Introduction
Tuberculosis is an infectious disease caused by
Mycobacterium tuberculosis or the other members of the
Mycobacterium complex such as Mycobacterium africanum
and Mycobacterium bovis. The disease remains a major threat
to global public health and presents increasing morbidity and
mortality rates worldwide (1,2). There
were 1.5 million cases of mortality and 9 million new cases of
tuberculosis in 2013 worldwide (3).
M. tuberculosis can invade numerous organs in humans, but
primarily affect the lung function (4). Cough and phlegm are the most common
early symptoms of tuberculosis, along with blood or blood clots in
the phlegm (5). Clinically, patients
with tuberculosis are admitted to intensive care units (ICU) to
monitor physical symptoms and inflammation (6,7). In the
last decade, more efficient drug targets for tuberculosis have been
explored and the pathological mechanisms of M. tuberculosis
have been investigated in order to understand its pathogenesis
(8,9). These drugs and mechanisms of pathology
of M. tuberculosis have contributed to clinical treatments
for patients with tuberculosis (10,11).
Notably, the most common pathology characteristic of M.
tuberculosis is inflammatory responses in patients.
Although previous reports have indicated the
significance of inhibition of inflammation responses in patients
with tuberculosis (12–14), the molecular mechanisms of the M.
tuberculosis-induced signaling pathway are seldom reported and
must be further analyzed in human pleural mesothelial cells
(hPMCs). M. tuberculosis infection commonly leads to
recruitment of leukocytes and formation of granulomas around the
infected macrophages, which results in limitation of the spread of
M. tuberculosis in the lungs (15). Previous work indicates that
inflammation responses are host-directed therapies for patients
with M. tuberculosis infection (16). Tsenova et al (17) demonstrated that inflammation
accelerates pathology in a rabbit model of active pulmonary
tuberculosis. Furthermore, inflammation in patients frequently
induces intraocular inflammation, chronic pulmonary heart disease
and other syndromes (18,19). These reports suggest that
inflammation may be a key inducer for aggravated pathology for
patients with tuberculosis.
Currently, tumor necrosis factor-α (TNF-α) and
matrix metalloproteinases (MMPs) are reported to be associated with
the pathological processes of tuberculosis (20). A previous study indicated that TNF-α
expression is associated with pathogenesis and progression of
patients with pulmonary tuberculosis (21). Mihaltan (22) also suggested that TNF-α blockers are
beneficial for the treatment of pulmonary tuberculosis. In
addition, MMP-1 polymorphism has been indicated as a risk factor
for fibrosis after pulmonary tuberculosis (23). Furthermore, the role of MMP-8 in 5′
adenosine monophosphate-activated protein kinase-dependent matrix
destruction in human pulmonary tuberculosis has been studied and
the results demonstrated that neutrophil-derived MMP-8 serves a key
role in the pathology of tuberculosis (24). These reports suggest that MMPs and
TNF-α are associated with the progression of tuberculosis.
In the present study, the inflammatory factors in
patients with pulmonary tuberculosis were investigated. The balance
of T helper cell (Th)1/Th2 cytokines and the expression levels of
interferon (IFN)-γ, interleukin (IL)-10, IL-12, and IL-4 were
analyzed. The TNF-α and MMP-induced extracellular-signal-regulated
kinase (ERK)/Akt signaling pathways were investigated in hPMCs
isolated from patients with pulmonary tuberculosis.
Materials and methods
Ethics statement
The study protocol was performed according to the
Guide for the Care and Use of Clinical Patients of Capital Medical
University (Beijing, China). The study was approved by the Ethics
Committee of Beijing Chest Hospital (Beijing, China). Informed
consent was provided by all participants. A total of 124 patients
(12–58 years old, 72 male and 52 female) with M.
tuberculosis infection who had been admitted to ICU were
recruited to analyze inflammatory cell and factor expression in
Beijing Tuberculosis and Thoracic Tumor Research Institute (Bejing,
China) between May 2012 and June 2014. A further 52 healthy
volunteers (21–46 years old, 32 male and 20 female) were recruited
as a control group between May 2012 and May 2013.
Cell culture
hPMCs were obtained from patients and healthy
volunteers and cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany). hPMCs were cultured in a 5%
CO2 incubator with a humidified atmosphere at 37°C.
ELISA
Blood samples (15 ml) were collected from clinical
patients and healthy volunteers via the jugular vein catheter.
Serum was obtained from blood via centrifugation at 6,000 × g at
4°C for 15 min. In the protein detection assay, human MMP-1
(DY901B), MMP-9 (DMP900), TNF-α (DTA00C), IL-1 (DLB50), IL-6
(D6050), IL-10 (D1000B), IL-12 (D1200), IL-4 (D4050), IL-2 (D2050),
IL-15 (DY247) and IFN-γ (DIF50; All Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) ELISA kits were used to determine serum levels
of the inflammatory factors. The procedures were performed
according to the manufacturer's protocols. The final results were
recorded at 450 nm on an ELISA plate reader (Bio-Rad Laboratories,
Inc.).
Small interfering RNA (siRNA)
transfection
hPMCs were cultured to 80% confluence and
transfected with siRNA that targeted TNF-α (si-TNF-α,
5′-UGGGGAACUCUUCCCUCUG-3′) or si-vector containing scrambled siRNA
(5′-CUCGUCUCAUUGATGACAGTT-3′) using Lipofectamine™ RNAi MAX
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A total of 100 pmol si-TNF-α and si-vector
(GenePharma Co., Ltd., Shanghai, China) were used for transfection.
The subsequent experimentation was performed after 48 h
transfection.
Western blot analysis
hPMCs were lysed in RIPA buffer (Sigma-Aldrich;
Merck KGaA) containing a phosphatase inhibitor and protease
inhibitor cocktail. Protein concentrations were determined by BCA
protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein
concentration was measured by a BCA protein assay kit (Thermo
Scientific, Inc.). A total of 20 µg protein extracts was subjected
to 12.5% SDS-PAGE and then transferred to polyvinylidene membrane
(EMD Millipore, Billerica, MA, USA). The primary antibodies of rat
anti-human anti-TNF-α (1:1,000 dilution, ab667), anti-MMP-1
(1:1,000 dilution, ab137332), anti-MMP-9 (1:1,000 dilution,
ab73734), high mobility group box-1 protein (HMGB1, 1:1,000
dilution, ab18256), ERK (1:2,000 dilution, ab196883, Abcam), pERK
(1:2,000 dilution, ab214362, Abcam), AKT (1:1,000 dilution, ab8805,
Abcam), pAKT (1:1,000 dilution, ab133458, Abcam), and β-actin
(1:1,000 dilution, ab8226; all Abcam, Cambridge, UK) were used to
incubate with the membranes for 120 min at 37°C. Then goat
anti-rabbit IgG mAb (1:5,000 dilution, PV-6001, OriGene
Technologies, Inc., Beijing, China) were added to the membranes for
60 min at 37°C. Following this, the membrane was washed three times
in TBST, and was developed using a chemiluminescence assay system
(Roche Diagnostics, Basel, Switzerland) and exposed to Kodak
exposure films. Densitometric quantification of the immunoblot data
was performed by using the software of Quantity-One (version 3.23,
Bio-Rad Laboratories, Inc.).
Histological assay
Lung specimens (n=3 in each group) obtained from
patients and healthy volunteers as previously indicated (25). Specimens were prepared and fixed in
4% paraformaldehyde for 2 h at 37°C. Paraffin-embedded tissue
sections (4 µm) were prepared and epitope retrieval was performed
using Tris-HCl buffer for heat-induced epitope retrieval
(AP-9005-050, Thermo Fisher Scientific, Inc.) for further analysis.
The paraffin sections were quenched with hydrogen peroxide (3%) for
10–15 min, and subsequently blocked with a blocking solution 5%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) for 10–15 min at
37°C. Finally, the sections were incubated with goat anti-human
anti-CD11b (1:1,000 dilution, ab133357, Abcam), anti-CD177 (1:1,000
dilution, ab203025, Abcam), or anti-CD31 (1:1,000 dilution,
ab28364, Abcam) at 4°C for 12 h. Sections were stained with the
rabbit anti-goat horseradish peroxidase-conjugated anti-rabbit IgG
(1:5,000 dilution, PV-6001, OriGene Technologies, Inc.) after
washing with PBS three times for 2 h at 37°C. Visualization was
achieved with peroxidase-labeled streptavidin-biotin and
diaminobenzidine (DAB, Advansta, Inc., Menlo Park, CA, USA) for ~5
min at 37°C. The slides were examined with a Keyence Biozero
BZ8100E microscope.
Flow cytometry
Serum was obtained as described above, and serum
levels of lymphocytes, plasmacytes, neutrophils and monocytes in
patients with pulmonary tuberculosis or healthy volunteers were
analyzed using a flow cytometer. For detecting Th1 and Th2 cells, a
Th1/Th2 (7 plex) Multiplex Immunoassay Kit (ab213389, Abcam) was
used to measure the percentage of Th1 and Th2 cells, and TH1/Th2
ratio. All procedures were performed as previously described and
the percentage of cells were analyzed using BD FACSCanto™ Software
(version 2.0; BD Biosciences San Jose, CA, USA) as described
previously (26).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Significance was established with the SPSS 19.0 statistical (IBM
Corp., Armonk, NY, USA) and GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). Comparisons between two groups
were conducted by Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Analysis of inflammatory cells in
serum in patients with M. tuberculosis infection
Clinical data from 124 patients with pulmonary
tuberculosis revealed that immune cells were upregulated in the
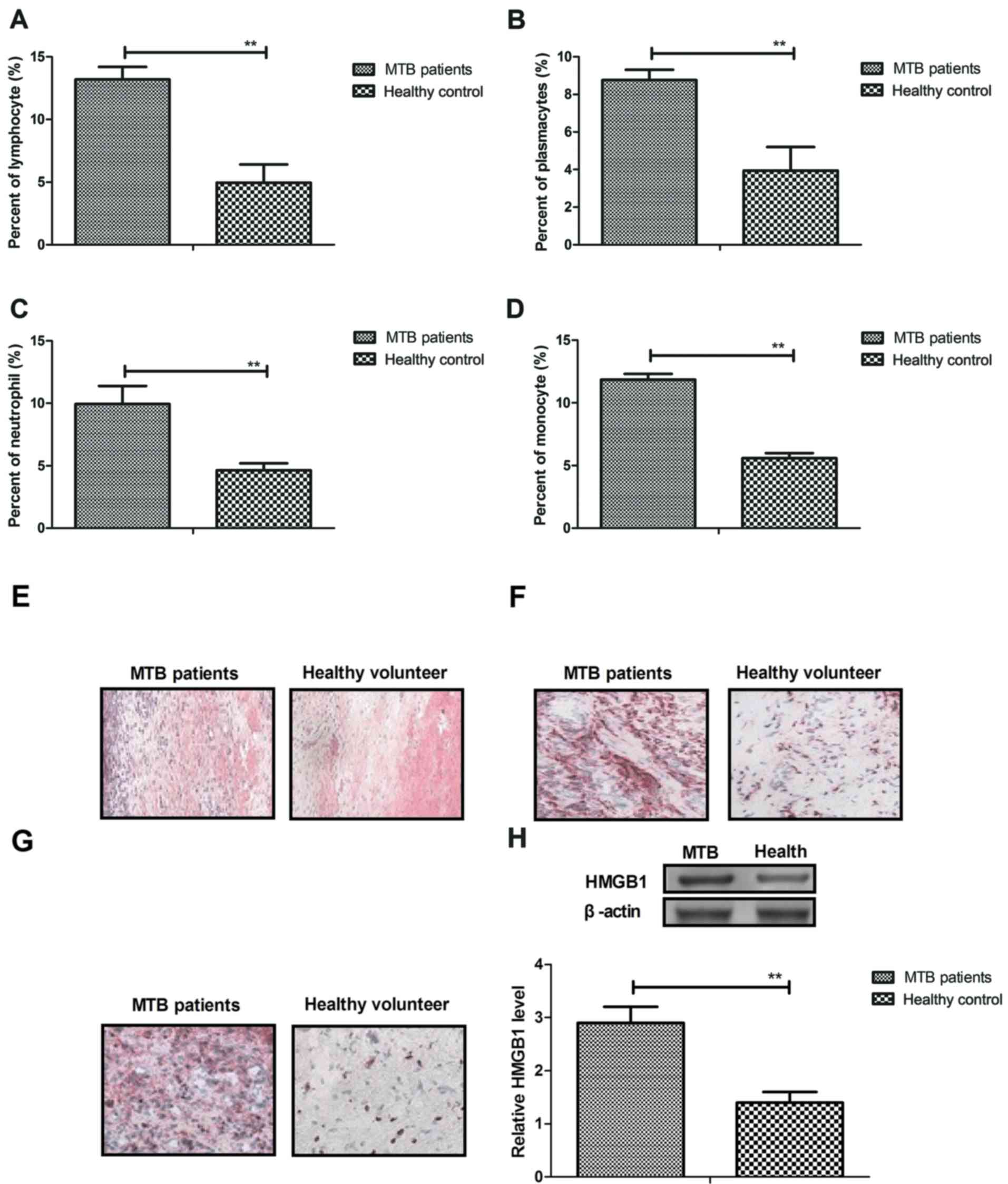
serum. As shown in Fig. 1A-D, the
results indicated that serum concentration levels of lymphocytes,
plasmacytes, neutrophils and monocytes were significantly increased
in the patients with M. tuberculosis compared with healthy
controls (P<0.01). In addition, outcomes indicated that
macrophages, mast cells and endothelial cells were also increased
in lung tissue in the patients with M. tuberculosis compared
with healthy controls (Fig. 1E-G).
Furthermore, it was observed that high HMGB1 expression levels were
significantly upregulated in lung tissue in patients with M.
tuberculosis compared with healthy controls (P<0.01;
Fig. 1H), which would contribute to
inflammation in patients. These outcomes suggest that inflammatory
cells were upregulated in patients with M. tuberculosis.
Analysis of inflammatory factors in
patients with M tuberculosis
To detect the association between inflammatory
factors and M. tuberculosis, serum levels of inflammatory
factors were measured in patients with pulmonary tuberculosis in
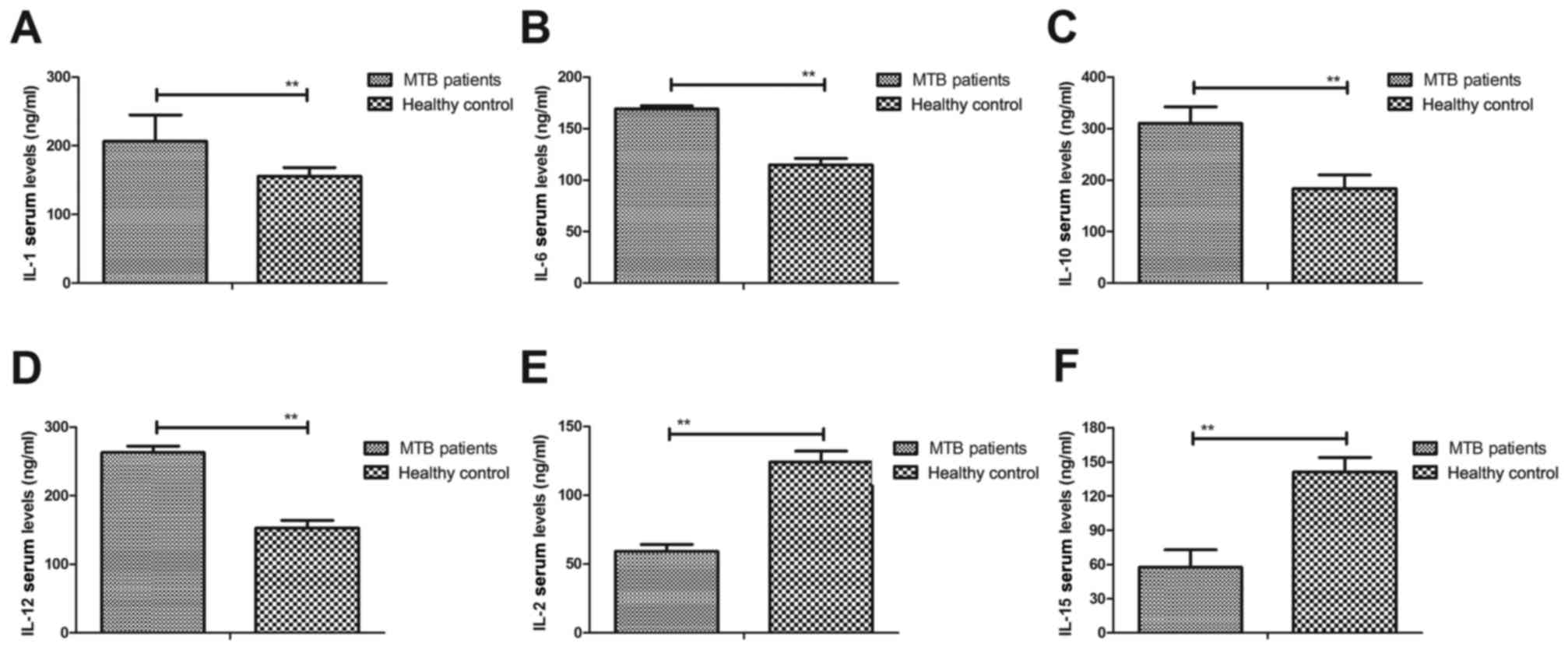
ICU, with healthy volunteers as the control. As shown in Fig. 2A-D, serum concentration levels of
IL-1, IL-6, IL-10 and IL-12 were significantly upregulated in
patients with pulmonary tuberculosis in ICU compared with healthy
controls (P<0.01). However, significantly lower serum levels of
IL-2 and IL-15 were observed in patients with pulmonary
tuberculosis compared with healthy controls (P<0.01; Fig. 2E and F). These outcomes suggest that
inflammatory factors were increased in the serum, while
anti-inflammatory factors were decreased in patients with pulmonary
tuberculosis in ICU.
Analysis of the balance of Th1/Th2
cytokines in patients with pulmonary tuberculosis
A previous study indicated that an imbalance of
Th1/Th2 cytokines is a key indicator of inflammation and serves a
critical function in the pathology of pulmonary tuberculosis in ICU
(27). In the present study, the
expression levels of Th1 and Th2 cytokines were measured in
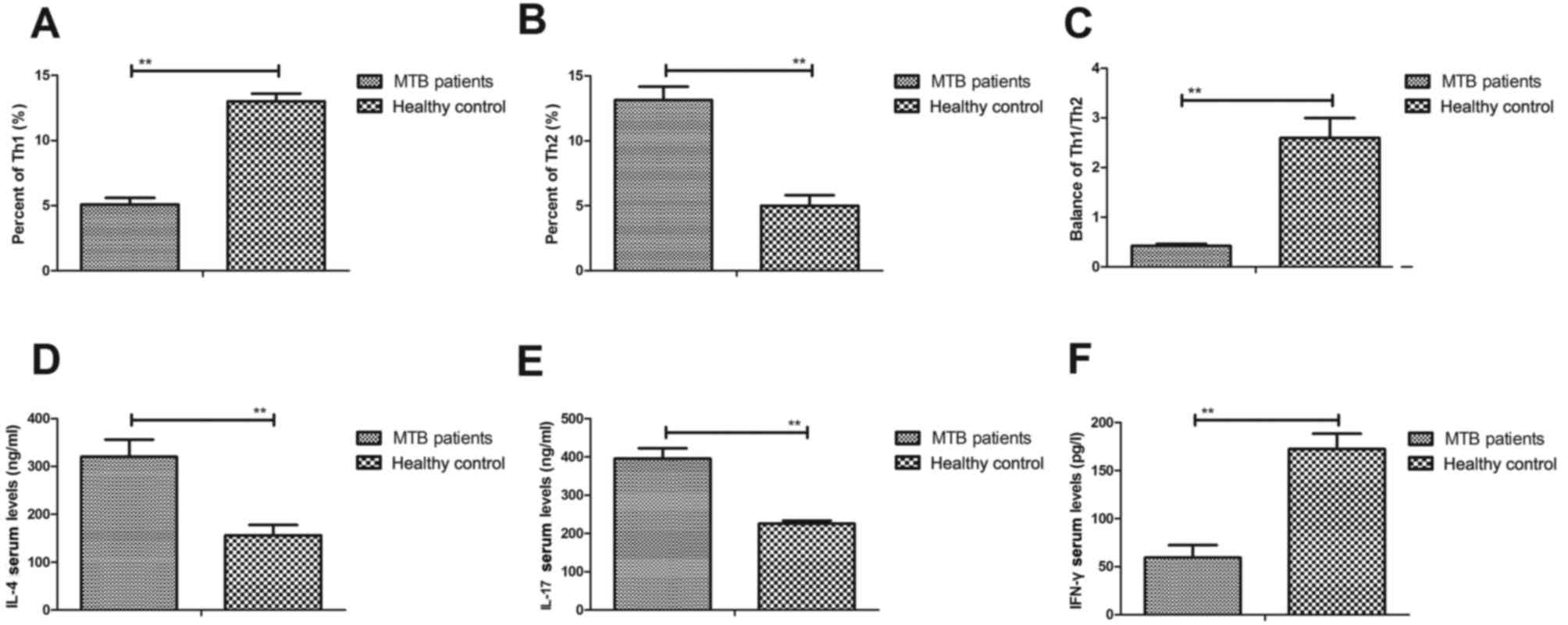
patients with pulmonary tuberculosis in ICU. As shown in Fig. 3A and B, significantly lower
expression of Th1 cytokines and significantly higher expression of
Th2 cytokines was observed in the serum of patients with pulmonary
tuberculosis compared with healthy controls (both P<0.01). In
addition, the ratio of Th1/Th2 cytokines was significantly
decreased in patients with pulmonary tuberculosis compared with
healthy controls (P<0.01; Fig.
3C). In addition, significantly higher IL-4 and IL-17 levels
(both P<0.01) and significantly lower serum levels of IFN-γ
(P<0.01) were observed (Fig.
3D-F), contributing to the imbalance of Th1/Th2 cytokines in
patients with pulmonary tuberculosis compared with healthy
controls. These outcomes suggest that an imbalance of Th1/Th2
cytokines is associated with pulmonary tuberculosis.
Analysis of TNF-α and MMP expression
in hPMCs isolated from patients with pulmonary tuberculosis
It has previously been reported that expression
levels of TNF-α and MMPs are correlated with the severity of
patients with pulmonary tuberculosis (28,29). In
the present study, the levels of TNF-α, MMP-1 and MMP-9 were
evaluated in serum and hPMCs isolated from patients with pulmonary
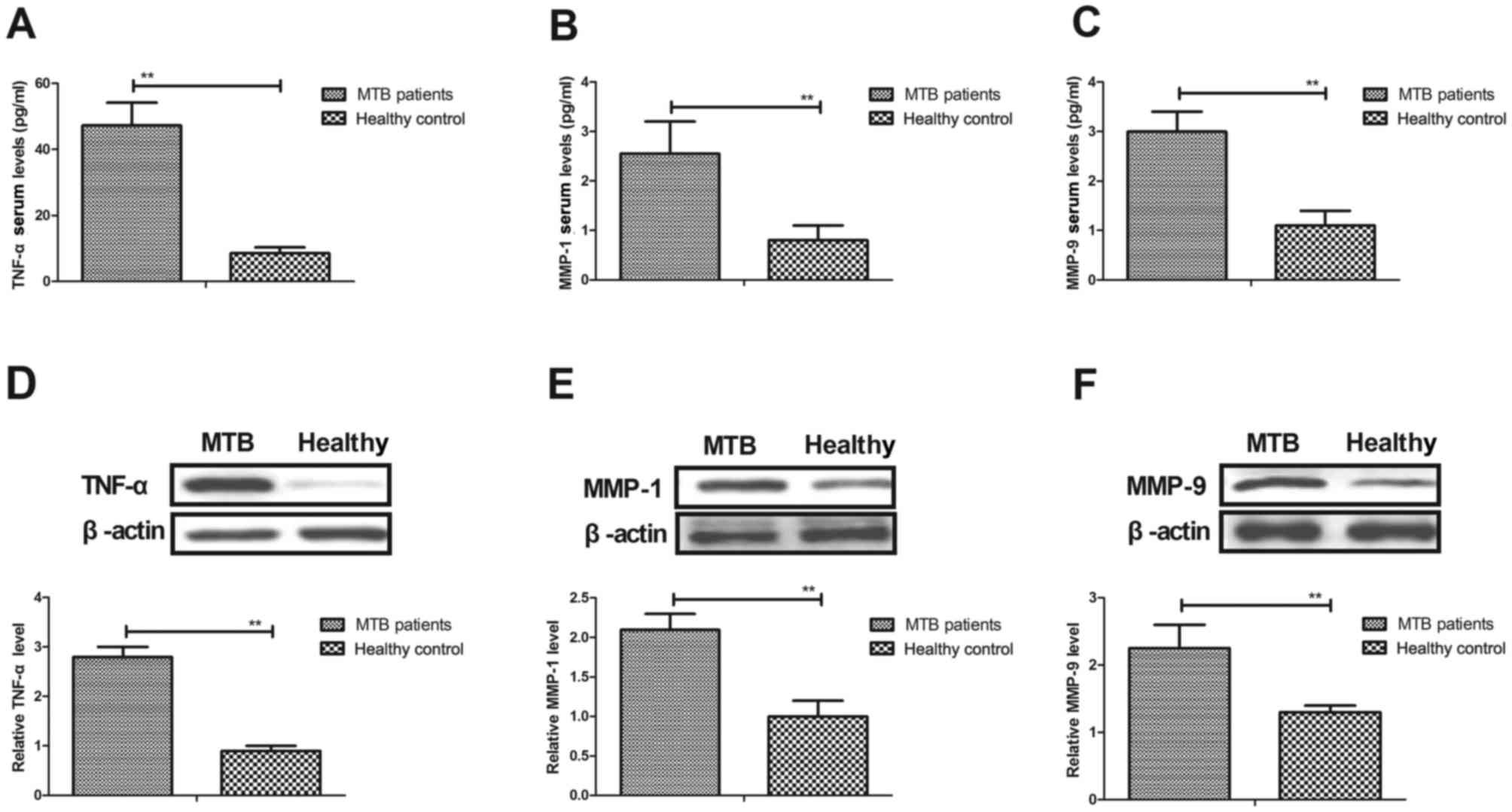
tuberculosis. As shown in Fig. 4A-C,
the results indicated that serum levels of TNF-α, MMP-1 and MMP-9
were significantly increased in patients with pulmonary
tuberculosis compared with healthy controls (all P<0.01). It was
also observed that protein expression levels of TNF-α, MMP-1 and
MMP-9 were significantly upregulated in hPMCs isolated from
patients with pulmonary tuberculosis (all P<0.01; Fig. 4D-F). These results indicate that
M. tuberculosis stimulates hPMCs to upregulate TNF-α, MMP-1
and MMP-9, which may aggravate the severity of pulmonary
tuberculosis.
Analysis of TNF-α-induced ERK/Akt
signaling pathway in hPMCs isolated from patients with pulmonary
tuberculosis
To further analyze the molecular mechanism of TNF-α,
MMP-1 and MMP-9 upregulation in hPMCs, the ERK/Akt signaling
pathway was evaluated in hPMCs isolated from patients with
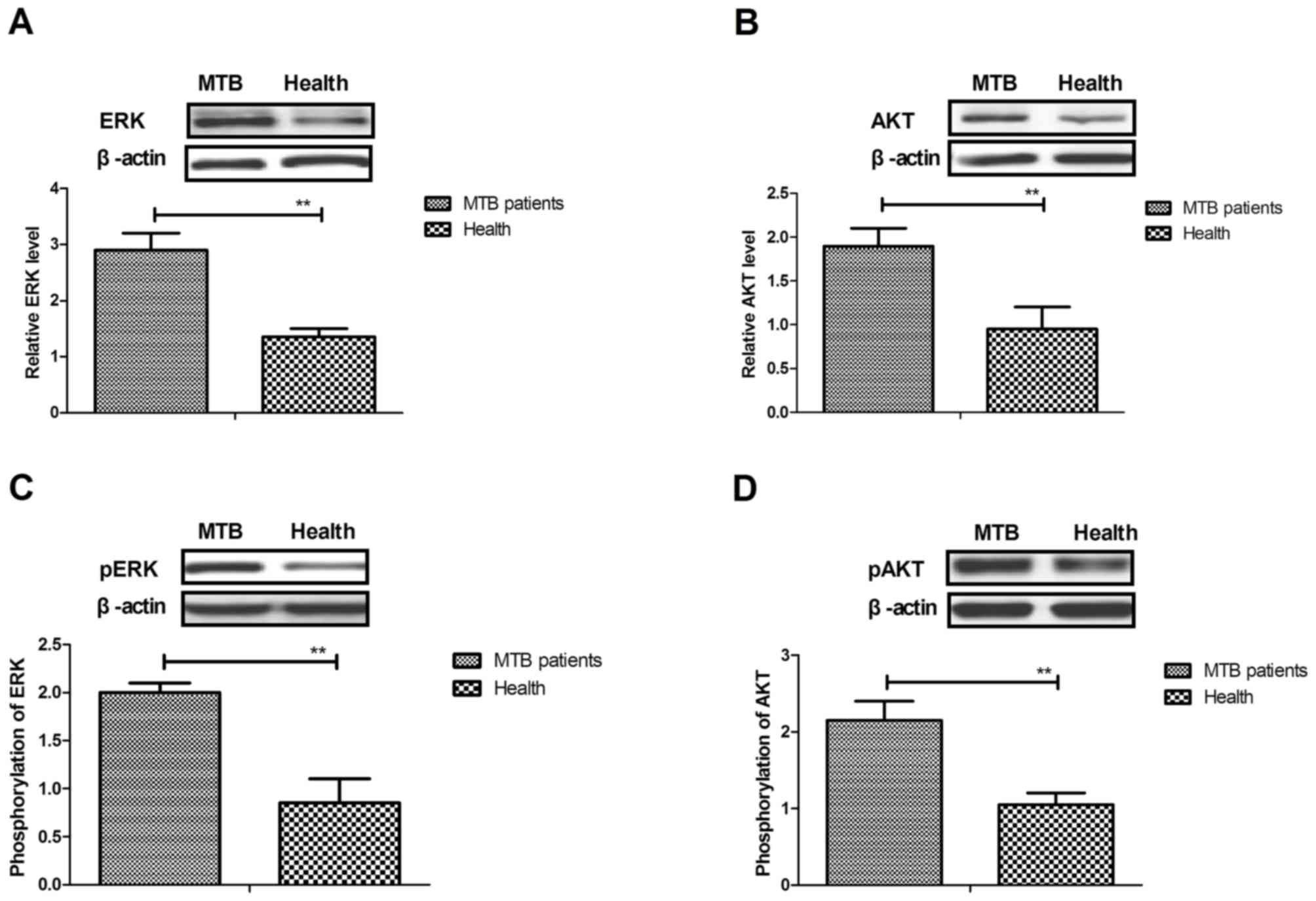
pulmonary tuberculosis. As shown in Fig.
5A and B, the results indicated that expression levels of ERK
and Akt were significantly upregulated in hPMCs isolated from
patients with pulmonary tuberculosis compared with healthy controls
(both P<0.01). In addition, phosphorylation levels of ERK and
Akt were significantly increased in hPMCs isolated from patients
compared with healthy controls (both P<0.01; Fig. 5C and D). Furthermore, it was
identified that inhibition of TNF-α expression suppressed ERK and
Akt expression and phosphorylation (Fig.
5E and F). MMP-1 and MMP-9 expression levels were also
significantly downregulated by TNF-α knockdown in hPMCs (P<0.01;
Fig. 5G and H). These results
suggest that TNF-α mediates the upregulation of MMP-1 and MMP-9
expression through the ERK/Akt signaling pathway in hPMCs in
patients with pulmonary tuberculosis.
Discussion
Pulmonary tuberculosis is a chronic infectious
disease, which is characterized by lung granulomatous lesion
formation and severe inflammatory responses (30,31). A
previous study suggested that inflammatory factors induced by
inflammatory responses in lung tissue contributed to the severity
of pulmonary tuberculosis when patients were infected with M.
tuberculosis (32). Clinically,
the activity of pulmonary tuberculosis inflammation caused by
Mycobacterium has been identified as an indicator for
patients' disease severity and drug tolerance (33). In the present study, inflammatory
responses were investigated in patients with pulmonary tuberculosis
in ICU. Serum levels of inflammatory cells, including lymphocytes,
plasmacytes, neutrophils and monocytes were studied in patients
with pulmonary tuberculosis in ICU. It was identified that
inflammatory responses are enhanced and inflammatory factors are
stimulated in patients with M. tuberculosis infection.
Notably, it was observed that the balance of Th1/Th2 cytokines was
disturbed in patients with pulmonary tuberculosis compared with
healthy controls. It was observed that pulmonary tuberculosis
upregulated TNF-α expression through the ERK/Akt signaling pathway
in hPMCs.
Inflammatory cells are the most common pathological
characteristics for patients with pulmonary tuberculosis infected
with Mycobacterium (34).
These inflammatory cells include lymphocytes, plasmacytes,
neutrophils, monocytes, macrophages, mast cells and endothelial
cells. Previous studies have demonstrated that the morphology and
function of plasmacytes are associated with the progression of
tuberculosis (35,36). In addition, Iliaz et al
(37) indicated that
neutrophil/lymphocyte ratio could be used as a reference in the
differential diagnosis of tuberculosis. Furthermore, previous
investigations have suggested that suppression of M.
tuberculosis growth can upregulate TNF-α, which increases the
levels of human monocytes in patients with pulmonary tuberculosis
(38,39). These reports suggest that inhibition
of inflammatory cells may be a potential target for the treatment
of pulmonary tuberculosis. The present results indicated that
inflammatory cells were increased in the serum of patients with
pulmonary tuberculosis.
Inflammatory factors secreted by inflammatory cells
are reported to be associated with pathogenicity and severity of
pulmonary tuberculosis, and may induce other tuberculosis-related
diseases (40). In the present
study, the expression levels of IL-1, IL-6, IL-10 and IL-12 were
investigated in patients with pulmonary tuberculosis. Katti
(41) assessed serum IL-1, IL-2 and
IFN-γ levels in patients with pulmonary tuberculosis and reported
that increased IL-1 is an indicator for Th1 responses. In the
present study, it was observed that IL-1 serum levels were
upregulated in patients with pulmonary tuberculosis. Serum levels
of IL-6 act as a potential biomarker of disease progression in
pulmonary tuberculosis following anti-tuberculosis drug therapy
(42). In the present study, IL-6
serum levels were increased in patients compared with the controls.
The present results also indicated that serum levels of IL-2 and
IL-15 were decreased in the serum of patients with pulmonary
tuberculosis, which may contribute to understanding the molecular
mechanism of inflammatory responses in pulmonary tuberculosis.
Furthermore, expression levels and polymorphisms of IL-10 and TNF-α
are affected in patients with pulmonary tuberculosis (43). In the present study, it was
demonstrated that serum levels of IL-10 and TNF-α were increased in
patients with pulmonary tuberculosis compared with healthy
controls, which may lead to inflammatory responses in patients.
Understanding the mechanisms of pulmonary
tuberculosis is beneficial for preventing and treating M.
tuberculosis infection (44). In
the present study, the ERK/Akt signaling pathway was analyzed in
hPMCs isolated from patients with M. tuberculosis infection.
The results indicated that M. tuberculosis induced higher
levels of TNF-α, mediated by the ERK/Akt signal pathway, resulting
in upregulation of MMP-1 and MMP-9 expression in hPMCs. Although
MMP-9 activity has been characterized in processes of granuloma
formation in pleural tuberculosis, to the best of our knowledge,
the signal pathway in inflammatory responses in tuberculosis
pleural disease has not yet been reported (45). The present investigations have
elaborated on the molecular mechanism of MMP-9 upregulation and
indicated that TNF-α-induced upregulation of MMP-1 and MMP-9
expression is mediated by the ERK/Akt signaling pathway in hPMCs in
patients with pulmonary tuberculosis.
In conclusion, the present study indicates that
inflammatory responses and inflammatory factors are upregulated in
patients with pulmonary tuberculosis. Notably, the present findings
suggest that M. tuberculosis-induced inflammatory responses
and factors are mediated by MMP-1 and MMP-9 expression via the
TNF-α-mediated ERK/Akt signaling pathway in hPMCs. These results
suggest that inhibition of inflammatory responses and inflammatory
factors may be beneficial for the treatment of patients with
pulmonary tuberculosis in ICU.
Acknowledgements
This study was supported by the Youth Programme of
Beijing Municipal Administration of Hospitals (grant no.
QML20151501) and the National Science and Technology Major Project
of China (grant nos. 2015ZX10004801-003 and
2016ZX10003001-011).
References
|
1
|
Mahmoud ES, Baharoon SA, Alsafi E and
Al-Jahdaly H: Acute respiratory distress syndrome complicating
community-acquired pneumonia secondary to mycobacterium
tuberculosis in a tertiary care center in Saudi Arabia. Saudi Med
J. 37:973–978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sood S, Yadav A and Shrivastava R:
Mycobacterium aurum is Unable to Survive Mycobacterium tuberculosis
latency associated stress conditions: Implications as non-suitable
model organism. Indian J Microbiol. 56:198–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
WHO: Tuberculosis, . http://www.who.int/mediacentre/factsheets/fs104/en/October.
2017
|
|
4
|
Maruri F, Sterling TR, Kaiga AW, Blackman
A, van der Heijden YF, Mayer C, Cambau E and Aubry A: A systematic
review of gyrase mutations associated with
fluoroquinolone-resistant Mycobacterium tuberculosis and a proposed
gyrase numbering system. J Antimicrob Chemother. 67:819–831. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Georghiou SB, Magana M, Garfein RS,
Catanzaro DG, Catanzaro A and Rodwell TC: Evaluation of genetic
mutations associated with Mycobacterium tuberculosis resistance to
amikacin, kanamycin and capreomycin: A systematic review. PLoS One.
7:e332752012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Phelippeau M and Petureau F: Severe
pulmonary tuberculosis in the ICU, diagnosis and treatment. Rev
Pneumol Clin. 71:294–296. 2015.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silva DR, Gazzana MB and Dalcin Pde T:
Severe tuberculosis requiring ICU admission. J Bras Pneumol.
38:386–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo H, Pang L and Xie J: Biosynthesis and
regulation of mycolic acids in Mycobacterium tuberculosis-a review.
Wei Sheng Wu Xue Bao. 52:146–151. 2012.PubMed/NCBI
|
|
9
|
Chiappini E, Bonsignori F, Accetta G,
Boddi V, Galli L, Biggeri A and De Martino M: Interferon-gamma
release assays for the diagnosis of Mycobacterium tuberculosis
infection in children: A literature review. Int J Immunopathol
Pharmacol. 25:335–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiappini E, Accetta G, Bonsignori F, et
al: Interferon-gamma release assays for the diagnosis of
Mycobacterium tuberculosis infection in children: a systematic
review and meta-analysis. Int J Immunopathol Pharmacol. 25:557–564.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lamrabet O and Drancourt M: Genetic
engineering of Mycobacterium tuberculosis: A review. Tuberculosis
(Edinb). 92:365–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raslan WF, Rabaan A and Al-Tawfiq JA: The
predictive value of Gen-Probe's amplified Mycobacterium
tuberculosis direct test compared with culturing in
paraffin-embedded lymph node tissue exhibiting granulomatous
inflammation and negative acid fast stain. J Infect Public Health.
7:251–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gunluoglu G, Yazar EE, Veske NS, Seyhan EC
and Altin S: Mean platelet volume as an inflammation marker in
active pulmonary tuberculosis. Multidiscip Respir Med. 9:112014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sahin O and Ziaei A: The role of
methotrexate in resolving ocular inflammation after specific
therapy for presumed latent syphilitic uveitis and presumed
tuberculosis-related uveitis. Retina. 34:1451–1459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dorhoi A and Kaufmann SH: Perspectives on
host adaptation in response to Mycobacterium tuberculosis:
Modulation of inflammation. Semin Immunol. 26:533–542. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zumla A, Rao M, Parida SK, Keshavjee S,
Cassell G, Wallis R, Axelsson-Robertsson R, Doherty M, Andersson J
and Maeurer M: Inflammation and tuberculosis: Host-directed
therapies. J Intern Med. 277:373–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsenova L, O'Brien P, Holloway J, Peixoto
B, Soteropoulos P, Fallows D, Kaplan G and Subbian S: Etanercept
exacerbates inflammation and pathology in a rabbit model of active
pulmonary tuberculosis. J Interferon Cytokine Res. 34:716–726.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caspers L, Makhoul D, Ebraert H, Michel O
and Willermain F: Clinical manifestations of patients with
intraocular inflammation and positive QuantiFERON-TB gold in-tube
test in a country nonendemic for tuberculosis. Am J Ophthalmol.
158:646–647. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aranday-Cortes E, Hogarth PJ, Kaveh DA,
Whelan AO, Villarreal-Ramos B, Lalvani A and Vordermeier HM:
Transcriptional profiling of disease-induced host responses in
bovine tuberculosis and the identification of potential diagnostic
biomarkers. PLoS One. 7:e306262012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen WL, Sheu JR, Chen RJ, Hsiao SH, Hsiao
CJ, Chou YC, Chung CL and Hsiao G: Mycobacterium tuberculosis
Upregulates TNF-α Expression via TLR2/ERK signaling and induces
MMP-1 and MMP-9 production in human pleural mesothelial cells. PLoS
One. 10:e01379792015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mayordomo L, Marenco JL, Gomez-Mateos J
and Rejon E: Pulmonary miliary tuberculosis in a patient with
anti-TNF-alpha treatment. Scand J Rheumatol. 31:44–45. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mihăltan F: TNF-alpha blockers, rheumatoid
arthritis and pulmonary tuberculosis. Pneumologia. 56:212–216.
2007.PubMed/NCBI
|
|
23
|
Wang CH, Lin HC, Lin SM, Huang CD, Liu CY,
Huang KH, Hsieh LL, Chung KF and Kuo HP: MMP-1(−1607G) polymorphism
as a risk factor for fibrosis after pulmonary tuberculosis in
Taiwan. Int J Tuberc Lung Dis. 14:627–634. 2010.PubMed/NCBI
|
|
24
|
Ong CW, Elkington PT, Brilha S, Ugarte-Gil
C, Tome-Esteban MT, Tezera LB, Pabisiak PJ, Moores RC,
Sathyamoorthy T, Patel V, et al: Neutrophil-Derived MMP-8 drives
AMPK-dependent matrix destruction in human pulmonary tuberculosis.
PLoS Pathog. 11:e10049172015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nowak K, Hanusch C, Kölbel HC, Schwarzbach
M, Post S, Beck G, Gebhard MM, Metzger R and Hohenberger P:
Alterations of tumor and normal tissue of human lung cancer
resection specimens after isolation perfusion. J Physiol Pharmacol.
58 Suppl 5:501–511. 2007.PubMed/NCBI
|
|
26
|
Bajnok A, Kaposi A, Kovacs L, Vasarhelyi
B, Balog A and Toldi G: Analysis by flow cytometry of calcium
influx kinetics in peripheral lymphocytes of patients with
rheumatoid arthritis. Cytometry A. 83:287–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Cai Y, Cheng Q, Hu Y and Xiao H:
Imbalance of Th1/Th2 cytokines in patients with pulmonary
tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi. 25:535–537. 2002.(In
Chinese). PubMed/NCBI
|
|
28
|
Cavalcanti YV, Brelaz MC, Neves JK, Ferraz
JC and Pereira VR: Role of TNF-Alpha, IFN-Gamma and IL-10 in the
development of pulmonary tuberculosis. Pulm Med. 2012:7454832012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Anand SP and Selvaraj P: Effect of 1, 25
dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and
the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol.
133:126–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gonzalez-Angulo Y, Wiysonge CS, Geldenhuys
H, Hanekom W, Mahomed H, Hussey G and Hatherill M: Sputum induction
for the diagnosis of pulmonary tuberculosis: A systematic review
and meta-analysis. Eur J Clin Microbiol Infect Dis. 31:1619–1630.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Badyal RK, Kataria AS, Sachdeva K and
Kapoor S: Macrolithiasis in pulmonary tuberculosis: An autopsy
report with review of literature. Indian J Pathol Microbiol.
55:119–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davis JL, Cattamanchi A, Cuevas LE,
Hopewell PC and Steingart KR: Diagnostic accuracy of same-day
microscopy versus standard microscopy for pulmonary tuberculosis: A
systematic review and meta-analysis. Lancet Infect Dis. 13:147–154.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elipashev AA, Nikol'skii VO and Shprykov
AS: Prognostic value of morphological signs of the activity of
tuberculous inflammation in patients with circumscribed
drug-resistant pulmonary tuberculosis. Arkh Patol. 72:40–43.
2010.(In Russian). PubMed/NCBI
|
|
34
|
Kaminskaia GO, Popov EV and Romanov VV:
Comparison of systemic manifestations of inflammation in torpid
pulmonary tuberculosis and respiratory sarcoidosis. Probl Tuberk
Bolezn Legk. 1–29. 2008.
|
|
35
|
Skogmar S, Schön T, Balcha TT, Sturegard
E, Jansson M and Björkman P: Plasma levels of neopterin and
C-reactive protein (CRP) in tuberculosis (TB) with and without HIV
coinfection in relation to CD4 cell count. PLoS One.
10:e01442922015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koguchi Y, Kawakami K, Uezu K, Fukushima
K, Kon S, Maeda M, Nakamoto A, Owan I, Kuba M, Kudeken N, et al:
High plasma osteopontin level and its relationship with
interleukin-12-mediated type 1 T helper cell response in
tuberculosis. Am J Respir Crit Care Med. 167:1355–1359. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iliaz S, Iliaz R, Ortakoylu G, Bahadir A,
Bagci BA and Caglar E: Value of neutrophil/lymphocyte ratio in the
differential diagnosis of sarcoidosis and tuberculosis. Ann Thorac
Med. 9:232–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Azfar SF and Islam N: Suppression of
mycobacterium tuberculosis induced reactive oxygen species and
tumor necrosis factor-alpha activity in human monocytes of systemic
lupus erythematosus patients by reduced glutathione. Oman Med J.
27:11–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim K, Sohn H, Kim JS, Choi HG, Byun EH,
Lee KI, Shin SJ, Song CH, Park JK and Kim HJ: Mycobacterium
tuberculosis Rv0652 stimulates production of tumour necrosis factor
and monocytes chemoattractant protein-1 in macrophages through the
Toll-like receptor 4 pathway. Immunology. 136:231–240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Worodria W, Menten J, Massinga-Loembe M,
Mazakpwe D, Bagenda D, Koole O, Mayanja-Kizza H, Kestens L, Mugerwa
R, Reiss P, et al: Clinical spectrum, risk factors and outcome of
immune reconstitution inflammatory syndrome in patients with
tuberculosis-HIV coinfection. Antivir Ther. 17:841–848. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Katti MK: Assessment of serum IL-1, IL-2
and IFN-γ levels in untreated pulmonary tuberculosis patients: Role
in pathogenesis. Arch Med Res. 42:199–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chowdhury IH, Choudhuri S, Sen A,
Bhattacharya B, Ahmed AM, Hazra A, Pal NK and Bahar B: Serum
interleukin 6 (IL-6) as a potential biomarker of disease
progression in active pulmonary tuberculosis following
anti-tuberculosis drug therapy. Mol Immunol. 63:601–602. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scola L, Crivello A, Marino V, Gioia V,
Serauto A, Candore G, Colonna-Romano G, Caruso C and Lio D: IL-10
and TNF-alpha polymorphisms in a sample of Sicilian patients
affected by tuberculosis: Implication for ageing and life span
expectancy. Mech Ageing Dev. 124:569–572. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Erdemli SB, Gupta R, Bishai WR, Lamichhane
G, Amzel LM and Bianchet MA: Targeting the cell wall of
Mycobacterium tuberculosis: Structure and mechanism of L,
D-transpeptidase 2. Structure. 20:2103–2115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sheen P, O'Kane CM, Chaudhary K, Tovar M,
Santillan C, Sosa J, Caviedes L, Gilman RH, Stamp G and Friedland
JS: High MMP-9 activity characterises pleural tuberculosis
correlating with granuloma formation. Eur Respir J. 33:134–141.
2009. View Article : Google Scholar : PubMed/NCBI
|