many reports have demonstrated that
both Th1 and Th17 cells are crucial for the development of
neutrophilic inflammation in the airways, an increasing evidence in
both humans and animal models suggest that a mixed Th2/Th17
response drives the development of more severe AHR (31,32). In
addition, in an earlier study, Hansen et al found that in
their mouse models, Th1 cells have been shown to function in a
cooperative manner with Th2 cells to mediate severe airway
inflammatory responses (33). Since
there is no study on the mechanism of mixed-granulocytic asthma,
our results may give a new hint about the pathogenesis of this
heterogeneous disease.
The evidence of epidemiology and clinic points to
the fact that asthma has several distinct subgroups need to be
treated differently. Since treatment and prevention strategies now
are almost entirely focused on eosinophilic asthma, an improved
asthma management which rely on clinically sustainable
classification of disease phenotypes seems to be important
(34,35). In this study, we developed three
mouse models of allergic inflammation of the airways that exhibits
several features of chronic asthma in clinic. We have checked the
repeatability of these developed different experimental asthma
mouse models, and the data are representative of three
independent-experiments. These mouse models might therefore allow
studying pathophysiological processes occurring in the subgroup of
persistent asthmatics with non-eosinophilic asthma responding to
inhaled steroids. Although we are aware that murine models cannot
reflect all features of a complex disorder such as asthma, we
believe that mimicking different types of inflammation and
assessing the relationship to the development of AHR will allow to
further dissect different asthma phenotypes.
Acknowledgements
The study was supported with funds from the Major
State Basic Research Development Program of China (973 Program)
(no. 2013CB530505).
References
|
1
|
Baarnes CB, Hansen AV and Ulrik CS:
Enrolment in an asthma management program during pregnancy and
adherence with inhaled corticosteroids: The ‘Management of Asthma
during Pregnancy’ Program. Respiration. 92:9–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uhm TG, Kim BS and Chung IY: Eosinophil
development, regulation of eosinophil-specific genes, and role of
eosinophils in the pathogenesis of asthma. Allergy Asthma Immunol
Res. 4:68–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thomson NC: Novel approaches to the
management of noneosinophilic asthma. Ther Adv Respir Dis.
10:211–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simpson JL, Scott R, Boyle MJ and Gibson
PG: Inflammatory subtypes in asthma: Assessment and identification
using induced sputum. Respirology. 11:54–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schleich FN, Manise M, Sele J, Henket M,
Seidel L and Louis R: Distribution of sputum cellular phenotype in
a large asthma cohort: Predicting factors for eosinophilic vs.
neutrophilic inflammation. BMC Pulm Med. 13:112013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MY, Seo CS, Lee NH, Ha H, Lee JA, Lee
H, Lee KY and Shin HK: Anti-asthmatic effect of schizandrin on
OVA-induced airway inflammation in a murine asthma model. Int
Immunopharmacol. 10:1374–1379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Douwes J, Gibson P, Pekkanen J and Pearce
N: Non-eosinophilic asthma: Importance and possible mechanisms.
Thorax. 57:643–648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peden DB: The epidemiology and genetics of
asthma risk associated with air pollution. J Allergy Clin Immunol.
115:213–219; quiz 220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang HS, Lee TH, Jun JA, Baek AR, Park
JS, Koo SM, Kim YK, Lee HS and Park CS: Neutrophilic inflammation
in asthma: Mechanisms and therapeutic considerations. Expert Rev
Respir Med. 11:29–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim KH, Jahan SA and Kabir E: A review on
human health perspective of air pollution with respect to allergies
and asthma. Environ Int. 59:41–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melgert BN, Postma DS, Kuipers I,
Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W
and Hylkema MN: Female mice are more susceptible to the development
of allergic airway inflammation than male mice. Clin Exp Allergy.
35:1496–1503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang HY, Mitzner W and Watson J:
Variation in airway responsiveness of male C57BL/6 mice from 5
vendors. J Am Assoc Lab Anim Sci. 51:401–406. 2012.PubMed/NCBI
|
|
13
|
Freeman CM, Crudgington S, Stolberg VR,
Brown JP, Sonstein J, Alexis NE, Doerschuk CM, Basta PV, Carretta
EE, Couper DJ, et al: Design of a multi-center immunophenotyping
analysis of peripheral blood, sputum and bronchoalveolar lavage
fluid in the Subpopulations and Intermediate Outcome Measures in
COPD Study (SPIROMICS). J Transl Med. 13:192015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakajima H and Hirose K: Role of IL-23 and
Th17 cells in airway inflammation in asthma. Immune Netw. 10:1–4.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pelaia G, Vatrella A, Busceti MT, Gallelli
L, Calabrese C, Terracciano R and Maselli R: Cellular mechanisms
underlying eosinophilic and neutrophilic airway inflammation in
asthma. Mediators Inflamm. 2015:8797832015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parameswaran K, Pizzichini E, Pizzichini
MM, Hussack P, Efthimiadis A and Hargreave FE: Clinical judgement
of airway inflammation versus sputum cell counts in patients with
asthma. Eur Respir J. 15:486–490. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Simpson JL, Scott RJ, Boyle MJ and Gibson
PG: Differential proteolytic enzyme activity in eosinophilic and
neutrophilic asthma. Am J Respir Crit Care Med. 172:559–565. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gasiuniene E, Lavinskiene S, Sakalauskas R
and Sitkauskiene B: Levels of IL-32 in serum, induced sputum
supernatant, and bronchial lavage fluid of patients with chronic
obstructive pulmonary disease. COPD. 13:569–575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Emmanouil P, Loukides S, Kostikas K,
Papatheodorou G, Papaporfyriou A, Hillas G, Vamvakaris I, Triggidou
R, Katafigiotis P, Kokkini A, et al: Sputum and BAL Clara cell
secretory protein and surfactant protein D levels in asthma.
Allergy. 70:711–714. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kerzel S, Wagner J, Rogosch T, Yildirim
AO, Sikula L, Fehrenbach H, Garn H, Maier RF, Schroeder HW Jr and
Zemlin M: Composition of the immunoglobulin classic antigen-binding
site regulates allergic airway inflammation in a murine model of
experimental asthma. Clin Exp Allergy. 39:591–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomson NC and Spears M: Inhaled
corticosteroids for asthma: On-demand or continuous use. Expert Rev
Respir Med. 7:687–699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raissy HH, Kelly HW, Harkins M and Szefler
SJ: Inhaled corticosteroids in lung diseases. Am J Respir Crit Care
Med. 187:798–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Simpson JL, Gibson PG, Yang IA, Upham J,
James A, Reynolds PN and Hodge S; AMAZES Study Research Group, :
Impaired macrophage phagocytosis in non-eosinophilic asthma. Clin
Exp Allergy. 43:29–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang SK, Zhang Q, Qiu Z and Chung KF:
Mechanistic impact of outdoor air pollution on asthma and allergic
diseases. J Thorac Dis. 7:23–33. 2015.PubMed/NCBI
|
|
25
|
Tosca MA, Silvestri M, Morandi F, Prigione
I, Pistorio A, Ciprandi G and Rossi GA: Impairment of lung function
might be related to IL-10 and IFN-γ defective production in
allergic children. Immunol Lett. 140:104–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Newcomb DC and Peebles RS Jr:
Th17-mediated inflammation in asthma. Curr Opin Immunol.
25:755–760. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Furukawa T, Sakagami T, Koya T, Hasegawa
T, Kawakami H, Kimura Y, Hoshino Y, Sakamoto H, Shima K, Tsukioka
K, et al: Characteristics of eosinophilic and non-eosinophilic
asthma during treatment with inhaled corticosteroids. J Asthma.
52:417–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manise M, Bakayoko B, Schleich F, Corhay
JL and Louis R: IgE mediated sensitisation to aeroallergens in an
asthmatic cohort: Relationship with inflammatory phenotypes and
disease severity. Int J Clin Pract. 70:596–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao P, Gibson PG, Baines KJ, Yang IA,
Upham JW, Reynolds PN, Hodge S, James AL, Jenkins C, Peters MJ, et
al: Anti-inflammatory deficiencies in neutrophilic asthma: Reduced
galectin-3 and IL-1RA/IL-1β. Respir Res. 16:52015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bafadhel M, McCormick M, Saha S, McKenna
S, Shelley M, Hargadon B, Mistry V, Reid C, Parker D, Dodson P, et
al: Profiling of sputum inflammatory mediators in asthma and
chronic obstructive pulmonary disease. Respiration. 83:36–44. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Al-Ramli W, Préfontaine D, Chouiali F,
Martin JG, Olivenstein R, Lemière C and Hamid Q: T(H)17-associated
cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin
Immunol. 123:1185–1187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lajoie S, Lewkowich IP, Suzuki Y, Clark
JR, Sproles AA, Dienger K, Budelsky AL and Wills-Karp M:
Complement-mediated regulation of the IL-17A axis is a central
genetic determinant of the severity of experimental allergic
asthma. Nat Immunol. 11:928–935. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hansen G, Berry G, DeKruyff RH and Umetsu
DT: Allergen-specific Th1 cells fail to counterbalance Th2
cell-induced airway hyperreactivity but cause severe airway
inflammation. J Clin Invest. 103:175–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hering T: Update of the
GINA-recommendations. MMW Fortschr Med. 159:63–64. 2017.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Isidoro-García M, Sánchez-Martín A,
García-Sánchez A, Sanz C, García-Berrocal B and Dávila I:
Pharmacogenetics and the treatment of asthma. Pharmacogenomics.
18:1271–1280. 2017. View Article : Google Scholar : PubMed/NCBI
|