Introduction
Mineral and bone disorder (MBD) is a common
complication associated with chronic kidney disease (CKD) (1). CKD-MBD is characterized by severe renal
injury-induced mineral and hormone metabolic disorders accompanied
by bone deteriorations (1). In the
progression of CKD, low glomerular filtration rate (GFR) is
associated with excessive phosphorus (P) levels, which accelerate
osteocyte-derived fibroblast growth factor-23 (FGF-23) and
parathyroid hormone (PTH) secretion and adversely affects bone
remodeling and resorption, eventually resulting in osteoporotic
bone complications (2). Previous
clinical studies have demonstrated that the severity of CKD is
associated with fracture rate (3,4).
Dysregulated mineral homeostasis is a typical feature of CKD
(3,4). Therefore, improving bone mineralization
and microstructure in patients with CKD is necessary to reduce the
risk of fracture.
Calcium (Ca) is important for bone health. Low Ca
intake is associated with an increased risk of osteopenia and bone
fractures, whereas high Ca intake has been indicated to protect
against osteoporosis (5).
Epidemiological studies have demonstrated that Ca supplementation
has beneficial effects on bone mineral content and bone mineral
density (BMD) (6,7). In rats, Ca supplementation prevented
the bone loss and decline in kidney function induced by a high-P
diet (8). Furthermore, a high
calcium diet (HCD) increases bone mineral (Ca and P) content in
high-fat diet-induced obese mice (9). However, the effects of an HCD on
CKD-induced bone have not previously been reported.
Previous studies have employed an 5/6 nephrectomy
(5/6 Nx) animal model to evaluate CKD-MBD (10,11). At
16 weeks following 5/6 Nx, diminished bone microarchitecture was
observed in the tibial trabecular bone of cluster of
differentiation 1 mice (11), which
is a well-established mouse model to produce moderate CKD with
high-turnover bone injury. In the present study, a CKD-MBD animal
model was established in C57BL/6J mice and the effects of an HCD on
mineral homeostasis and trabecular bone properties were
investigated.
Materials and methods
Animal model
The present study was approved by the Ethics
Committee of Shandong Wendeng Orthopedic Hospital (Wendeng, China)
and was performed in accordance with institutional guidelines. A
total of 36 8-week-old male C57BL/6J mice (body weight, 20±2 g)
were obtained from Shanghai Laboratory Animal Center (Shanghai,
China) and acclimated to the environment for 1 week. Normal dietary
Ca (TD.04200, containing 0.6% calcium carbonate and 0.4% phosphate
by weight) and high dietary Ca (TD.96348, containing 2% calcium
carbonate and 1.25% phosphate by weight) were purchased from Harlan
Teklad (Madison, WI, USA). The mice individually caged in a
temperature-controlled environment (23±2°C; humidity, 60±5%) with
an artificial 12-h light/dark cycle and were provided with free
access to food and tap water. The 5/6 Nx mouse model of CKD was
established without angiotensin II infusion according to the method
published by Souza et al (12) and upregulation of blood urea nitrogen
and serum creatinine verified successful establishment of the
animal model (12). The mice were
randomly divided into three groups (n=12 in each group): Sham
group, sham-operated mice (without removal of kidneys) treated with
normal dietary Ca following sham surgery for 12 weeks; 5/6 Nx
group, 5/6 Nx mice treated with normal dietary Ca following Nx
surgery for 12 weeks; and 5/6 Nx+HCD group, 5/6 Nx mice treated
with HCD following Nx surgery for 12 weeks. All mice were
sacrificed at week 12 via an intraperitoneal injection of sodium
pentobarbital (200 mg/kg; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Blood was harvested from the heart and centrifuged at
1,500 × g for 15 min at 4°C to obtain serum. Serum (~800 µl), urine
(~2 ml) and lumbar vertebrae (L3-L5) tissues were immediately
collected and maintained at −80°C for further analysis.
Biochemical markers in serum and
urine
The concentrations of Ca (cat. no. C004-3; Nanjing
Jiancheng Biology Engineering Institute, Nanjing, China) and
creatinine (Cr; cat. no. C011-1; Nanjing Jiancheng Biology
Engineering Institute) from the serum and urine were measured using
standard colorimetric methods (13)
and picric acid methods (14),
respectively. The level of urinary Ca (UCa) was corrected according
to the concentration of urinary Cr (UCr). Serum Cr (SCr; cat. no.
C011-1; Nanjing Jiancheng Biology Engineering Institute) and blood
urea nitrogen (BUN; cat. no. C013-2; Nanjing Jiancheng Biology
Engineering Institute) levels were measured using an autoanalyzer
and an enzymatic kinetic method with commercial kits following the
manufacturer's protocol. Serum levels of FGF-23 (cat. no. 60-6800;
Immutopics, Inc., San Clemente, CA, USA), PTH (cat. no.
E-EL-M0709c; Elabscience Biotechnology Co., Ltd., Wuhan, China),
tartrate resistant acid phosphatase-5b (TRAP-5b; cat. no.
SB-TR201A; Immunodiagnostic Systems, Scottsdale, AZ, USA) and
alkaline phosphatase (ALP; cat. no. E-EL-M0200c; Elabscience
Biotechnology Co., Ltd.) were detected using murine ELISA assays
with a SpectraMax M5 ELISA plate reader (Molecular Devices, LLC,
Sunnyvale, CA, USA).
Bone Ca
The lumbar vertebrae were incinerated using a muffle
furnace (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 800°C
for 12 h and 10 mg of bone ash was dissolved in 1 ml of 37% HCl
diluted with Milli-Q® water. The calcium content was
determined using a kit (cat. no. C004-3; Nanjing Jiancheng Biology
Engineering Institute).
Histomorphology
Kidney tissues were collected immediately following
sacrifice and fixed with 4% formalin at room temperature for 24 h
and paraffin-embedded. Tissues were cut into ~5 µm-thick sections,
which were stained with Masson's trichrome (cat. no. SBJ-0290;
Nanjing SenBeiJia Biological Technology Co., Ltd., Nanjing, China)
as previously described (15) and
visualized under a microscope (magnification, ×200; Leica DM 2500;
Leica Microsystems GmbH, Wetzlar, Germany). Renal injury was
assessed using a previously described 0–4 scale (16) as follows: 0, none; 1, <10; 2,
10–25; 3, 25–75; or 4, >75%.
The lumbar vertebrae were collected immediately
following sacrifice and fixed with 4% formalin at room temperature
for 24 h, and then decalcified in 0.5 M EDTA (pH=8.0) and embedded
in paraffin according to standard histological procedures. Sections
of ~5 µm were cut and stained with hematoxylin and eosin (H&E;
cat. no. C0105; Beyotime Institute of Biotechnology,) prior to
being visualized under a microscope (magnification, ×200; Leica DM
2500).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from lumbar vertebrae was extracted using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. cDNA was synthesized by RT reactions
with 2 µg of total RNA using moloney murine leukemia virus reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc.)
following 37°C for 50 min and 70°C for 15 min. A total of 1 µl cDNA
was used for PCR using a DNA Engine (ABI) with SYBR Green PCR
Master Mix (Invitrogen; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were applied: 95°C for 10 min
followed by 40 cycles of 95°C for 15 sec, 58°C for 30 sec and 72°C
for 30 sec. The primers were synthesized by Sangon Biotech Co.,
Ltd. (Shanghai, China) as follows: osteoprotegerin (OPG), forward
5′-GCATTATGACCCAGAAACT-3′ and reverse 5′-ACCTGAGAAGAACCCATC-3′;
receptor activator of nuclear factor κB ligand (RANKL), forward
5′-AACCAAGATGGCTTCTATTACC-3′ and reverse
5′-AAGGGTTGGACACCTGAATG-3′; and GAPDH, forward
5′-TCACTGCCACCCAGAAGA-3′ and reverse 5′-AAGTCGCAGGAGACAACC-3′.
Signals were assessed using Quantity One® software
version 4.5 (Bio Rad Laboratories, Inc., Hercules, CA, USA) and
normalized to the GAPDH. Analysis of relative gene expression data
used real-time quantitative PCR and the quantitation cycle (Cq)
method (17).
Statistical analysis
Data are presented as the mean + or ± standard
deviation as indicated for each group. All statistical analyses
were performed using GraphPad Prism software version 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Inter-group differences were
analyzed using one-way analysis of variance with a post hoc Tukey
test for multiple comparisons. P<0.05 was considered to indicate
a statistically significant difference.
Results
HCD supplementation has no significant
effect on renal dysfunction in 5/6 Nx mice
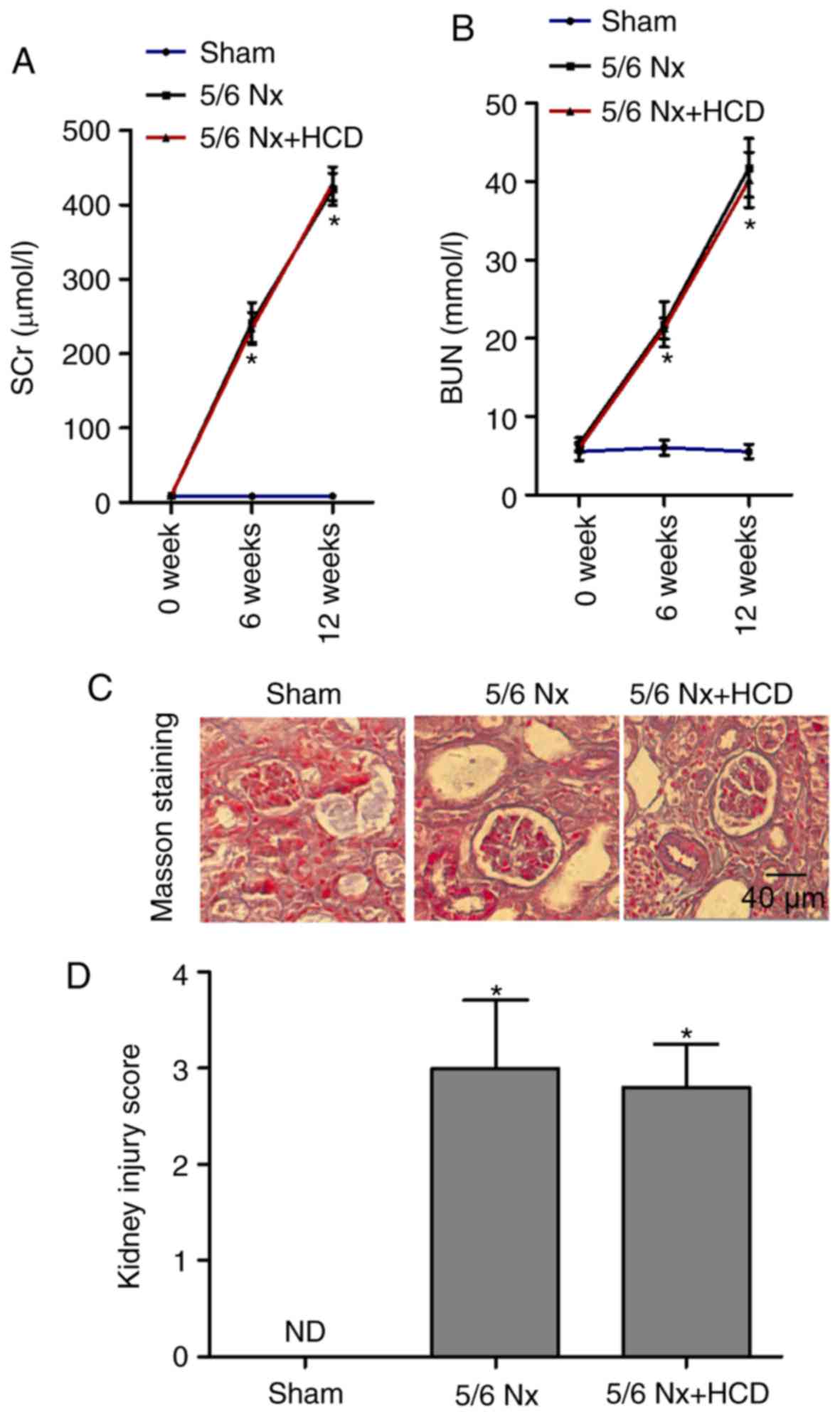
To evaluate the role of HCD supplementation in 5/6
Nx-induced renal dysfunction, serum levels of SCr and BUN were
compared across groups. The levels of SCr and BUN were
significantly increased in the 5/6 Nx group compared with the
sham-operated group at week 6 and week 12 (P<0.05; Fig. 1A and B); however, HCD supplementation
in 5/6 Nx mice did not significantly improve these parameters
(Fig. 1A and B). Masson staining was
performed to observe the extent of renal interstitial fibrosis. As
indicated in Fig. 1C and D,
increased matrix deposition, interstitial fibrosis and renal
lesions were observed in the kidneys from 5/6 Nx mice compared with
sham mice, and injury scores were significantly increased
(P<0.05; Fig. 1D). Furthermore,
HCD supplementation had no marked effect on renal pathological
changes in 5/6 Nx mice. These data suggest that the CKD mice models
were successfully established and that HCD did not improve renal
function in 5/6 Nx mice.
HCD supplementation regulates bone
metabolism-associated biomarkers
Acute kidney injury (AKI) or CKD-induced GFR
regression predisposes to bone metabolism disturbances via
upregulating serum P (18), which
accelerates FGF-23 and PTH secretion. Previous studies have
demonstrated that increased FGF-23 is associated with CKD or AKI
severity (19,20). FGF-23 is a peptide released from
osteocytes and osteoblasts, whilst PTH is associated with Ca, P and
FGF-23 levels in CKD (21). To
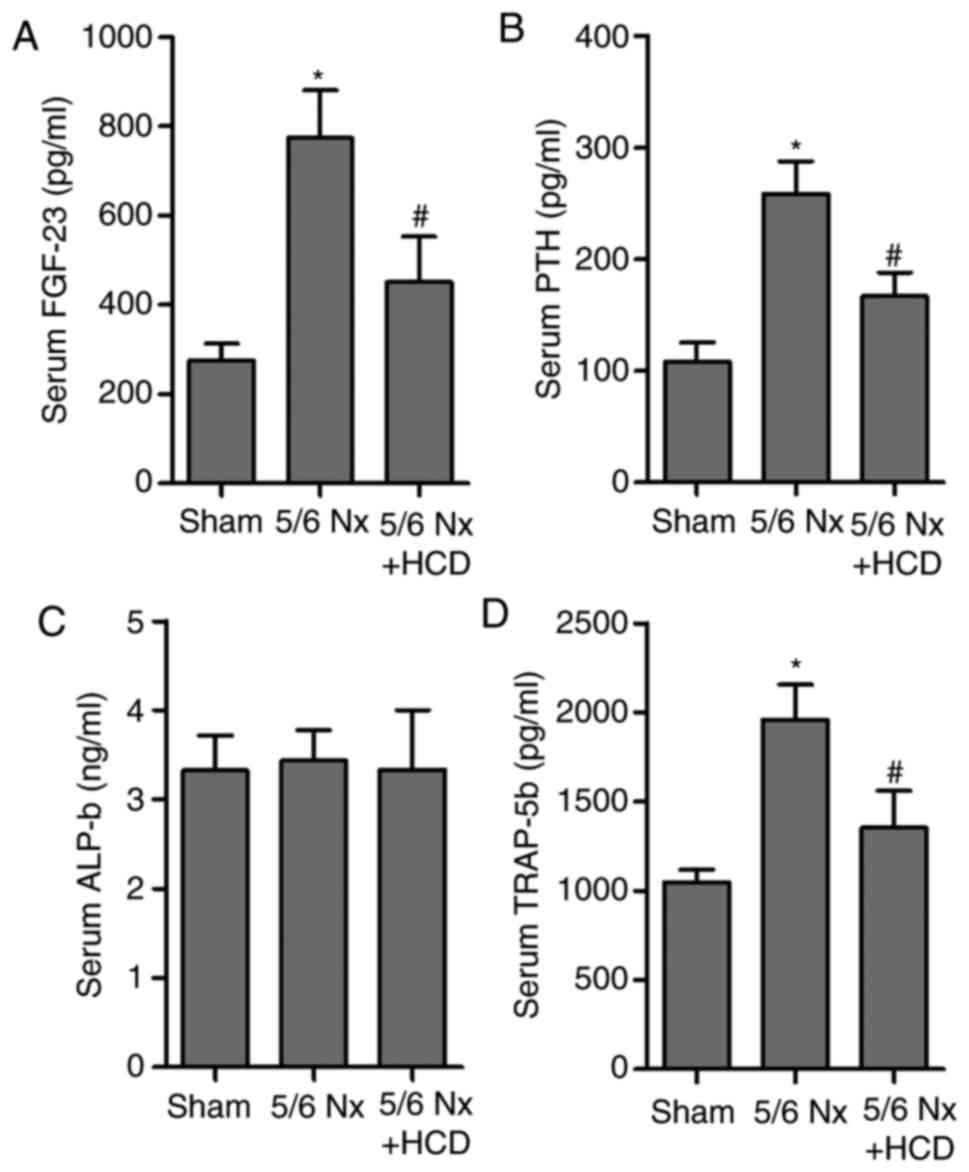
evaluate the effect of an HCD in CKD-MBD, the levels of FGF-23 and
PTH in the serum of 5/6 Nx mice were measured. Results indicated
that serum FGF-23 and PTH levels were significantly increased in
the 5/6 Nx group compared with the sham group (P<0.05; Fig. 2A and B). Notably, HCD significantly
reversed CKD-induced upregulation of serum FGF-23 and PTH levels in
5/6 Nx mice (P<0.05; Fig. 2A and
B). In addition, the effect of 5/6 Nx on markers of bone
resorption and formation, ALP and TRAP-5b (22,23), in
the serum of 5/6 Nx mice was assessed (Fig. 2C and D). 5/6 Nx induced a significant
increase in serum TRAP-5b concentration compared with the sham
group; however, HCD supplementation significantly decreased TRAP-5b
levels in CKD mice (P<0.05). No significant difference in ALP
concentration was observed in any of the experimental groups.
HCD supplementation regulates Ca
metabolism and bone remodeling
HCD has an important role in the bone-kidney axis
and the regulation of calcium homeostasis in CKD (24,25). The
Ca content in serum, urine and bone was measured in CKD mice.
Results indicated that 5/6 Nx mice exhibited decreased SCa levels
and an ~3-fold increase in UCa excretion compared with the sham
group, whereas HCD supplementation significantly increased SCa
content and decreased UCa excretion in CKD mice (P<0.05;
Fig. 3A and B). At week 12,
comparison of the results of bone Ca content between sham and 5/6
Nx groups indicated 5/6 Nx significantly decreased bone Ca content;
however, the bone calcium content was significantly increased in
5/6 Nx mice with HCD supplementation (both P<0.05; Fig. 3C). A similar result was obtained for
Ca content in the ash of lumbar vertebrae (Fig. 3D). Furthermore, H&E staining was
conducted to observe the trabecular bone microstructure of lumbar
vertebrae. As indicated in Fig. 3E,
a loss of network connection in the trabecular bone was observed in
the 5/6 Nx group compared with the sham group; however, the
increased disconnections and separation among trabecular bone
network were improved by HCD supplementation in CKD mice.
HCD supplementation regulates the
OPG/RANKL ratio
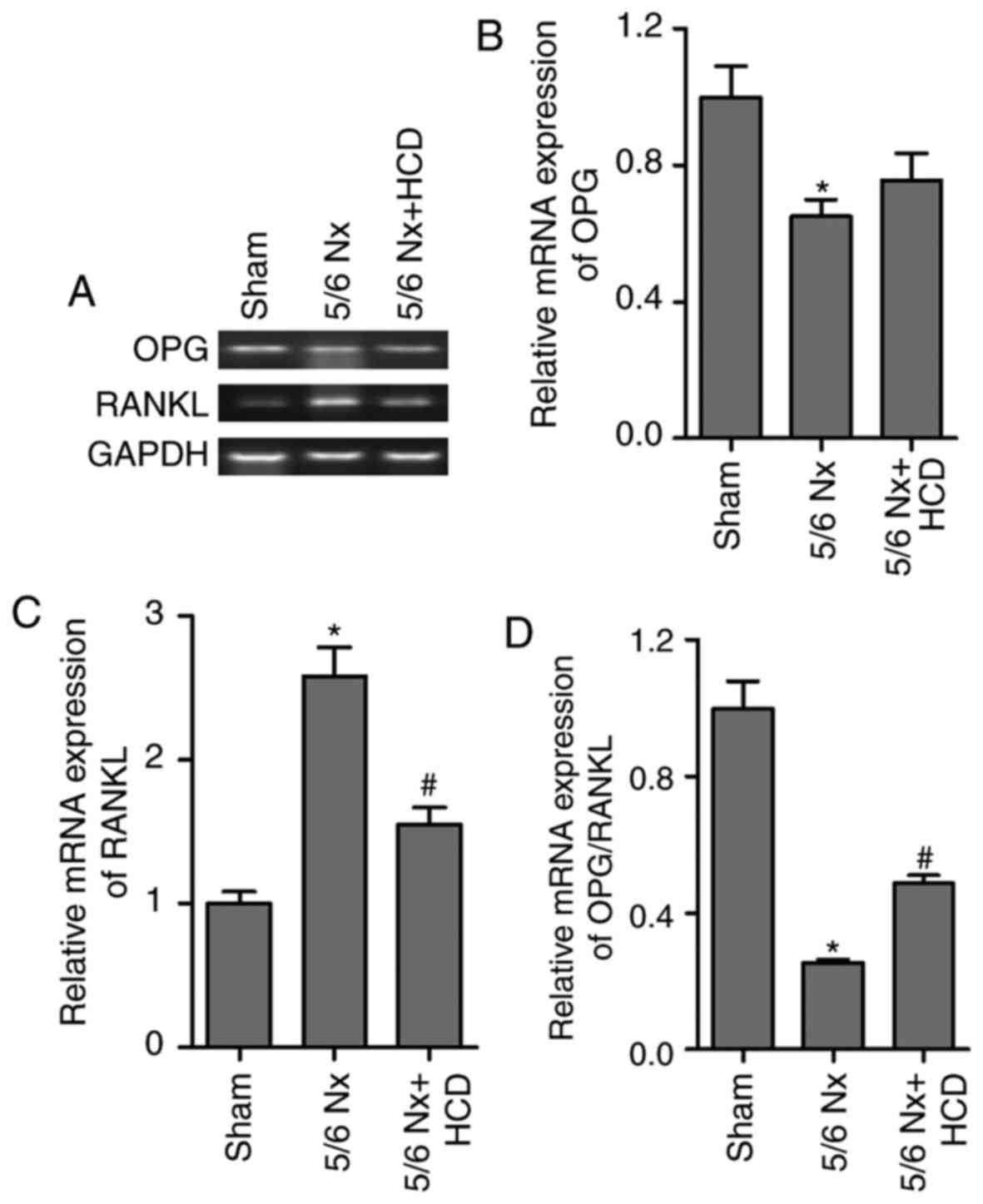
The maturation and formation of osteoclasts is
primarily regulated by the balance of extracellular OPG and RANKL
(26). OPG and RANKL cytokines
affect the activity of osteoblast cells and osteoclastogenesis
(26). OPG cytokine binds to RANKL
and prevents RANKL from binding to the RANK receptor on osteoclast
cells, subsequently inhibiting bone resorption and
osteoclastogenesis (27). Thus, the
ratio of OPG/RANKL expression in lumbar vertebrae was determined in
the present study (Fig. 4). RT-qPCR
results revealed a significant decrease in the OPG mRNA expression
levels and ratio of OPG/RANKL compared with the sham group
(P<0.05; Fig. 4A, B and D).
Furthermore, RANKL mRNA expression levels were significantly
increased in mice treated with 5/6 Nx compared with the sham group
(P<0.05; Fig. 4A and C). HCD
supplementation significantly reversed the effects of 5/6 Nx on
OPG/RANKL and RANKL mRNA expression (P<0.05; Fig. 4C and D). However, no significant
difference was observed between the mRNA expression levels of OPG
in the 5/6 Nx and 5/6 Nx + HCD groups.
Discussion
Patients with CKD exhibit marked disruptions in bone
and mineral metabolism accompanied with biochemical alterations,
including elevated FGF-23 and PTH, decreased 1, 25-dihydroxyvitamin
D3 (vitamin D3), elevated serum P and decreased SCa (28). Oral Ca carbonate affects the positive
Ca balance in stage 3 and 4 CKD (29). Ca (1,200 mg) combined with vitamin D3
(800 IU) supplementation protects against BMD loss in women with
moderate CKD (30). Furthermore,
dietary Ca supplementation has been indicated to prevent bone loss
in several animal models, notably ovariectomized and renal injury
rodents (31,32). These findings suggest that dietary Ca
supplementation may serve as an adjunctive therapy for CKD-induced
bone loss.
The lumbar vertebra is a hypersensitive site that is
susceptible to bone injury (33). In
clinical practice, kidney transplant recipients have a lower lumbar
spine trabecular bone score, which is associated with an increased
incidence of fractures (34). This
suggests that Nx is associated with lumbar vertebrae injury. In the
present study, 5/6 Nx significantly decreased SCa content and
increased serum PTH and FGF-23 concentration. It is known that PTH
stimulates the expression of RANKL, a mediator of
osteoclastogenesis and bone resorption (35). In the present study, it was observed
that RANKL mRNA expression was significantly increased and OPG mRNA
expression was decreased in the lumbar vertebrae of 5/6 Nx mice
compared with the sham group. These findings indicate that bone
resorption and bone loss are enhanced in mice following 5/6 Nx.
However, HCD supplementation significantly increased SCa, bone Ca
content and Ca/Ash, whilst bone loss of lumbar vertebrae in 5/6 Nx
mice was ameliorated. A previous study indicated that dietary Ca
supplementation inhibits RANKL-mediated osteoclastic bone
resorption via the suppression of PTH (8). Thus, these findings suggest that HCD
supplementation may increase bone Ca content and bone remodeling
via inhibiting RANKL-induced bone resorption in 5/6 Nx mice. Piri
et al (36) assessed the
levels of OPG and RANKL associated with dietary supplements of Ca,
vitamin D and estrogen, revealing that Ca intake led to an increase
in OPG and reduction in RANKL, which ultimately caused an increase
in BMD. According to the results of the present study, OPG/RANKL
signaling is associated with 5/6 Nx-induced bone deterioration of
lumbar vertebrae in mice, which suggests that OPG/RANKL may be an
important target for the treatment of CKD-induced bone injury.
FGF-23 is a member of the fibroblast growth factor
family and is associated with P homeostasis (37). A recent study has revealed that Ca
regulates bone FGF-23 expression and that HCD increases serum
FGF-23 concentration in Cyp27b1−/− and
Gcm2−/− mice (38). In
parathyroidectomized rats, dietary Ca supplementation increased SCa
and FGF-23 concentration (39).
However, Ca has no effect on FGF-23 promoter activity in cultured
osteoblasts (40). Furthermore, it
has been reported that serum FGF-23 concentration is positively
correlated with serum P levels (41). In the present study, 5/6 Nx mice had
high serum P levels, which was associated with high serum FGF-23
despite hypocalcemia. These data suggest that Ca regulation of
FGF-23 expression is also controversial. In the present study, it
was identified that dietary Ca supplementation significantly
decreased serum P and FGF-23 in CKD mice.
Ca and P are substrates for bone mineralization that
are tightly regulated by several hormones, including FGF-23, PTH
and vitamin D3 (42). Dysregulation
of one hormone in this system results in the dysregulation of Ca
and P homeostasis; particularly, active vitamin D synthesis is
stimulated by hypophosphatemia in the kidney (42). FGF-23 is increased in the primary
stage of CKD; however, renal tubular injury accelerates FGF23
secretion and results in a decrease in serum vitamin D3 levels,
which in turn increases serum phosphate and decreases ionized Ca
(43). In a clinical setting, foods
rich in Ca and low in phosphate are permitted in patients with CKD
(44). In the present study of 5/6
Nx mice with CKD, serum FGF-23 and PTH were significantly
decreased, whereas serum Ca and bone Ca were increased in response
to dietary Ca supplementation, ameliorating CKD-induced network
connection loss in trabecular bone.
In conclusion, these findings provide a novel
insight into the pathophysiological and nutritional role of an HCD
in the regulation of Ca homeostasis and bone metabolism-associated
hormones in 5/6 Nx mice. In addition, HCD has an anabolic potential
to enhance novel bone formation and suppress bone resorption in 5/6
Nx mice. The present study provides the theory base for dietary Ca
supplementation beneficial for the balance of bone metabolism in
patients with CKD. However, cross-sectional and longitudinal
studies are required to elaborate the standards, rules and
alimental interaction in routine clinical practice.
References
|
1
|
Lin W, Li Y, Chen F, Yin S, Liu Z and Cao
W: Klotho preservation via histone deacetylase inhibition
attenuates chronic kidney disease-associated bone injury in mice.
Sci Rep. 7:461952017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kochanek M, Said A and Lerma EV: Mineral
metabolism in chronic kidney disease. Dis Mon. 61:425–433. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Naylor KL, McArthur E, Leslie WD, Fraser
LA, Jamal SA, Cadarette SM, Pouget JG, Lok CE, Hodsman AB, Adachi
JD and Garg AX: The three-year incidence of fracture in chronic
kidney disease. Kidney Int. 86:810–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El Desoky S, Farag YM, Safdar E, Shalaby
MA, Singh AK and Kari JA: Prevalence of hyperparathyroidism,
mineral and bone disorders in children with advanced chronic kidney
disease. Indian J Pediatr. 83:420–425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Q and Sergeev IN: Calcium and vitamin
D in obesity. Nutr Res Rev. 25:130–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnston CC Jr, Miller JZ, Slemenda CW,
Reister TK, Hui S, Christian JC and Peacock M: Calcium
supplementation and increases in bone mineral density in children.
N Engl J Med. 327:82–87. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee WT, Leung SS, Wang SH, Xu YC, Zeng WP,
Lau J, Oppenheimer SJ and Cheng JC: Double-blind, controlled
calcium supplementation and bone mineral accretion in children
accustomed to a low-calcium diet. Am J Clin Nutr. 60:744–750. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Katsumata S, Matsuzaki H, Uehara M and
Suzuki K: Effects of dietary calcium supplementation on bone
metabolism, kidney mineral concentrations, and kidney function in
rats fed a high-phosphorus diet. J Nutr Sci Vitaminol (Tokyo).
61:195–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song Q and Sergeev IN: High vitamin D and
calcium intakes increase bone mineral (Ca and P) content in
high-fat diet-induced obese mice. Nutr Res. 35:146–154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrari GO, Ferreira JC, Cavallari RT,
Neves KR, dos Reis LM, Dominguez WV, Oliveira EC, Graciolli FG,
Passlick-Deetjen J, Jorgetti V and Moysés RM: Mineral bone disorder
in chronic kidney disease: Head-to-head comparison of the 5/6
nephrectomy and adenine models. BMC Nephrol. 15:692014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heveran CM, Ortega AM, Cureton A, Clark R,
Livingston EW, Bateman TA, Levi M, King KB and Ferguson VL:
Moderate chronic kidney disease impairs bone quality in C57Bl/6J
mice. Bone. 86:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Souza AC, Tsuji T, Baranova IN, Bocharov
AV, Wilkins KJ, Street JM, Alvarez-Prats A, Hu X, Eggerman T, Yuen
PS and Star RA: TLR4 mutant mice are protected from renal fibrosis
and chronic kidney disease progression. Physiol Rep. 3:e125582015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Löwe A, Breuer J and Palkowitsch P:
Evaluation of the effect of two gadolinium-containing
contrast-enhancing agents, gadobutrol and gadoxetate disodium, on
colorimetric calcium determinations in serum and plasma. Invest
Radiol. 46:366–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Massoomi F, Mathews HG III and Destache
CJ: Effect of seven fluoroquinolones on the determination of serum
creatinine by the picric acid and enzymatic methods. Ann
Pharmacother. 27:586–588. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun D, Feng J, Dai C, Sun L, Jin T, Ma J
and Wang L: Role of peritubular capillary loss and hypoxia in
progressive tubulointerstitial fibrosis in a rat model of
aristolochic acid nephropathy. Am J Nephrol. 26:363–371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan HY, Qi D, Yu C, Zhao F, Liu T, Zhang
ZK, Yang MY, Zhang LM, Chen DQ and Du Y: Paeonol protects
endotoxin-induced acute kidney injury: Potential mechanism of
inhibiting TLR4-NF-kappaB signal pathway. Oncotarget.
7:39497–39510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kakareko K, Rydzewska-Rosolowska A,
Brzosko S, Gozdzikiewicz-Lapinska J, Koc-Zorawska E, Samocik P,
Kozlowski R, Mysliwiec M, Naumnik B and Hryszko T: The effect of
nephrectomy on Klotho, FGF-23 and bone metabolism. Int Urol
Nephrol. 49:681–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nitta K, Nagano N and Tsuchiya K:
Fibroblast growth factor 23/klotho axis in chronic kidney disease.
Nephron Clin Pract. 128:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Christov M, Waikar SS, Pereira RC, Havasi
A, Leaf DE, Goltzman D, Pajevic PD, Wolf M and Jüppner H: Plasma
FGF23 levels increase rapidly after acute kidney injury. Kidney
Int. 84:776–785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lavi-Moshayoff V, Wasserman G, Meir T,
Silver J and Naveh-Many T: PTH increases FGF23 gene expression and
mediates the high-FGF23 levels of experimental kidney failure: A
bone parathyroid feedback loop. Am J Physiol Renal Physiol.
299:F882–F889. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Lee JW, Uy L, Abosaleem B, Gunn H,
Ma M and DeSilva B: Tartrate-resistant acid phosphatase (TRACP 5b):
A biomarker of bone resorption rate in support of drug development:
Modification, validation and application of the BoneTRAP kit assay.
J Pharm Biomed Anal. 49:1203–1212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haussler MR, Whitfield GK, Kaneko I,
Forster R, Saini R, Hsieh JC, Haussler CA and Jurutka PW: The role
of vitamin D in the FGF23, klotho, and phosphate bone-kidney
endocrine axis. Rev Endocr Metab Disord. 13:57–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin A and Quarles LD: Evidence for
FGF23 involvement in a bone-kidney axis regulating bone
mineralization and systemic phosphate and vitamin D homeostasis.
Adv Exp Med Biol. 728:65–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pitari MR, Rossi M, Amodio N, Botta C,
Morelli E, Federico C, Gullà A, Caracciolo D, Di Martino MT,
Arbitrio M, et al: Inhibition of miR-21 restores RANKL/OPG ratio in
multiple myeloma-derived bone marrow stromal cells and impairs the
resorbing activity of mature osteoclasts. Oncotarget.
6:27343–27358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walsh MC and Choi Y: Biology of the
RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol.
5:5112014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hill Gallant KM and Spiegel DM: Calcium
balance in chronic kidney disease. Curr Osteoporos Rep. 15:214–221.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hill KM, Martin BR, Wastney ME, McCabe GP,
Moe SM, Weaver CM and Peacock M: Oral calcium carbonate affects
calcium but not phosphorus balance in stage 3–4 chronic kidney
disease. Kidney Int. 83:959–966. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bosworth C, de Boer IH, Targher G,
Kendrick J, Smits G and Chonchol M: The effect of combined calcium
and cholecalciferol supplementation on bone mineral density in
elderly women with moderate chronic kidney disease. Clin Nephrol.
77:358–365. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Charoenphandhu N, Tudpor K, Thongchote K,
Saengamnart W, Puntheeranurak S and Krishnamra N: High-calcium diet
modulates effects of long-term prolactin exposure on the cortical
bone calcium content in ovariectomized rats. Am J Physiol
Endocrinol Metab. 292:E443–E452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fonseca D and Ward WE: Daidzein together
with high calcium preserve bone mass and biomechanical strength at
multiple sites in ovariectomized mice. Bone. 35:489–497. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naitoh M, Takada ST, Kurosu Y, Inagaki K,
Mitani A and Ariji E: Relationship between findings of mandibular
cortical bone in inferior border and bone mineral densities of
lumbar vertebrae in postmenopausal women. Okajimas Folia Anat Jpn.
91:49–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Naylor KL, Lix LM, Hans D, Garg AX, Rush
DN, Hodsman AB and Leslie WD: Trabecular bone score in kidney
transplant recipients. Osteoporos Int. 27:1115–1121. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Heerden B, Kasonga A, Kruger MC and
Coetzee M: Palmitoleic acid inhibits RANKL-induced
osteoclastogenesis and bone resorption by suppressing NF-kappaB and
MAPK signalling pathways. Nutrients. 9:E4412017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Piri F, Khosravi A, Moayeri A, Moradipour
A and Derakhshan S: The effects of dietary supplements of calcium,
vitamin D and estrogen hormone on serum levels of OPG and RANKL
cytokines and their relationship with increased bone density in
Rats. J Clin Diagn Res. 10:AF01–AF04. 2016.PubMed/NCBI
|
|
37
|
Stubbs J, Liu S and Quarles LD: Role of
fibroblast growth factor 23 in phosphate homeostasis and
pathogenesis of disordered mineral metabolism in chronic kidney
disease. Semin Dial. 20:302–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
David V, Dai B, Martin A, Huang J, Han X
and Quarles LD: Calcium regulates FGF-23 expression in bone.
Endocrinology. 154:4469–4482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodriguez-Ortiz ME, Lopez I,
Muñoz-Castañeda JR, Martinez-Moreno JM, Ramírez AP, Pineda C,
Canalejo A, Jaeger P, Aguilera-Tejero E, Rodriguez M, et al:
Calcium deficiency reduces circulating levels of FGF23. J Am Soc
Nephrol. 23:1190–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu S, Tang W, Zhou J, Stubbs JR, Luo Q,
Pi M and Quarles LD: Fibroblast growth factor 23 is a
counter-regulatory phosphaturic hormone for vitamin D. J Am Soc
Nephrol. 17:1305–1315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Imanishi Y, Inaba M, Nakatsuka K, Nagasue
K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T,
et al: FGF-23 in patients with end-stage renal disease on
hemodialysis. Kidney Int. 65:1943–1946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Penido MGMG and Alon US: Phosphate
homeostasis and its role in bone health. Pediatr Nephrol.
27:2039–2048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nickolas TL and Jamal SA: Bone kidney
interactions. Rev Endocr Metab Disord. 16:157–163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Spiegel DM and Brady K: Calcium balance in
normal individuals and in patients with chronic kidney disease on
low- and high-calcium diets. Kidney Int. 81:1116–1122. 2012.
View Article : Google Scholar : PubMed/NCBI
|