Introduction
In 2004, the effectiveness of gold nanoparticles
(GNPs) as a radiosensitizer in vivo was first demonstrated
by Hainfeld et al (1). Over
the next decade, numerous studies were performed to investigate the
optimal treatment parameters (2–6). The
mechanism underlying the radiosensitizing effect of GNPs is not
fully understood and a number of mechanisms have been suggested,
including increased photoelectric photon absorption with a high-Z
material, or an insult to the tumor blood vessels via an
anti-angiogenic effect (3,7,8). In a
previous study, GNPs were administered via intravenous (IV)
injection or direct intratumoral (IT) injection (5); however, to the best of our knowledge,
no studies have been performed to compare these two routes of
administration.
When GNPs of an appropriate size are intravenously
administered, particles accumulate in the tumor via the enhanced
permeability and retention effect (9). However, the particles are primarily
incorporated into other organs, and only a limited amount reaches
the tumor (1). Intratumoral
injection of particles increases the density of GNPs within the
tumor, which may increase the treatment effect; however, this
method is disadvantageous as it results in heterogeneous
distribution (10).
The degree of GNP-induced radiosensitization is also
reported to differ among various cell types (11,12).
Malignant melanoma is radiation-resistant and typically develops on
the skin surface. Therefore, low-energy X-ray treatment is possible
and malignant melanoma may allow a good indication for the
successful clinical application of this treatment method.
Previously, two studies on the radiosensitization effects of GNPs
in malignant melanoma bearing mice have been reported (13,14).
Although the experiments of the two studies were performed under
similar conditions, their results were inconsistent regarding the
radiosensitizing effect, with one study indicating enough
radiosensitizing effect of GNP, but the other study unable to
replicate the same results. For further understanding, the present
study performed experiments under treatment conditions that
differed in tumor size from the two previous studies.
Recently, the modification of GNPs with polyethylene
glycol (PEG)-binding (PEGylation) has been reported to achieve a
favorable treatment effect (15–18). As
a foreign body, GNPs are rapidly incorporated and removed by the
reticuloendothelial system; PEGylation inhibits this reaction
(18) and may lead to a
tumor-specific treatment effect. The influence of PEGylation was
not evaluated in the previous studies using malignant melanoma
bearing mice (13,14). Therefore, it was included in the
present investigation.
Materials and methods
Animal model
A total of 50, 6-week-old nude mice (body weight
18.3±1.5 g) BALB/C-nu/nu (female), were purchased from CLEA Japan
Inc. (Tokyo, Japan). Housing conditions for all mice were as
follows: Temperature 20–26°C and humidity 40–60%. A 12-h light/dark
cycle was used. Food and water were provided ad libitum).
Experiments were performed at Tokai University Animal Experiment
Center (Kanagawa, Japan) in accordance with the Tokai University
guidelines and the experimental protocol was approved by the
Institutional Animal Care and Use Committee (Department of
Radiation Oncology, Tokai University, Kanagawa, Japan) prior to the
start of the study. The murine malignant melanoma cell line, B16F10
(American Type Culture Collection, Manassas, VA, USA), was cultured
in Dulbecco's modified Eagle medium (DMEM; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) at 37°C in a 5% CO2
atmosphere. The culture media contained penicillin (50 IU/ml) and
streptomycin (50 µg/ml) and was supplemented with 10% fetal calf
serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). B16F10 cells
were cultured for 4 days at a density of 1×105 cells in
7 ml DMEM in a 100-mm culture dish (Eppendorf, Hamburg, Germany) at
37°C in a 5% CO2 atmosphere. Following two washes with
phosphate-buffered saline (PBS) for ~5 min, the B16F10 cells were
harvested with 0.25 w/v% trypsin-1 mmol/l EDTA-4Na solution with
phenol red (Wako Pure Chemical Industries, Ltd.). Harvested B16F10
cells were suspended in PBS, and the cell density was adjusted
(2×106 cells/100 µl). A total of 2×105 cells
were subcutaneously inoculated in the femoral region of all
mice.
Treatment groups
Two types of GNPs were used: PEGylated GNPs
(Peg-GNPs) and naked GNPs (N-GNPs). In total, 2.8 mg/ml
methyl-terminated 5000 PEG-coated GNPs (diameter, 5 nm;
Sigma-Aldrich; Merck KGaA) were used as Peg-GNPs and GNPs at a
concentration of 200 mg Au/cc (diameter, 15 nm; Nanoprobes Inc.,
Yaphank, NY, USA) were used as N-GNPs.
Eight treatment groups were established, as follows:
i) Control (n=10); ii) Peg-GNP IV-alone (n=5); iii) Peg-GNP
IT-alone (n=5); iv) radiotherapy (RT)-alone (n=10); v) Peg-GNP IV +
RT (n=5); vi) Peg-GNP IT + RT (n=5); vii) N-GNP IV + RT (n=5) and
viii) N-GNP IT + RT (n=5). Each group consisted of 5 mice for each
experiment. Mice were randomly allocated so as to prevent variation
in the mean tumor volume among the groups. The experiment was
performed in two stages: The first stage included groups i-vi; the
second stage included groups i, iv, vii and viii. Accordingly,
Groups i and iv consisted of 10 mice. Treatment was initiated when
the tumor diameter reached ~1 cm at 12–19 days following
transplantation (Fig. 1).
To ensure that the growth ability of the grafted
tumors was uniform, 19 animals with a tumor volume of ≤200 or
≥1,300 mm3 at 12–19 days following cell transplantation
were excluded from the analysis. Finally, the number of mice for
the analysis is as follows: i) Control (n=7); ii) Peg-GNP IV-alone
(n=3); iii) Peg-GNP IT-alone (n=3); iv) radiotherapy (RT)-alone
(n=5); v) Peg-GNP IV + RT (n=3); vi) Peg-GNP IT + RT (n=3); vii)
N-GNP IV + RT (n=4) and viii) N-GNP IT + RT (n=3).
GNPs were injected via the tail vein or directly
into the tumor using a 27-G needle attached to a 1-ml syringe
(Terumo Corp., Tokyo, Japan). Injection volumes (both IV and IT) of
Peg-GNPs and N-GNPs were 0.3 and 0.2 ml per mouse, respectively.
With respect to IV of N-GNPs, the dose and concentrations were
almost equal to that of Hainfeld et al's research (1). As the concentration of PEG-GNPs is
lower than that of N-GNPs, PEGylation was expected to lead to a
much higher accumulation of GNPs in the tumor when compared with
N-GNPs.
X-ray irradiation
A MBR-1520R-3 (Hitachi Medical Corp., Tokyo, Japan)
device was used to perform X-ray irradiation (settings, 150 kv and
20 mA, which are commonly used in such experiments.). A filter
(0.5-mm-thick aluminum + 0.1-mm-thick copper) was used, and the
distance between the radiation source and the skin was set at 55
cm. With the exception of the femoral region on the affected side,
the mouse body was protected with a 2-cm thick block prepared with
a low-melting-point lead alloy (cerrobend block). Irradiation was
performed without anesthesia or sedation using a retainer prepared
with a 50-ml conical tube. A dose of 10 Gy was delivered in a
single fraction.
Tumor assessment
Following treatment, the major and short axes of the
tumor were measured over time, and the tumor volume was calculated
using the following approximation formula: Tumor volume = (major
axis × short axis2)/2 (mm3). Tumor diameter
was measured on alternate days, three times per week for three
weeks, and the tumor volume ratio at each measurement time-point to
the tumor volume at the time of treatment initiation was calculated
as the tumor volume ratio (TVR) using the following formula: TVR =
(tumor volume at the measurement time point)/(tumor volume
immediately prior to treatment initiation). TVR was used as an
index of the tumor growth rate. The time-course of the tumor volume
ratio was evaluated, and compared among the groups.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 23; IBM Corp., Armonk, NY, USA). Mean values were
compared using Student's t-test (two-sided). Survival analysis was
performed using the Kaplan-Meier method, and the survival rate was
compared using the log-rank test. The data is presented as the mean
± standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
Suppression of tumor growth
Fig. 2 presents the
mean TVR values (Fig. 2A) and tumor
volume (Fig. 2B) of each group over
the time-course of the experiment. The TVR value was highest in the
control group, followed by the groups that were treated with the
drug alone without irradiation. The mean values of the four groups
that were treated with a combination of the drug and irradiation
were similar. Significant differences were observed between the RT
groups (RT-alone, RT + Peg-GNP IV, RT + Peg-GNP IT and RT + N-GNP
IT) and the control groups, and the RT groups and the drug alone
groups (Fig. 2A).
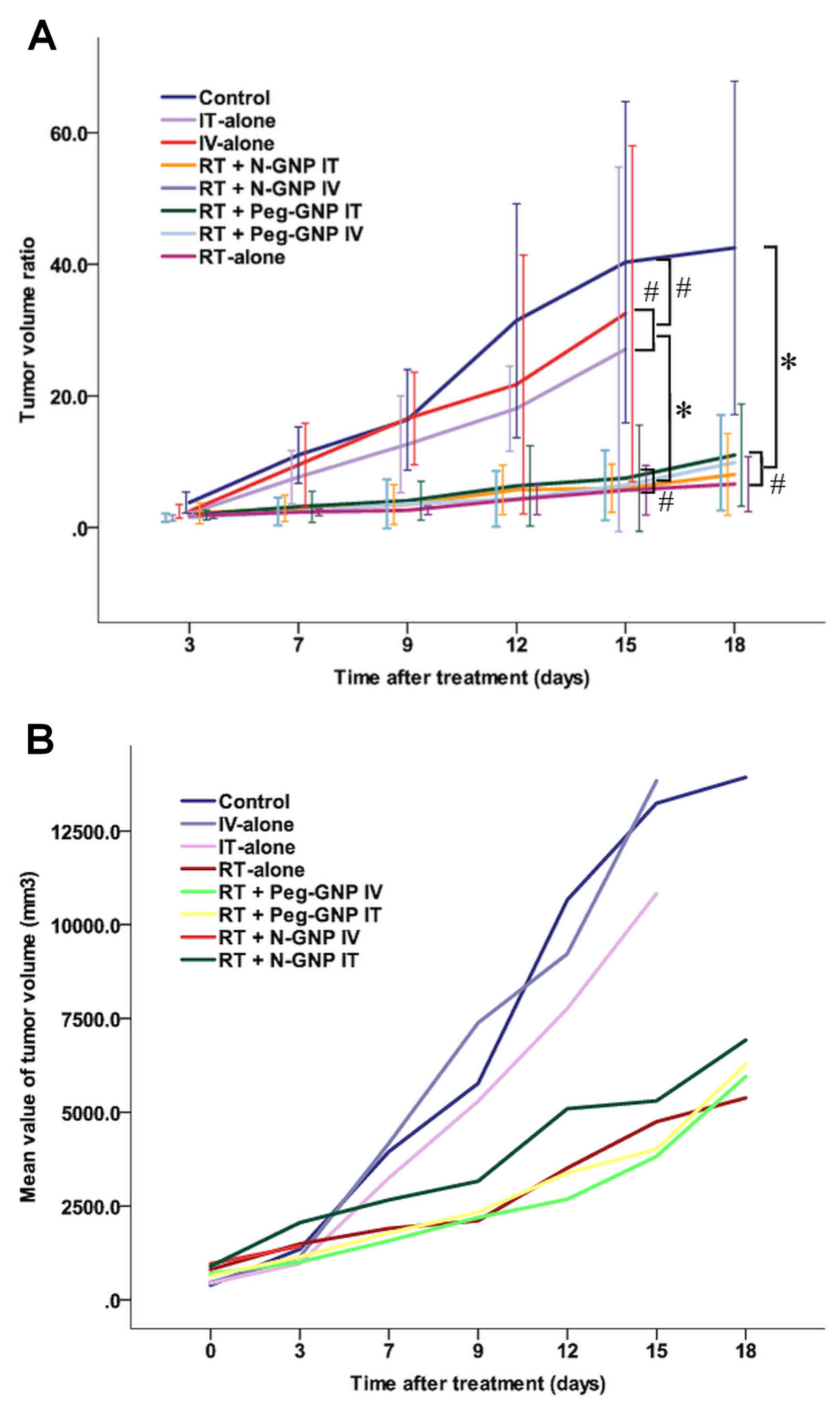 | Figure 2.Tumor growth following treatment. (A)
Mean tumor volume ratio values of each group over the time-course
of the experiment. The tumor volume ratio indicates the ratio of
the tumor volume at each time-point to the initial tumor volume.
The error bars indicate the 95% confidence intervals. Among the RT
groups (RT-alone, RT + Peg-GNP IV, RT + Peg-GNP IT and RT + N-GNP
IT), no statistically significant differences were observed. (B)
Mean tumor volume of each group over the time-course of the
experiment. *P<0.05 RT groups vs. control or Peg-GNP alone,
#P>0.05 IV-alone vs. IT alone, control vs. Peg-GNP
alone. Data are presented as mean ± standard deviation. RT,
radiotherapy; GNP, gold nanoparticles; N-GNP, naked GNP; Peg-GNP,
polyethylene glycol-binding GNP; IV, intravenous injection; IT,
intratumoral injection. |
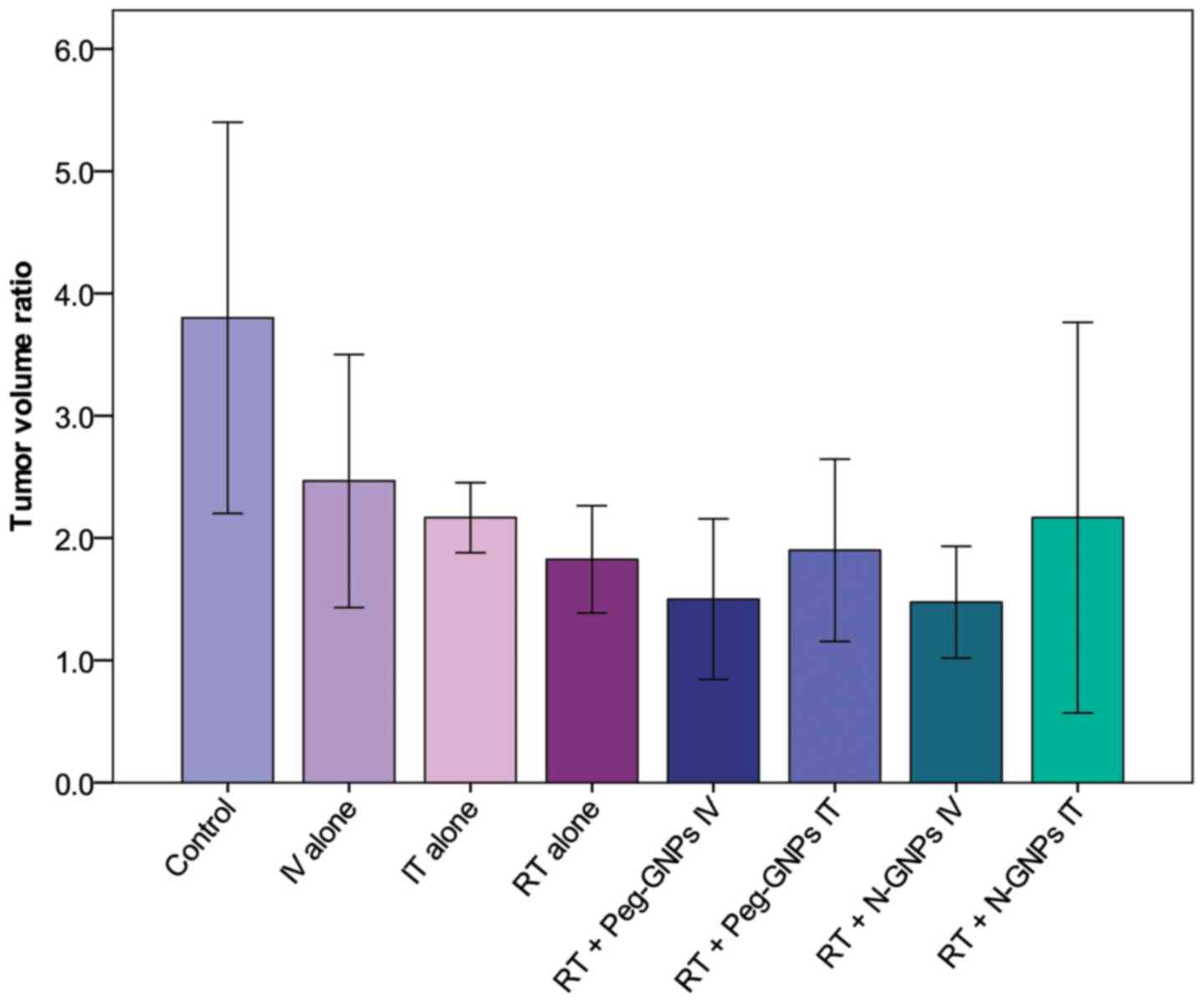
Fig. 3 presents a
comparison of the TVR among the treatment groups on day 3 after the
initiation of treatment. A tumor growth-inhibitory effect was
observed in all of the treatment groups when compared with the
control group. The mean values of the groups in which IV-injected
GNPs were administered in combination with RT compared with the
RT-alone group and the Peg-GNP and N-GNP groups demonstrated a
slight radiosensitization effect, observed as a decrease in tumor
volume.
Table I presents the
results of the analysis of significance for inter-group differences
in the TVR on days 3 and 15. As 3 and 1 of the 4 mice in the N-GNP
IV + RT group succumbed to their symptoms on days 5 and 6 after the
initiation of treatment, respectively, this group was not included
in the evaluations after day 7. In this group, one mouse was
initially excluded due to a large tumor volume (2,176
mm3) 12 days following cell transplantation. Tumor
volume of other mice in this group were 600, 726, 1,224, 1,296
mm3. The anti-tumor effect of the drug alone was
evaluated by comparing the control group to the Peg-GNP IV-alone
and Peg-GNP IT-alone groups. A significant tumor-inhibitory effect
was observed on day 3 in the IT group (P=0.047); however, the
difference on day 15 was no longer significant. The
radiosensitization effects with GNPs and the influence of the
administration methods were evaluated in the Peg-GNP IT + RT,
Peg-GNP IT + RT, N-GNP IV + RT and N-GNP IT + RT groups vs. the
RT-alone group. On day 3, the mean TVR was reduced in the Peg-GNP
IT + RT and N-GNP IV + RT groups in comparison with the RT-alone
group (1.8 vs. 1.5 and 1.8 vs. 1.4, respectively); however the
differences were not statistically significant (P=0.177 and
P=0.129, respectively). Thereafter, the growth rate increased in
the Peg-GNP IV+ RT group and the values were reversed on day 15.
The Peg-GNP IT-alone, Peg-GNP IT + RT and N-GNP IT + RT Peg-GNP
IV-alone groups were compared with the Peg-GNP IT + RT group to
assess the differences in the effects that occurred due to the
variation in the administration route. No significant differences
due to the variation in the administration route were observed in
either the drug-alone group (Peg-GNP IT-alone), or in the
irradiation-combined groups, (Peg-GNP IT + RT or N-GNP IT + RT). In
the N-GNP IV + RT and N-GNP IT + RT groups, the difference due to
variations in the drug type with the same route of administration
was assessed. The drug type was not observed to have an impact on
the treatment effect.
 | Table I.Comparison of the different treatment
groups at 3 and 15 days. |
Table I.
Comparison of the different treatment
groups at 3 and 15 days.
|
| 3 days | 15 days |
|---|
|
|
|
|
|---|
| Group | Mean value | P-value | Mean value | P-value |
|---|
| Control vs. RT
alone |
| 0.024 |
| 0.014a |
| Control | 3.8 |
| 40.3 |
|
| RT-alone | 1.8 |
| 5.6 |
|
| Control vs. Peg-GNP
IV-alone |
| 0.091 |
| 0.605 |
| Control | 3.8 |
| 40.3 |
|
| Peg-GNP IV-alone | 2.4 |
| 32.5 |
|
| Control vs. Peg-GNP
IT-alone |
| 0.047a |
| 0.392 |
| Control | 3.8 |
| 40.3 |
|
| Peg-GNP IT-alone | 2.1 |
| 27 |
|
| RT-alone vs. Peg-GNP
IV + RT |
| 0.177 |
| 0.68 |
| RT-alone | 1.8 |
| 5.6 |
|
| Peg-GNP IV + RT | 1.5 |
| 6.4 |
|
| RT-alone vs. Peg-GNP
IT + RT |
| 0.745 |
| 0.424 |
| RT-alone | 1.8 |
| 5.6 |
|
| Peg-GNP IT + RT | 1.9 |
| 7.5 |
|
| RT-alone vs. N-GNP IV
+ RT |
| 0.129 |
|
|
| RT-alone | 1.8 |
|
|
|
| N-GNP IV + RT | 1.4 |
|
|
|
| RT-alone vs. N-GNP IT
+ RT |
| 0.375 |
| 0.859 |
| RT-alone | 1.8 |
| 5.6 |
|
| N-GNP IT + RT | 2.1 |
| 5.9 |
|
| Peg-GNP IV-alone
vs. Peg-GNP IT-alone |
| 0.295 |
| 0.568 |
| Peg-GNP
IV-alone | 2.4 |
| 32.5 |
|
| Peg-GNP
IT-alone | 2.1 |
| 27 |
|
| Peg-GNP IV + RT vs.
Peg-GNP IT + RT |
| 0.158 |
|
|
| Peg-GNP IV +
RT | 1.5 |
| 6.4 | 0.659 |
| Peg-GNP IT +
RT | 1.9 |
| 7.5 |
|
| N-GNP IV + RT vs.
N-GNP IT + RT |
| 0.108 |
|
|
| N-GNP IV + RT | 1.4 |
|
|
|
| N-GNP IT + RT | 2.1 |
|
|
|
| Peg-GNP IV + RT vs.
N-GNP IV + RT |
| 0.911 |
|
|
| Peg-GNP IV +
RT | 1.5 |
|
|
|
| N-GNP IV + RT | 1.4 |
|
|
|
| Peg-GNP IT + RT vs.
N-GNP IT + RT |
| 0.551 |
| 0.497 |
| Peg-GNP IT +
RT | 1.9 |
| 7.5 |
|
| N-GNP IT + RT | 2.1 | | 5.9 |
|
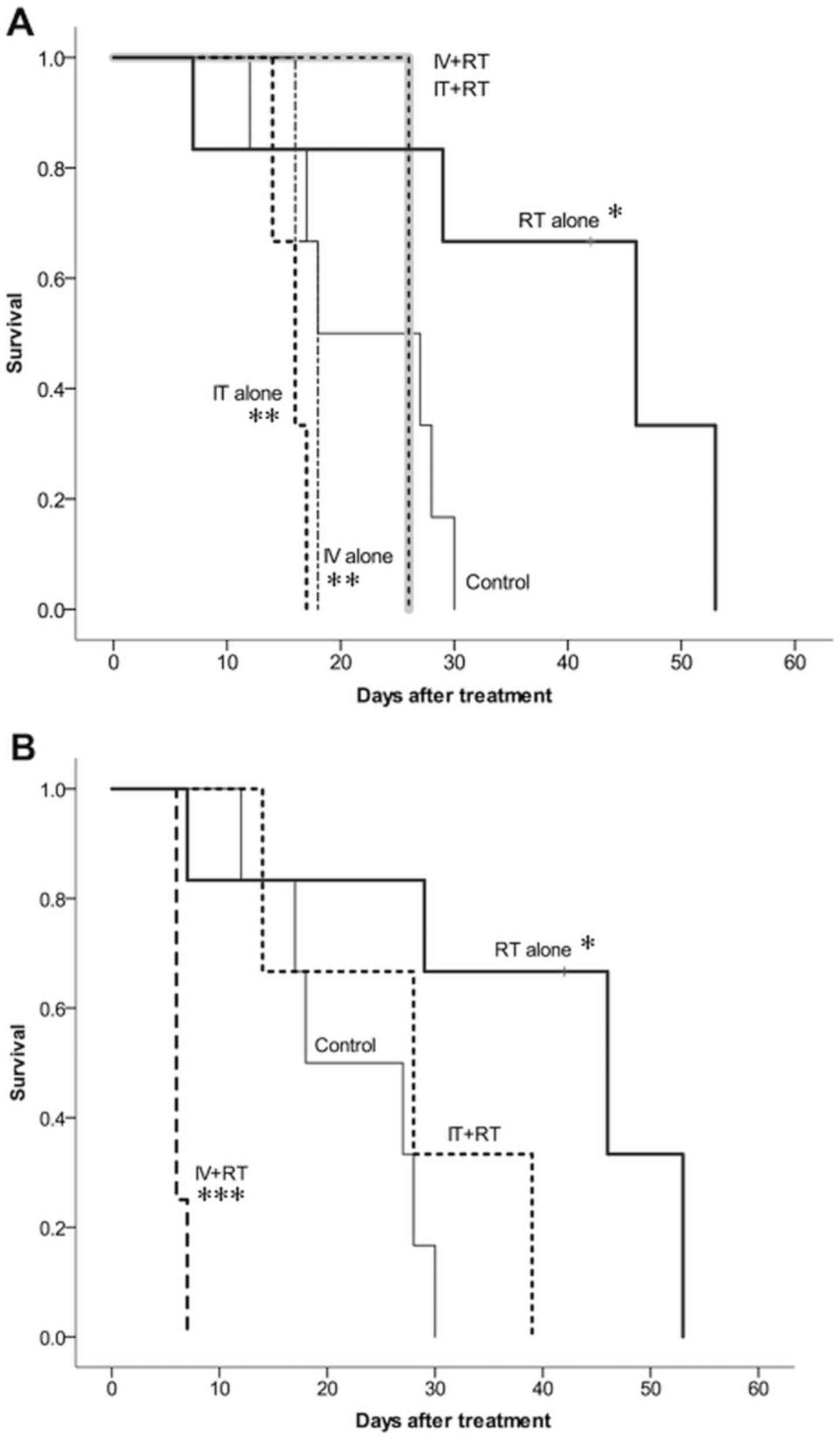
Survival time
Fig. 4 presents the
survival time of the groups treated with Peg-GNPs (Fig. 4A) and N-GNPs (Fig. 4B) employing each administration
method. The addition of GNPs did not improve the survival time. The
results of the inter-group comparisons, which were performed using
the log-rank test, were as follows: i) A significant difference was
observed between the control and RT-alone groups (P=0.023), and the
survival time was longer in the RT-alone group. ii) Combined
treatment with GNPs and radiotherapy led to a significantly more
favorable survival time in comparison to the GNP-alone groups
(Peg-GNP IT-alone vs. Peg-GNP IT + RT, P=0.025; Peg-GNP IV-alone
vs. Peg-GNP IV + RT, P=0.030). iii) Combined treatment with GNPs
and irradiation did not lead to a significant improvement in
survival time when compared with the RT-alone group (RT-alone vs.
Peg-GNP IV + RT, P=0.070; RT-alone vs. Peg-GNP IT + RT, P=0.070;
RT-alone vs. N-GNP IT + RT, P=0.094). The survival time was
significantly reduced in the N-GNPs IV + RT group (RT-alone vs.
N-GNPs IV + RT, P=0.004).
Discussion
GNP-induced radiosensitization in vivo was
initially reported by Hainfeld et al in 2004 (1). Subsequent studies have clarified that
the degree of radiosensitization is influenced by radiation energy,
drug concentration, particle size and cell type (2,6,11,19).
Since malignant melanoma is a superficial radiation-resistant
tumor, it may be a good indicator for the success of this treatment
method; however, very few in vivo studies have been
performed to investigate the effects in melanoma.
In 2008, Chang et al (13) reported GNP-induced radiosensitization
with electron beams in mice that were implanted with cells from the
B16F10 melanoma cell line (13).
Previous studies clarified that the degree of radiosensitization is
proportionate to the concentration of GNPs (8); however the dose of the drug in the
present study was 40- to 50-fold higher than in the studies of
Chang et al (13) or Mousavie
Anijdan et al (14). In
addition to the drug concentration, there were differences in other
experimental conditions, including the radiation energy, radiation
dose and the timing of treatment initiation. In a previous study,
treatment was initiated at a high dose (single dose of electron
beam treatment: 25 Gy) 7 days after transplantation, and the tumor
volume at this time-point was 50–90 mm3, which was
markedly smaller than that at the start of the current experiment
(13).
In 2013, Mousavie Anijdan et al (14) performed an experiment under similar
conditions, using megavoltage X-rays, but only partial
radiosensitization was observed (14). In the present experiment, the mean
TVR value 3 days after intravenous injection was slightly lower in
the IV-combined (Peg-GNPs and N-GNPs) groups than in the RT-alone
group, which suggests the presence of slight radiosensitization;
however the difference was not statistically significant. The
results reported by Mousavie Anijdan et al (14) were similar to the findings of the
present study in that GNP-induced radiosensitization was observed
in the first month, but overall, it did not lead to a significant
difference (14).
Table II compares
the results of the present experiment with those of previous
studies using malignant melanoma-implanted mice. However, whether
the ineffectiveness of GNPs as a radiosensitizer observed in the
current study was caused by the physical aspects (radiation dose or
energy), or the biological aspects of the experiments, remains to
be elucidated.
 | Table II.Summary of the previous studies on
the radiosensitization effect in B16F10 bearing mice. |
Table II.
Summary of the previous studies on
the radiosensitization effect in B16F10 bearing mice.
|
| Studies |
|---|
|
|
|
|---|
| Parameter | Chang et al,
2008 (13) | Mousavie Anijdan
et al, 2013 (14) | Present study
(Naked GNP cases) |
|---|
| Diameter of GNP,
nm | 13 | 50 | 15 |
| Concentration and
amount of GNP | 200 (nM)x0.2
(ml) | 5 (mg/ml)x0.1–0.2
(ml) | 200 (mg/ml)x0.2
(ml) |
| Administration
route | Intravenous | Intratumoral | Intravenous and
Intratumoral |
| Tumor volume,
mm3 | 50–90 | 400–600 | 200–1300 |
| Radiation
energy | 6 MeV
electrons | 6 and 18 MV
X-ray | 150 kv X-ray |
| Radiation dose,
Gy/1 fraction | 25 | 20 | 10 |
| Tumor growth | Suppressed | Partially
suppressed | Not suppressed |
| Statistical
survival benefit | Yes | No | No |
| Effect of GNP
alone | No | No | Not performed but
partially observed in Peg-GNP |
Mousavie Anijdan et al (14) stated that the tumor volume may
markedly influence the radiosensitization effect.
There were also differences in a number of other
conditions; however, the findings of the present study did not
contradict previous conclusions. In highly malignant tumors, such
as melanoma, the tumor volume may influence the radiosensitization
effect. Therefore, the association between the tumor size and the
radiosensitizing effect should be evaluated in future studies.
Regarding the effect of GNPs alone, Peg-GNPs were
only observed to have an antitumor effect in the early period after
treatment initiation. Treatment with GNPs alone was not reported to
have an effect by Mousavie Anijdan et al (14); however, Chang et al (13) reported a slight increase in the
apoptosis activity level in comparison with the control group. A
number of studies have reported that GNPs themselves decrease
clonogenic survival, increase apoptosis and induce DNA damage
(20–22). Furthermore, Butterworth et al
(23) demonstrated that these
cytotoxic effects were cell-type specific. The results of the
present study may reflect these toxic effects. As the present
experiment included mice with tumors that were large in comparison
to those of previous studies, a generous amount of GNPs at a
commercially-available dose was administered. This may have had a
negative effect on survival. Whether the decreased survival was
associated with the impact of the initial tumor size at the time of
treatment or the toxicity of GNPs itself was not determined. These
issues should be further studied with a view toward the clinical
application of this treatment in the future.
In conclusion, the current study could not confirm
the radiosensitization effect of GNPs in melanoma bearing mice with
tumors that were larger in size in comparison to previous
experiments. However, Peg-GNP-alone demonstrated a slight tumor
suppression effect in the early stage of treatment. Further
research is required to validate the radiosensitizing effect on
large tumors.
Acknowledgements
The current study was supported by the Japan Society
for the Promotion of Sciences, Tokyo, Japan (grant no.
JP25461928).
References
|
1
|
Hainfeld JF, Slatkin DN and Smilowitz HM:
The use of gold nanoparticles to enhance radiotherapy in mice. Phys
Med Biol. 49:N309–N315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hainfeld JF, Dilmanian FA, Slatkin DN and
Smilowitz HM: Radiotherapy enhancement with gold nanoparticles. J
Pharm Pharmacol. 60:977–985. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mesbahi A: A review on gold nanoparticles
radiosensitization effect in radiation therapy of cancer. Rep Pract
Oncol Radiother. 15:176–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain S, Hirst DG and O'Sullivan JM: Gold
nanoparticles as novel agents for cancer therapy. Br J Radiol.
85:101–113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cooper DR, Bekah D and Nadeau JL: Gold
nanoparticles and their alternatives for radiation therapy
enhancement. Front Chem. 2:862014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Babaei M and Ganjalikhani M: The potential
effectiveness of nanoparticles as radio sensitizers for
radiotherapy. Bioimpacts. 4:15–20. 2014.PubMed/NCBI
|
|
7
|
Rahman WN, Bishara N, Ackerly T, He CF,
Jackson P, Wong C, Davidson R and Geso M: Enhancement of radiation
effects by gold nanoparticles for superficial radiation therapy.
Nanomedicine. 5:136–142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamada M, Foote M and Prow TW: Therapeutic
gold, silver and platinum nanoparticles. Wiley Interdiscip Rev
Nanomed Nanobiotechnol. 7:428–445. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang AZ, Langer R and Farokhzad OC:
Nanoparticle delivery of cancer drugs. Annu Rev Med. 63:185–198.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herold DM, Das IJ, Stobbe CC, Iyer RV and
Chapman JD: Gold microspheres: A selective technique for producing
biologically effective dose enhancement. Int J Radiat Biol.
76:1357–1364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coulter JA, Jain S, Butterworth KT,
Taggart LE, Dickson GR, McMahon SJ, Hyland WB, Muir MF, Trainor C,
Hounsell AR, et al: Cell type-dependent uptake, localization, and
cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine.
7:2673–2685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jain S, Coulter JA, Hounsell AR,
Butterworth KT, McMahon SJ, Hyland WB, Muir MF, Dickson GR, Prise
KM, Currell FJ, et al: Cell-specific radiosensitization by gold
nanoparticles at megavoltage radiation energies. Int J Radiat Oncol
Biol Phys. 79:531–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang MY, Shiau AL, Chen YH, Chang CJ,
Chen HH and Wu CL: Increased apoptotic potential and dose-enhancing
effect of gold nanoparticles in combination with single-dose
clinical electron beams on tumor-bearing mice. Cancer Sci.
99:1479–1484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mousavie Anijdan SH, Mahdavi SR, Shirazi
A, Zarrinfard MA and Hajati J: Megavoltage X-ray dose enhancement
with gold nanoparticles in tumor bearing mice. Int J Mol Cell Med.
2:118–123. 2013.PubMed/NCBI
|
|
15
|
Zhang XD, Wu D, Shen X, Chen J, Sun YM,
Liu PX and Liang XJ: Size-dependent radiosensitization of
PEG-coated gold nanoparticles for cancer radiation therapy.
Biomaterials. 33:6408–6419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joh DY, Sun L, Stangl M, Al Zaki A, Murty
S, Santoiemma PP, Davis JJ, Baumann BC, Alonso-Basanta M, Bhang D,
et al: Selective targeting of brain tumors with gold
nanoparticle-induced radiosensitization. PLoS One. 8:e624252013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hainfeld JF, Smilowitz HM, O'Connor MJ,
Dilmanian FA and Slatkin DN: Gold nanoparticle imaging and
radiotherapy of brain tumors in mice. Nanomedicine (Lond).
8:1601–1609. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang M and Thanou M: Targeting
nanoparticles to cancer. Pharmacol Res. 62:90–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brun E, Sanche L and Sicard-Roselli C:
Parameters governing gold nanoparticle X-ray radiosensitization of
DNA in solution. Colloids Surf B Biointerfaces. 72:128–134. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vecchio G, Galeone A, Brunetti V, Maiorano
G, Sabella S, Cingolani R and Pompa PP: Concentration-dependent,
size-independent toxicity of citrate capped AuNPs in Drosophila
melanogaster. PLoS One. 7:e299802012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi SY, Jeong S, Jang SH, Park J, Park
JH, Ock KS, Lee SY and Joo SW: in vitro toxicity of serum
protein-adsorbed citrate-reduced gold nanoparticles in human lung
adenocarcinoma cells. Toxicol In Vitro. 26:229–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Noël C, Simard JC and Girard D: Gold
nanoparticles induce apoptosis, endoplasmic reticulum stress events
and cleavage of cytoskeletal proteins in human neutrophils. Toxicol
In Vitro. 31:12–22. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Butterworth KT, Coulter JA, Jain S, Forker
J, McMahon SJ, Schettino G, Prise KM, Currell FJ and Hirst DG:
Evaluation of cytotoxicity and radiation enhancement using 1.9 nm
gold particles: Potential application for cancer therapy.
Nanotechnology. 21:2951012010. View Article : Google Scholar : PubMed/NCBI
|