Introduction
The human immunodeficiency virus (HIV) is the main
cause of the acquired immune deficiency syndrome (AIDS) (1). HIV is an RNA retrovirus that infects
immune cells and uses the host cell transcriptional and
translational machineries to proliferate and, at the same time,
undermine the health of the human host. By 2015 there were nearly
40 million AIDS patients worldwide (2), with an increasing trend and a shift
towards the younger population. By the end of 2015, ~1 million
people had AIDS in China, with the population below 45 years
accounting for 86.4% of all patients. Therefore, strengthening the
diagnosis and treatment of AIDS is critical to control this global
epidemic. Clinical research shows that Mycobacterium
tuberculosis (M. tuberculosis) is the main pathogen causing
AIDS mortality (3). M.
tuberculosis can proliferate in AIDS patients and destruct the
respiratory system and other organs. Studies have shown that the
main clinical features of AIDS are CD4+ T cell
reduction, excessive immune activation, and rapid increase in HIV
(4). As the main target of HIV/AIDS,
CD4+ T cells play an important role in the occurrence
and development of AIDS. In recent years, research has shown that
differentiation and maturation of T helper 17 (Th17) and T
regulatory (Treg) cells from CD4+ T cells play key roles
in resistance to amplification and invasion of the HIV virus.
Interleukin-17 (IL-17) promotes the expression of
cytokines and plays an important role in the inflammatory immune
response. Th17 cells are thought to have a significant inhibitory
effect on the replication and amplification of the HIV. Research
shows that Th17 in the late stage of infection and the early stage
of inflammation can promote the expression of immune factors, link
innate and adaptive immunity, and improve overall immunity
(5). The main role of Treg is to
inhibit the effect of T lymphocytes to prevent excessive autoimmune
symptoms, thereby reducing the body resistance to external
pathogens. Under normal circumstances, the Th17/Treg ratio is
relatively stable, but inflammation and other immune conditions
disturb this balance (6). For
example, during inflammation, TGF-β can promote Treg cell
production and promote the differentiation of Th17 and other cells.
In the later stages of the inflammatory reaction, TGF-β can inhibit
the immune response by inhibiting the proliferation of Treg cells,
so the Th17/Treg balance is critical to maintain the normal immune
function. There are few reports about the effect of Th17/Treg cell
immune imbalance on HIV replication in patients with AIDS
complicated with tuberculosis (TB) (7). Here, we studied for the first time the
Th17/Treg cell immune imbalance effect on virus replication in AIDS
patients with TB. Overall, we want to provide the theoretical and
experimental basis to diagnose and treat patients with AIDS
complicated with TB.
Materials and methods
Patient information
We recruited 32 patients treated for AIDS combined
with TB infection in Xiangya Hospital from January 2011 to March
2015. Among them, 18 were male and 14 were female. The average age
was 35.3±12.5 years. Thirty healthy individuals were also recruited
as controls, including 18 males and 14 females, with an average age
of 32.7±13.2 years. The studywas approved by the Ethics Committee
of Xiangya Hospital and informed consents were signed by the
patients and/or guardians.
Inclusion criteria: i) HIV patients with TB; and ii)
ages between 20 and 50 years. Exclusion criteria: i) Other
inflammatory diseases; ii) suffering from other immune system
diseases; and iii) ages below 20 years and over 50 years.
Reagents and instruments
Main reagents: Fetal bovine serum (FBS) and
L-glutamic acid (both from Hyclone, Logan, UT, USA), PBS buffer
solution (Alfa, Suzhou, China), monoclonal first antibody of IL-17,
IL-6 and IL-10, and HRP-labeled polyclonal second antibody (both
from Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Main instruments: Flow cytometry, biological safety
cabinet and nitrogen canister (all from Thermo Fisher Scientific,
Inc.), protein electrophoresis apparatus (Beijing 61 Instrument
Factory, Beijing, China), low-temperature high-speed centrifuge
(Hitachi, Tokyo, Japan), and cell counter (Thermo Fisher
Scientific, Inc.).
Sample collection
We collected 5 ml of elbow vein blood through the
EDTA anticoagulation (Applied Biosystems, Foster City, CA, USA)
vacuum collected blood vessels, and carried on the examination in
12 h.
Cell count
We used flow cytometry to carry out technical
statistics on different cells. The method was first to take the 15
µl CD4-FTTC monoclonal antibody (Acris Antibodies, Inc., San Diego,
CA, USA) into the sample tube of the flow cytometry, and then add
50 µl of each blood sample, gently mix evenly, and preservation
avoiding light and store it at room temperature for 10 min. Then,
the percentage of CD4+ T cells and the total cells were
detected by flow cytometry (8).
Enzyme-linked immune response
Total protein samples were obtained and the
expression of IL-17, IL-6 and IL-10 were determined by ELISA, and
the specific operation was performed in accordance with the ELISA
kit (Qiagen GmbH, Hilden, Germany) instructions. The standard curve
was made according to the ELISA standard curve step and the protein
samples were diluted in elution buffer with a ratio of 1:100. After
diluting the samples with sterilized PBS (pH 7.2) with a ratio of
1:200, 100 µl were added to a 96-well plate, and then 50 µl were
added to each well. After incubation at room temperature for 2 h,
the TMB substrate was added, with the determination of absorbance
at 495 nm. Then, the IL-17, IL-6 and IL-10 concentration in each
sample were calculated according to the standard curve (9).
Treg cell expression level
detection
Two flow type tubes were used as the test and
control tubes. The liquid transfer gun was used to add 100 µl cells
at 106/ml and 10 µl CD4-PE/CD25-APC (Becton-Dickinson,
San Jose, CA, USA), placed at 4°C for 30 min, 1 ml fixative
solution (BD Biosciences, Franklin Lakes, NJ, USA) was added and
mixed well, incubated at 4°C avoiding light for 30 min, and
centrifuge washing with permeabilization buffer. Then, 1 µl 1 rat
serum was added with 4°C incubation for 15 min, 2.5 µl PE
anti-mouse/rat Foxp3 antibody (BD Biosciences) and 2.5 µl PE rat
lgG2a homotype were added with 4°C incubation in the dark for 30
min. After three washes, 500 µl of Flow Cytometry Staining Buffer
(BD Biosciences) was added. Finally, flow cytometry was used to
analyze the expression level of Treg on CD4+ T
lymphocytes (10).
Th7 cell expression detection
Peripheral venous blood (10 ml) was collected,
peripheral mononuclear cells (PBMC) were separated by Ficoll
density gradient centrifugation, PBMC density was adjusted to
2×106/ml, and resuspended in RPMI-1640 culture medium
(Invitrogen Life Technologies, Carlsbad, CA, USA). PBMC suspension
2×106/ml was added into the 24-well culture plates, 1
ml/well, 20 ng/ml phorbol myristate acetate (PMA) and 1 µg/ml
ionomycin (both from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), were cultivated for 2 h in the culture box (37°C, 5%
CO2), then the monensin 2 nmol/ml (Sigma-Aldrich; Merck
KGaA), was added and cultured for 2 h. The cells were collected,
washed 3 times in PBS and divided into control and test tubes. Each
tube received 10 µl CD4-FITC (Becton-Dickinson), then incubated for
15 min at room temperature, adding 100 µl fixative with mixing at
room temperature for 15 min, after PBS washing 3 times, 100 µl of
film developing agent was added, gently mixed evenly, and placed at
room temperature in the dark for 5 min. In the experimental tube,
we added 5 µl of anti-human IL-17 mAb (BD Biosciences). Into the
control tubes, 5 µl of control antibody IgG-PE was added. The tubes
were mixed at room temperature for 30 min in the dark. After 3 PBS
washes, the cells were detected by flow cytometry with 500 µl PBS
suspension cells, and the expression level of Th17 cells was
analyzed by CD4+ T lymphocytes (10).
Viral load measurement
Viral loads in different blood samples were measured
by COBAS kit (Roche Diagnostics, Indianapolis, IN, USA). The kit
could be used for quantitative determination of viral load
(fluorescent quantitative PCR), and the process was carried out
following the instructions of the manufacturer.
Statistical analysis
The experimental data were processed with SPSS 20.0
(SPSS, Inc., Chicago, IL, USA) statistical software. The
experimental data are expressed as mean ± SD. Single factor
analysis method was used to analyze the data between different
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
CD4+ T cell count in
peripheral blood lymphocytes
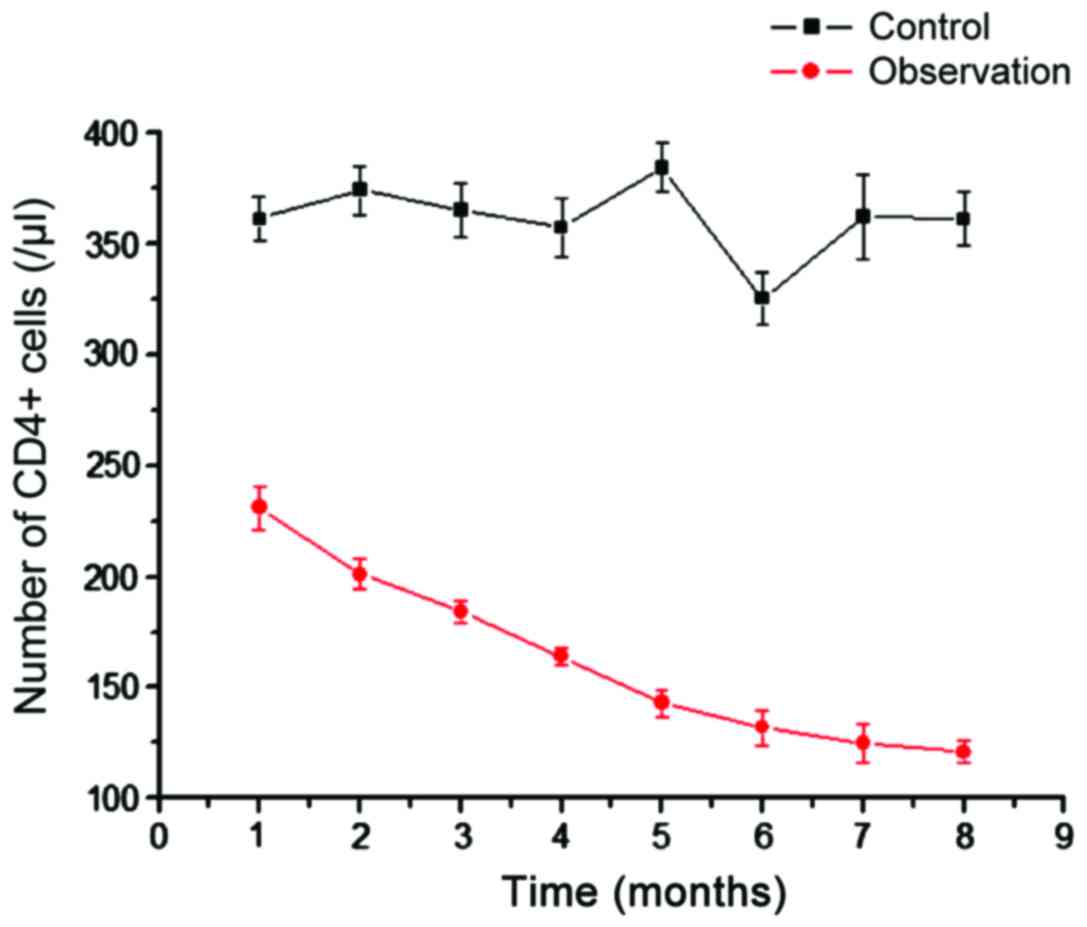
We analyzed the number of CD4+ T cells in
the control and observation groups (Fig.
1). Compared with CD4+ T cells in the blood of
healthy people, the CD4+ T cells in the observation
group were significantly reduced, and the difference was more
significant with time (Fig. 1). This
shows that AIDS with TB infection can reduce the number of
CD4+ T cells. As the main immune cells infected by HIV,
the gradual decrease in the number of CD4+ T cells can
explain the increasing number of viral particles.
Baseline expression of Th17 cells in
peripheral blood lymphocytes
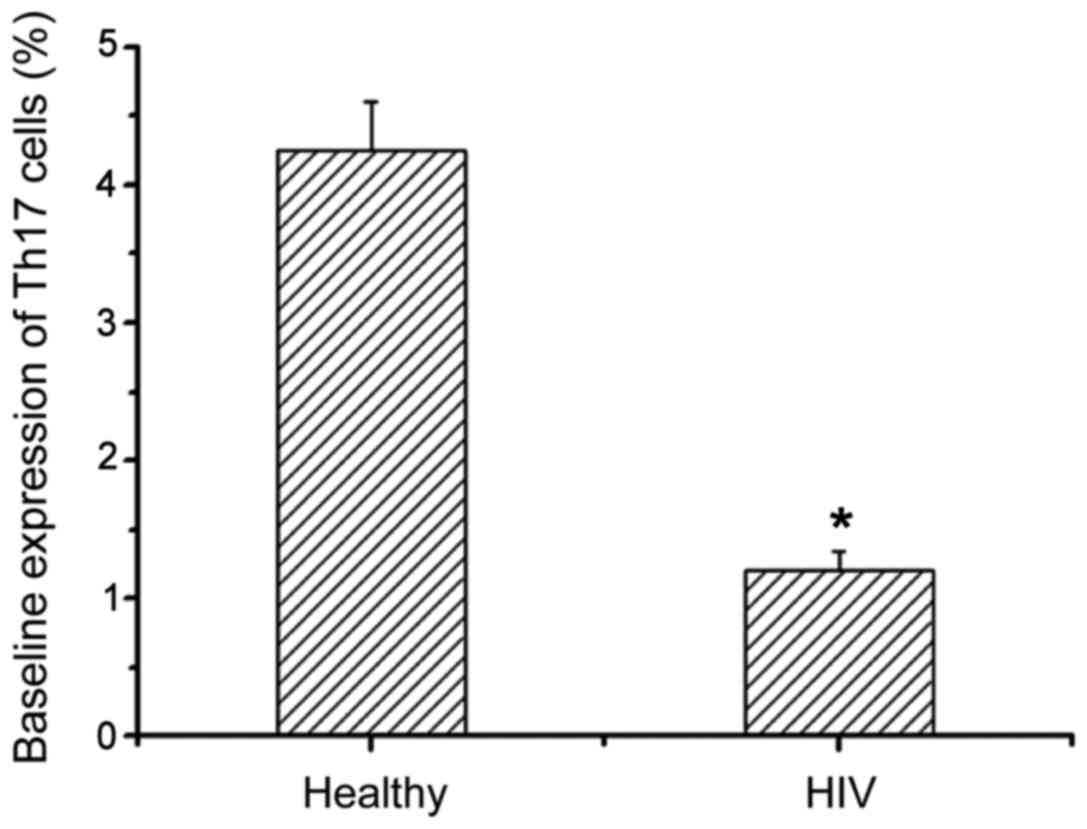
We next examined the expression levels of Th17 in
healthy people and patients with AIDS complicated with TB infection
by flow cytometry (Table I).
Compared with healthy people, Th17 expression levels in AIDS
patients with TB infection were significantly decreased, and there
was a significant difference between them (Fig. 2). This suggests that the HIV virus
can reduce the level of Th17 cells and thereby disrupt the immune
system.
 | Table I.Baseline expression of Th17 cells in
peripheral blood lymphocytes. |
Table I.
Baseline expression of Th17 cells in
peripheral blood lymphocytes.
| Groups | No. of cases | CD4+
(cells/µl) | Th17 cells (%) |
|---|
| Healthy
population | 30 | 912±254 | 4.24±0.36 |
| HIV complicated with
tuberculosis infection | 32 | 145±38 | 1.21±0.13 |
Expression of Treg in peripheral blood
lymphocytes
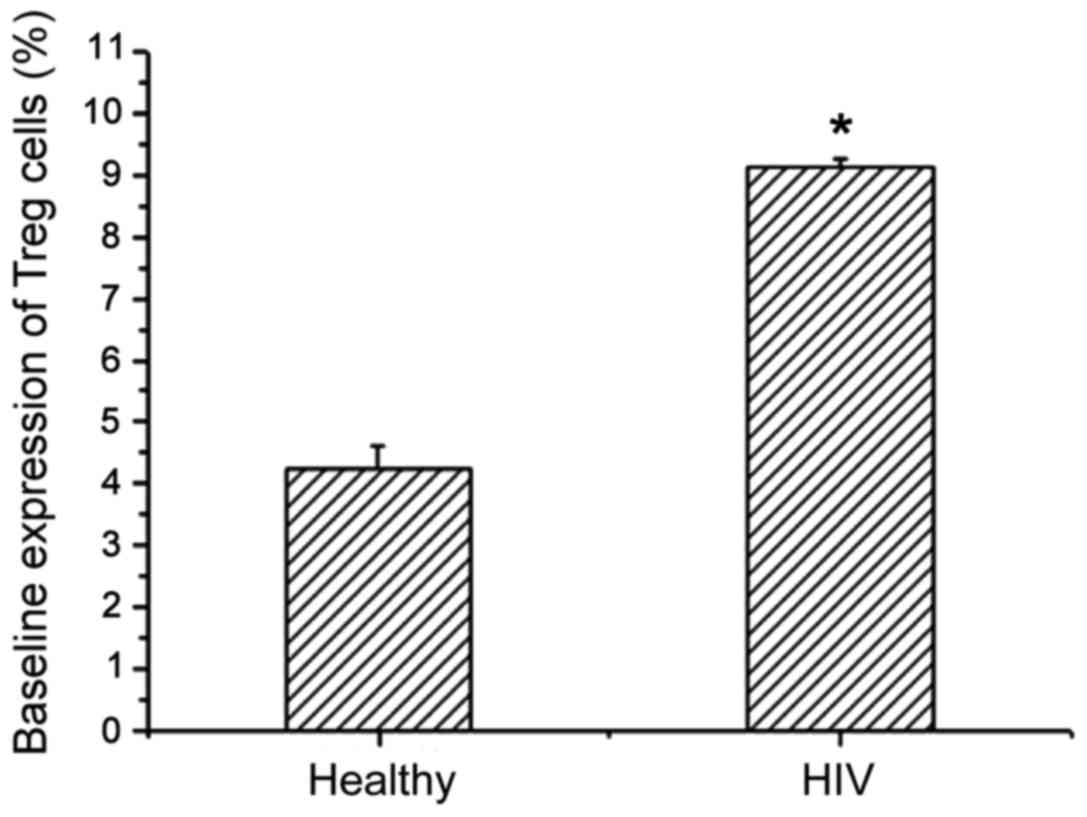
Treg expression levels in healthy people and AIDS
patients with TB infection were detected by flow cytometry
(Table II). Compared with healthy
people, the Treg level in AIDS patients with TB infection showed an
increasing trend, and there was a significant difference between
the two (Fig. 3). This shows that
the immune system can enhance immunity by increasing the amount of
Treg expression, thereby enhancing the ability to remove HIV.
 | Table II.Baseline expression of Treg cells in
peripheral blood lymphocytes. |
Table II.
Baseline expression of Treg cells in
peripheral blood lymphocytes.
| Groups | No. of cases | CD4+
(cells/µl) | Treg cells (%) |
|---|
| Healthy
population | 30 | 912±254 | 4.24±0.36 |
| HIV complicated with
tuberculosis infection | 32 | 1.832±316 | 9.14±1.31 |
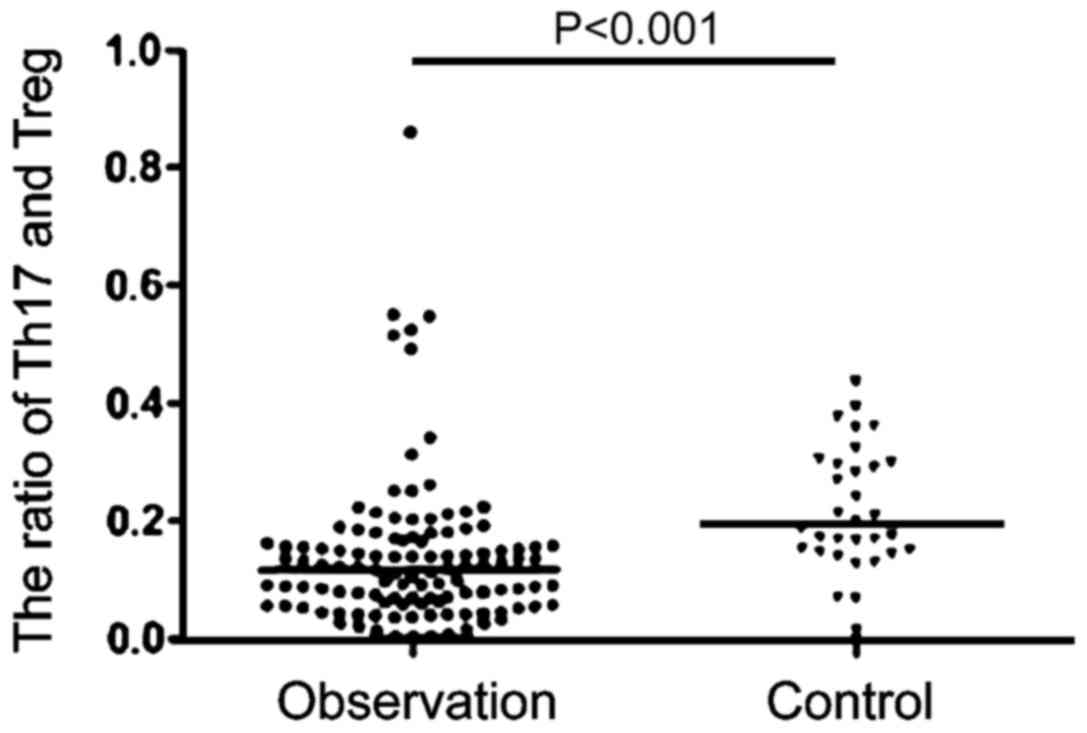
Changes of Th17/Treg ratio in
peripheral blood lymphocytes
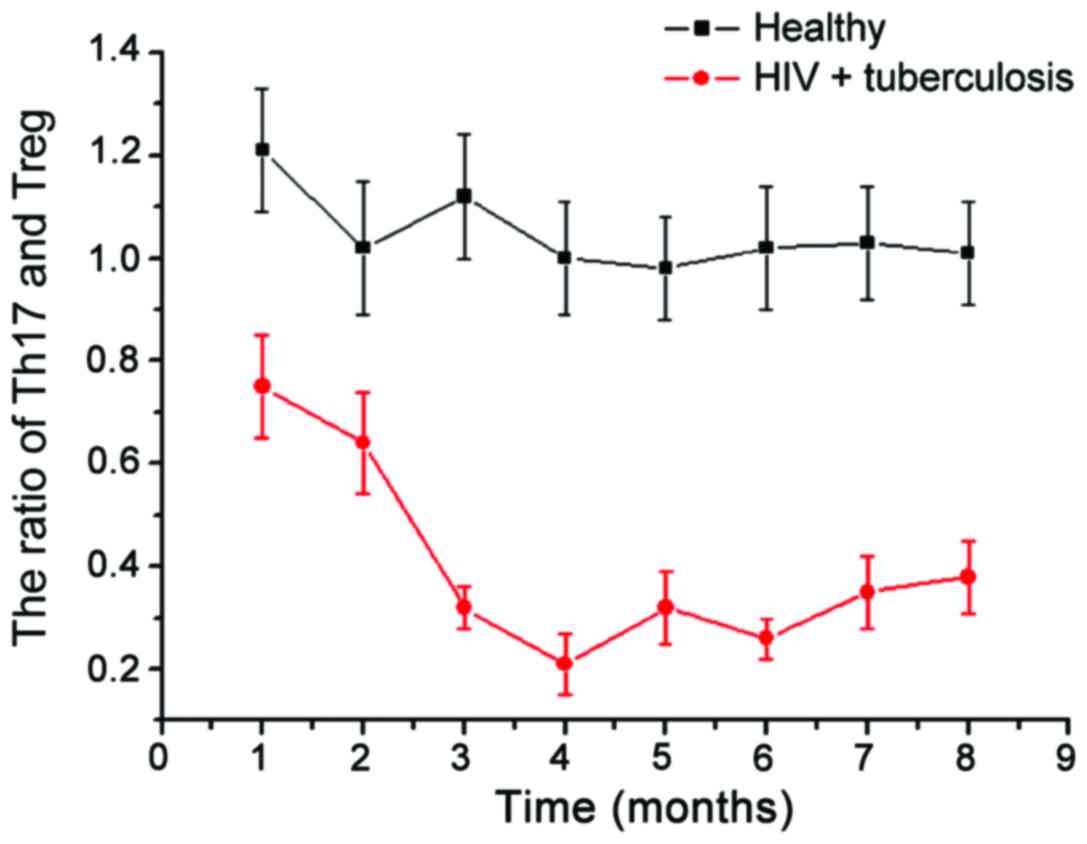
We then examined the changes in Th17/Treg in healthy
people and patients with HIV (Fig.
4). Compared with stable ratio of Th17/Treg in the healthy
population, the Th17/Treg ratio in patients with AIDS complicated
with TB infection had greater variability. In the initial period of
HIV infection, there was no significant change in the proportion of
Th17/Treg, but over time, the Th17/Treg ratio showed a gradual
downward trend. In the late stage, the Th17/Treg ratio showed a
gradual upward trend; the Th17/Treg ratio strongly correlates with
the stability of the collective immune system. With the Th17/Treg
immune imbalance gradually increasing in the observation group, the
difference in viral load was even more remarkable, which indicates
that there is significant correlation between Th17/Treg immune
imbalance and AIDS complicated with TB infection (Fig. 5).
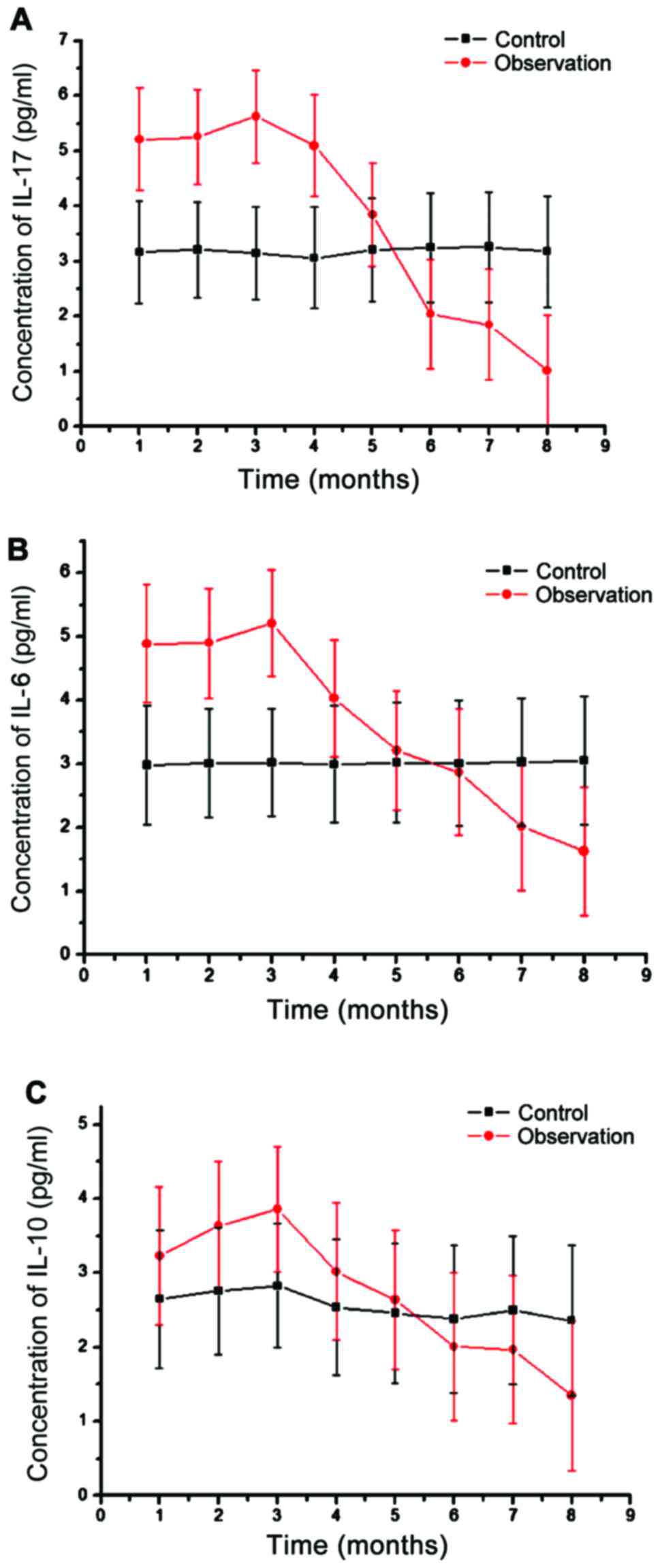
Changes of IL-17, IL-6 and IL-10 in
peripheral blood lymphocytes
Finally, we detected the changes of IL-17, IL-6 and
IL-10 by ELISA (Fig. 6). Compared
with the levels of IL-17, IL-6 and IL-10 in healthy subjects
(3.02±0.48, 3.13±0.76, 2.89±0.83 pg/ml), patients with AIDS
complicated with TB infection showed significantly higher levels,
and the differences were significant (Fig. 6). With the aggravation of the
disease, IL expression decreased gradually, with the later stage
showing significantly lower levels of IL-17, IL-6 and IL-10 than in
the healthy population.
Discussion
TB infection is the main cause of death in AIDS
patients and it also leads to many diseases following HIV infection
(11). HIV can also promote the
progression of TB in lung degeneration (12). Our study showed that co-infection of
HIV with M. tuberculosis can accelerate the differentiation
of immune cells. In this process, T cells induced by M.
tuberculosis can activate virus replication to promote HIV
transformation of non-infectious into infectious, and finally
promote the transition to AIDS and the subsequent deterioration. In
recent years, research has shown that HIV associated with TB
infection decreases the number of CD4+ T cells and leads
to the lack of related functions (13). The number of CD4+ T cells
is often used as an indicator to measure the immune system
function. CD4+ T cells can be divided into Th17 cells
and Treg cells (14). The number of
Th17 and Treg cells and the proportion of CD4+ T cells
are related to the development of the disease. For instance, in
patients with acute respiratory distress syndrome (15), the CD4+ T cells gradually
decreased as the condition gradually deteriorated and the Th17/Treg
ratio gradually decreased (16).
Here, we found that the Th17/Treg ratio also showed a tendency to
decrease as AIDS/TB progressed. Study on Treg cells has shown that
TGF-β1 can promote the differentiation of CD4+ T cells
into Treg cells, whereas Treg cells can block and inhibit viral
replication, and thus play a therapeutic role in the treatment of
disease (17). At the same time,
Th17 cells are mainly involved in the inflammatory reaction
(7), the abnormal increase of Th17
cells can induce autoimmune diseases (18,19).
Here, we measured Treg, Th17, and CD4+ T cell changes
and found that CD4+ T cells showed a gradual downward
trend as AIDS with TB progressed. Also, the Treg/Th17 ratio was
significantly reduced compared to the healthy group. This indicates
that Treg/Th17 is imbalanced in patients with HIV combined with
M. tuberculosis, and this immune imbalance leads to
increased HIV replication. HIV mainly destroys the immune system
and immune cells, so it eventually leads to a decrease in the
number of major immune cells, such as CD4+ T cells,
showing that the imbalance of Treg/Th17 can largely lead to
aggravation of AIDS with TB infection.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh A, Vajpayee M, Ali SA and Chauhan
NK: Cellular interplay among Th17, Th1, and Treg cells in HIV-1
subtype ‘C’ infection. J Med Virol. 86:372–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heydenreich B, Bellinghausen I, König B,
Becker WM, Grabbe S, Petersen A and Saloga J: Gram-positive
bacteria on grass pollen exhibit adjuvant activity inducing
inflammatory T cell responses. Clin Exp Allergy. 42:76–84. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Witte E, Witte K, Warszawska K, Sabat R
and Wolk K: Interleukin-22: A cytokine produced by T NK and NKT
cell subsets, with importance in the innate immune defense and
tissue protection. Cytokine Growth Factor Rev. 21:365–379. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petrovas C, Yamamoto T, Gerner MY, Boswell
KL, Wloka K, Smith EC, Ambrozak DC, Sandler NG, Timmer KJ, Sun X,
et al: CD4 T follicular helper cell dynamics during SIV infection.
J Clin Invest. 122:3281–3294. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim CJ, Nazli A, Rojas OL, Chege D,
Alidina Z, Huibner S, Mujib S, Benko E, Kovacs C, Shin LYY, et al:
A role for mucosal IL-22 production and Th22 cells in
HIV-associated mucosal immunopathogenesis. Mucosal Immunol.
5:670–680. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Paz B, Prado C, Alperi-López M,
Ballina-García FJ, Rodriguez-Carrio J, López P and Suárez A:
Effects of glucocorticoid treatment on CD25FOXP3+
population and cytokine-producing cells in rheumatoid arthritis.
Rheumatology (Oxford). 51:1198–1207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eyerich S, Wagener J, Wenzel V, Scarponi
C, Pennino D, Albanesi C, Schaller M, Behrendt H, Ring J,
Schmidt-Weber CB, et al: IL-22 and TNF-α represent a key cytokine
combination for epidermal integrity during infection with Candida
albicans. Eur J Immunol. 41:1894–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zeng M, Haase AT and Schacker TW: Lymphoid
tissue structure and HIV-1 infection: Life or death for T cells.
Trends Immunol. 33:306–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng M, Smith AJ, Wietgrefe SW, Southern
PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G,
Lifson JD, et al: Cumulative mechanisms of lymphoid tissue fibrosis
and T cell depletion in HIV-1 and SIV infections. J Clin Invest.
121:998–1008. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chege D, Sheth PM, Kain T, Kim CJ, Kovacs
C, Loutfy M, Halpenny R, Kandel G, Chun TW, Ostrowski M, et al
Toronto Mucosal Immunology Group, : Sigmoid Th17 populations, the
HIV latent reservoir, and microbial translocation in men on
long-term antiretroviral therapy. AIDS. 25:741–749. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mai J, Wang H and Yang XF: Th 17 cells
interplay with Foxp3+ Tregs in regulation of
inflammation and autoimmunity. Front Biosci (Landmark Ed).
15:986–1006. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caccamo N, Pietra G, Sullivan LC, Brooks
AG, Prezzemolo T, La Manna MP, Di Liberto D, Joosten SA, van
Meijgaarden KE, Di Carlo P, et al: Human CD8 T lymphocytes
recognize Mycobacterium tuberculosis antigens presented by HLA-E
during active tuberculosis and express type 2 cytokines. Eur J
Immunol. 45:1069–1081. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai HC, Velichko S, Hung LY and Wu R:
IL-17A and Th17 cells in lung inflammation: An update on the role
of Th17 cell differentiation and IL-17R signaling in host defense
against infection. Clin Dev Immunol. 2013:2679712013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Y, Zhang J, Li X, Yang Y, Zhang Y, Ma J
and Xi L: Penicillium marneffei infection: An emerging disease in
mainland China. Mycopathologia. 175:57–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Basile G, Paffumi I, D'Angelo AG,
Figliomeni P, Cucinotta MD, Pace E, Ferraro M, Saitta S, Lasco A
and Gangemi S: Healthy centenarians show high levels of circulating
interleukin-22 (IL-22). Arch Gerontol Geriatr. 54:459–461. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Engelhardt KR and Grimbacher B: Mendelian
traits causing susceptibility to mucocutaneous fungal infections in
human subjects. J Allergy Clin Immunol. 129:294–307. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Presicce P, Shaw JM, Miller CJ, Shacklett
BL and Chougnet CA: Myeloid dendritic cells isolated from tissues
of SIV-infected Rhesus macaques promote the induction of regulatory
T cells. AIDS. 26:263–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salgado M, Rallón NI, Rodés B, López M,
Soriano V and Benito JM: Long-term non-progressors display a
greater number of Th17 cells than HIV-infected typical progressors.
Clin Immunol. 139:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matthews K, Wilkinson KA, Kalsdorf B,
Roberts T, Diacon A, Walzl G, Wolske J, Ntsekhe M, Syed F, Russell
J, et al: Predominance of interleukin-22 over interleukin-17 at the
site of disease in human tuberculosis. Tuberculosis (Edinb).
91:587–593. 2011. View Article : Google Scholar : PubMed/NCBI
|