Introduction
Myocardial infarction (MI) refers to the myocardial
necrosis caused by the serious and persistent acute myocardial
ischemia based on the coronary artery disease, whose high morbidity
and mortality rates have made it one of the important causes for
the death of patients with cardiovascular disease. After MI,
myocardial cell loss and cardiac remodeling lead to the impaired
cardiac function, seriously affecting the life quality of patients,
so the timely and effective treatment is particularly important.
The statin drug is one of the commonly-used drugs in the clinical
treatment of MI (1,2). Preventive effect of atorvastatin on
ventricular remodeling post myocardial infarction has been
demonstrated by Reichert et al, Martin et al and Tang
et al (3–5). In recent years, some scholars have
found that statins can not only regulate the lipids, but also have
the effects of anti-inflammation, anti-oxidative stress, inhibiting
cell proliferation and alleviating cardiac remodeling after the
application in treatment of MI (6–10). We
used of two-dimensional speckle tracking imaging (2D-STI) to
investigate the effects of atorvastatin on left ventricular
systolic function. 2D-STI technology is a new technology developed
in recent years to evaluate myocardial function, especially cardiac
systolic and diastolic function under pathological conditions. This
study aimed to evaluate the effect of atorvastatin (Ator) on left
ventricular systolic function in MI rats using the two-dimensional
speckle tracking imaging (2D-STI) technique.
Materials and methods
Experimental materials
Experimental subjects
Forty clean-grade healthy adult male Sprague-Dawley
(SD) rats at the same age weighing 200–300 g with an average of
261.92±18.81 g were selected from the Experimental Animal Center of
Hubei University of Medicine [animal production license: SCXK
(Hubei) 2016-0008; animal use license: SYXK (Hubei) 2016-0031]. The
animal experimental program was approved by the Ethics Committee of
Xiangyang No. 1 People's Hospital, Hubei University of Medicine
(Xiangyang, China); the disposal of animal in the experimental
process conformed to the Guidelines on the Ethical Treatment of
Animal issued by the Ministry of Science and Technology, People's
Republic of China in 2006.
Instruments and reagents
Mindray Resona 7S color Doppler ultrasound
diagnostic instrument (L14-5WU probe; Mindray Bio-Medical
Electronics Co., Ltd., Shenzhen, China); TomTec 2D-STI analysis
software (TomTec Imaging Systems GmbH, Unterschleissheim, Germany),
small animal ventilator (RWD Life Technology Co., Ltd., Shenzhen,
China); electrocardiogram (ECG) machine with BL-420 bio-signal
collection system (Chengdu Techman Technology Co., Ltd., Chengdu,
China); 10% chloral hydrate (prepared by Xiangyang No. 1 People's
Hospital, Hubei University of Medicine); H&E dye liquor
(Nanjing Jiancheng Biological Engineering Institute); Ator calcium
tablets (trade name: Lipitor, Pfizer Pharmaceutical Co., Ltd.).
Experimental methods
Experimental grouping and drug
treatment
Forty rats, in accordance with the random number
table, were randomly divided into 4 groups with 10 rats in each
group. In Ator group, left anterior descending coronary artery
(LAD) was ligated to establish the MI model; after 24 h, Ator was
administrated (10 mg/kg/day) intragastrically for 4 weeks after
being dissolved in normal saline. In MI group, at 24 h after
establishment of MI model, the same amount of normal saline was
administrated intragastrically for 4 weeks. In sham-operation
group, LAD was looped with string, but not ligated. The normal
group received no treatment.
Establishment of MI model (11)
Preoperative preparation
The rats were numbered, weighed, anesthetized with
intraperitoneal injection of 10% chloral hydrate (3 ml/kg), and
fixed under the supine position, followed by skin preparation and
conventional disinfection. Connect the BL-420 bio-signal
acquisition system to monitor the ECG. The catheter was inserted on
the cervical trachea and connected with respirator.
LAD ligation
After the breath was stable, a 2 cm-long
longitudinal incision was made on the 3rd-4th intercostal space in
left chest, the subcutaneous fascia and each layer of muscle were
separated, and the chest was opened. The pericardium was gently
torn to show the LAD accompanying veins at the junction of
pulmonary arterial cone and left aurcle. LAD was ligated with 6-0
swaged needle from 2–3 mm below the left aurcle and the left side
of LAD accompanying veins; the needle was withdrawn near the
interventricular groove.
Chest closure
After the successful ligation, the hematocele and
foreign body in chest cavity were cleared, and the chest was closed
coated with erythromycin ointment. After the spontaneous breathing
was restored, the tracheal catheter was removed.
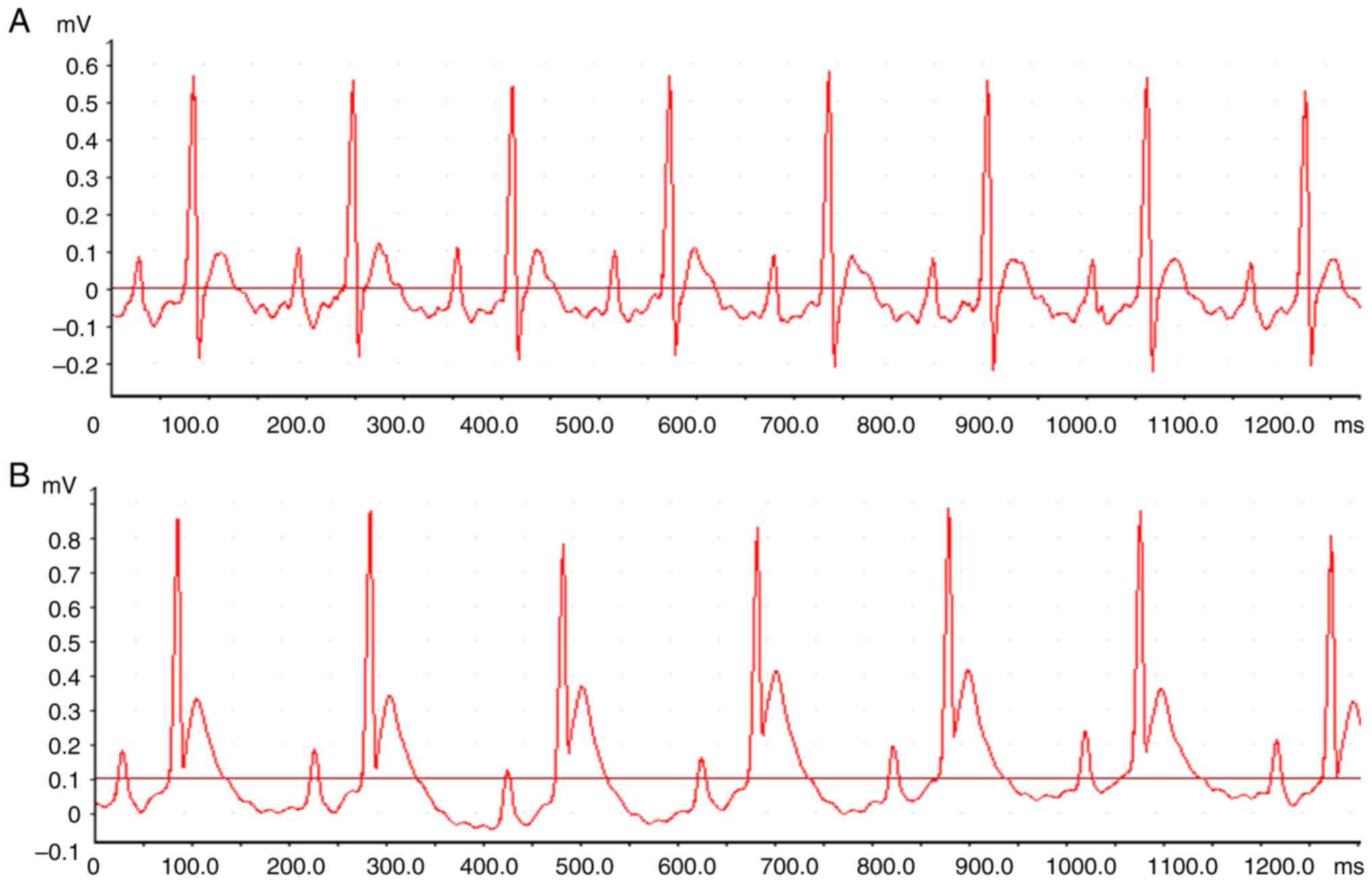
Signs of successful modeling
After the LAD ligation, the pale myocardium was
visible in the blood supplying area in the distal ligated vessels,
and the myocardial movement was weakened; in ECG, the persistent
ST-segment elevation (Fig. 1) could
be seen for at least 30 min.
Modeling in sham-operation group
The myocardium around the LAD was looped with 6-0
swaged needle, but not ligated; other operation steps were the same
as above.
Cardiac ultrasound image acquisition and analysis in
rats
Cardiac ultrasound images
Cardiac ultrasound images were collected from rats
in Ator group, MI group and sham-operation group before and after
operation once per week for 4 weeks. Rats in normal group received
the cardiac ultrasonography once per week for 5 weeks.
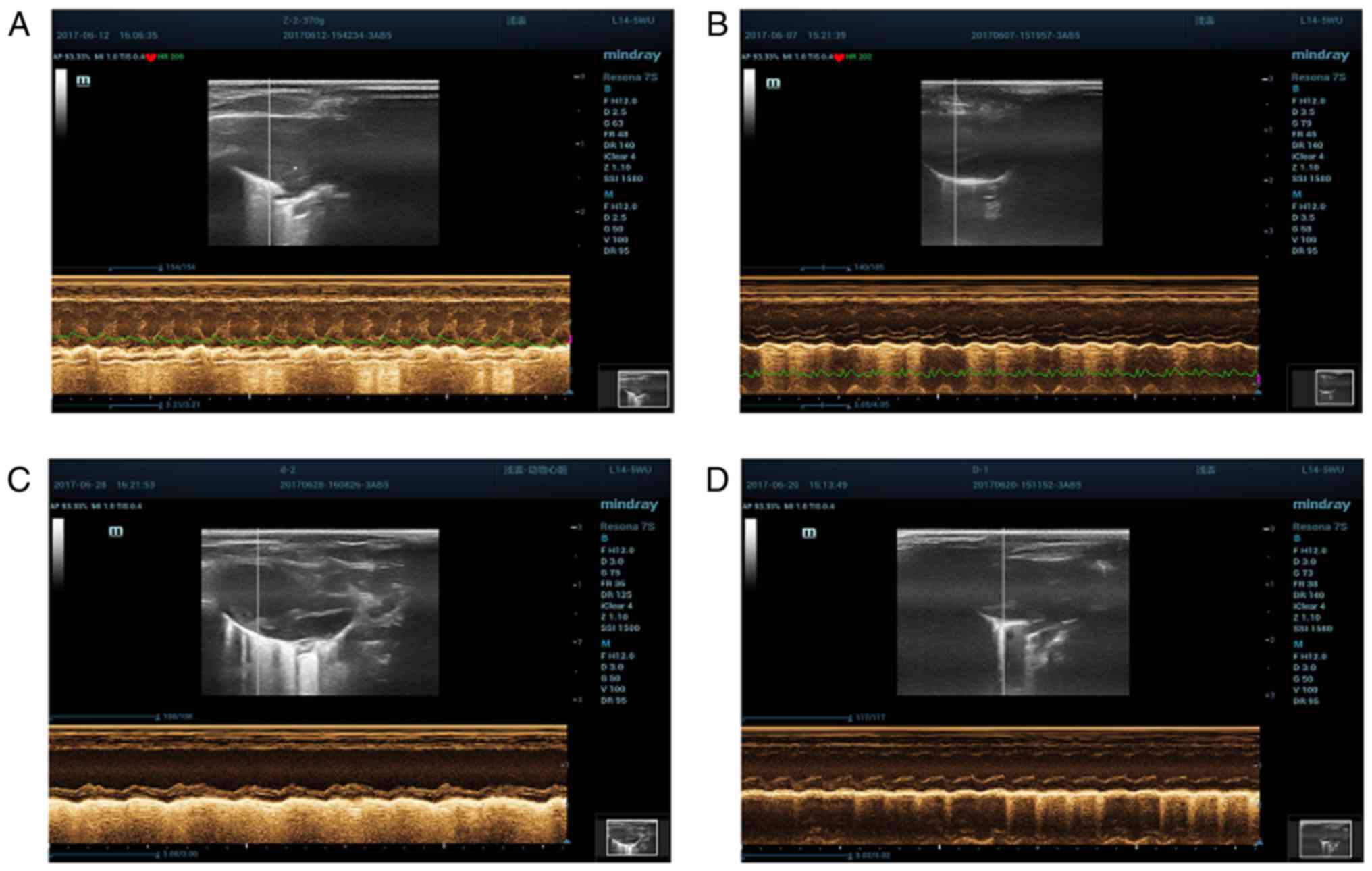
Cardiac ultrasound detection
methods
Rats were anesthetized, and fixed under the supine
position, followed by skin preparation; ECG was recorded
simultaneously; left ventricular end-diastolic diameter (LVEDD),
left ventricular end-systolic diameter (LVESD), left ventricular
ejection fraction (LVEF) and left ventricular fractional shortening
(LVFS) were measured via the M-mode ultrasound images using the
L14-5WU probe (Fig. 2). All indexes
were measured for 3 times and the averages were taken.
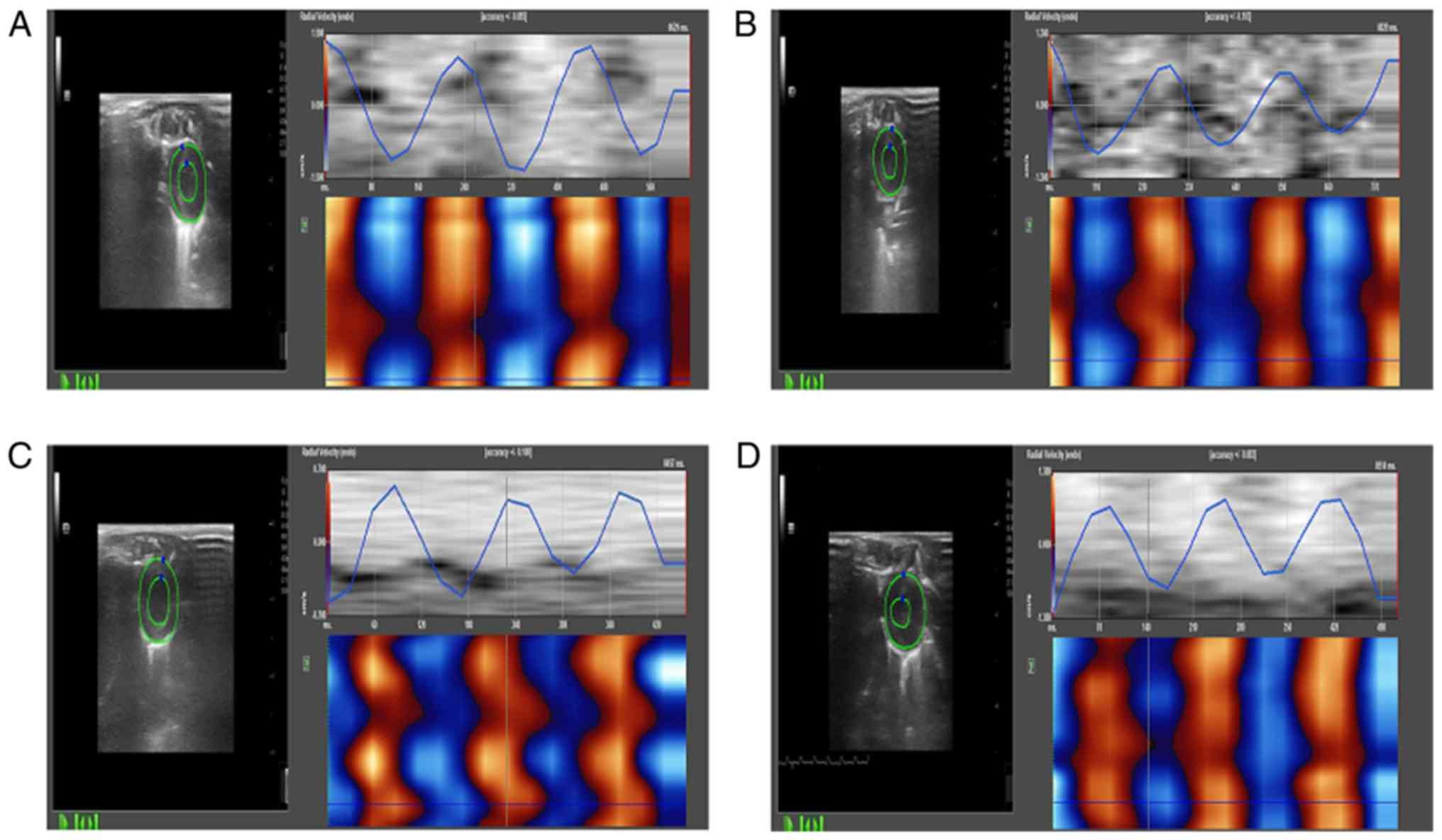
Two-dimensional dynamic images
The two-dimensional dynamic images of at least three
consecutive intact cardiac cycles were collected and stored at the
views of parasternal left ventricle short axis papillary muscles
and left ventricular short axis cardiac apex, left ventricular long
axis view, apical two-chamber view, and apical four-chamber view,
and analyzed off-line using the TomTec software. The clearest
one-frame image of left ventricular endocardium was frozen, the
left ventricular endometrial surface was checked manually, after
automatically tracking the changes in myocardial motion; the
tracking of endocardium curve in the entire cardiac cycle was
adjusted. The radial, circumferential and longitudinal myocardial
systolic peak velocities, strain and strain rates of left
ventricular anterior, lateral, posterior, inferior, anteroseptal,
posteroseptal walls and apex were obtained (Fig. 3). All the analysis and measurement
were performed by two ultrasound doctors in a double-blind way, and
the average was taken.
Pathological examination
After 4 weeks, all rats were euthanized; the hearts
were taken out, and washed in the precooled normal saline to remove
the blood and connective tissues outside the heart, followed by
fixation in 10% neutral formalin, paraffin embedding, section
cutting, hematoxylin-eosin (H&E) staining, and observation
under the light microscope (×400).
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Measurement data were presented
as mean ± standard deviation (mean ± SD). The one-way analysis of
variance (ANOVA) was used for the within-group comparison at
different time, while the repeated measures ANOVA was used for the
between-group comparison at the same time. p<0.05 suggested that
the difference was statistically significant.
Results
Survival of SD rats
During the experiment, 7 rats died. Finally, 10 rats
were enrolled into the normal group, 9 rats into the sham-operation
group, 7 rats into the MI group and 7 rats into the Ator group.
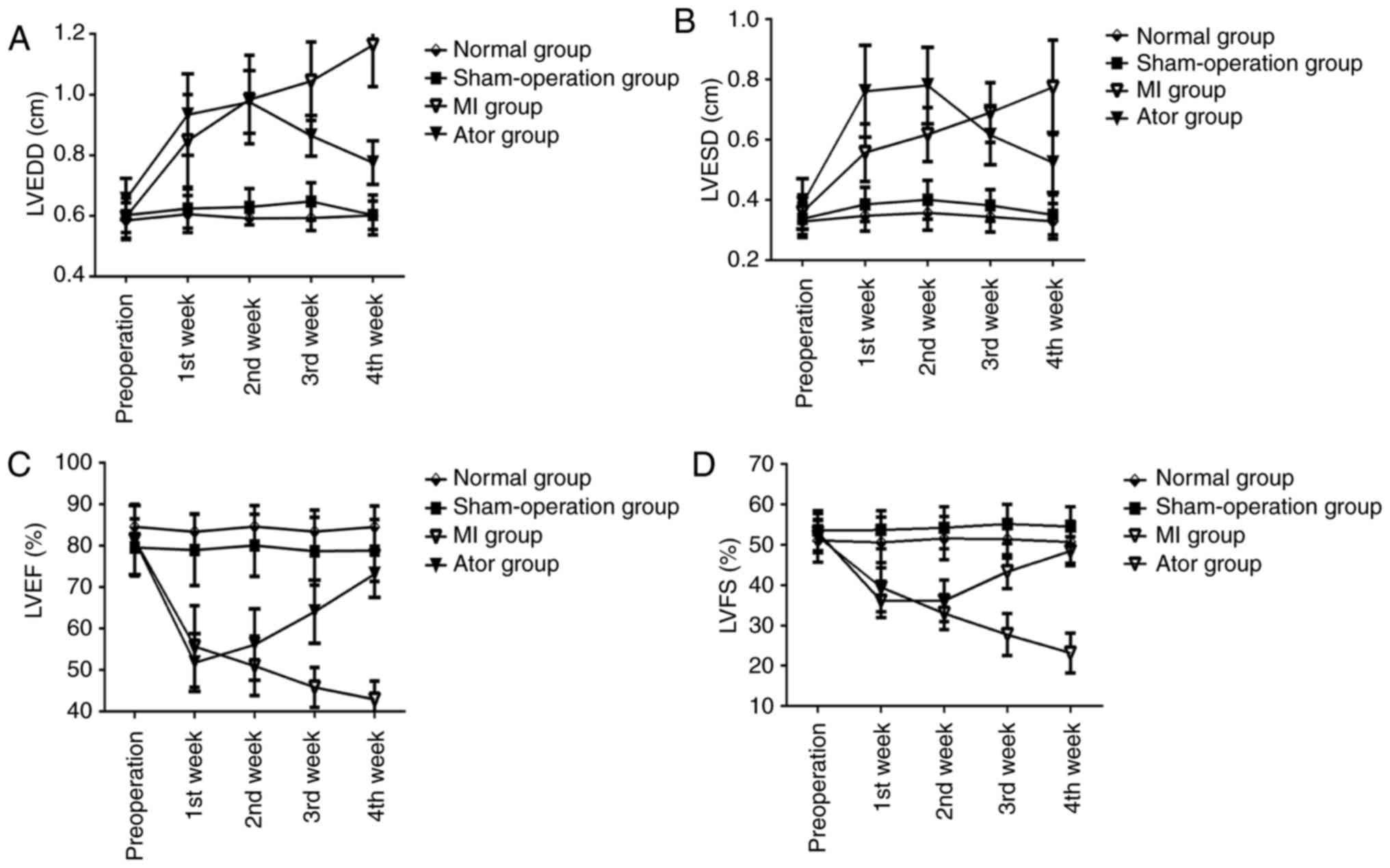
Comparisons of conventional ultrasound measurement
results (Table I and Fig. 4)
 | Table I.Echocardiogram measurement results of
the four groups (mean ± SD). |
Table I.
Echocardiogram measurement results of
the four groups (mean ± SD).
| Group | LVEDD (cm) | LVESD (cm) | LVEF (%) | LVFS (%) |
|---|
| Normal group |
|
Preoperation |
0.59±0.06 |
0.33±0.05 |
84.58±5.09 |
51.06±5.30 |
| The 1st
week |
0.61±0.06 |
0.35±0.05 |
83.39±4.30 |
50.55±6.27 |
| The 2nd
week |
0.59±0.02 |
0.36±0.06 |
84.61±5.09 |
51.59±5.38 |
| The 3rd
week |
0.59±0.04 |
0.34±0.05 |
83.42±5.15 |
51.40±4.60 |
| The 4th
week |
0.60±0.05 |
0.33±0.06 |
84.52±5.07 |
50.67±5.23 |
| Sham-operation
group |
|
Preoperation |
0.60±0.06 |
0.34±0.05 |
79.60±6.90 |
53.54±4.94 |
| The 1st
week |
0.62±0.06 |
0.39±0.06 |
78.96±8.60 |
53.70±4.74 |
| The 2nd
week |
0.63±0.06 |
0.40±0.06 |
80.08±7.50 |
54.21±5.23 |
| The 3rd
week |
0.65±0.06 |
0.38±0.05 |
78.65±8.15 |
55.15±4.81 |
| The 4th
week |
0.60±0.07 |
0.35±0.07 |
78.84±7.48 |
54.54±4.94 |
| MI group |
|
Preoperation |
0.60±0.08 |
0.36±0.06 |
81.49±8.46 |
52.03±4.02 |
| The 1st
week |
0.85±0.15a–c |
0.56±0.10a–c |
55.68±9.86a–c |
39.47±6.07a–c |
| The 2nd
week |
0.98±0.15a–c |
0.62±0.09a–c |
51.00±7.18a–c |
32.97±4.03a–c |
| The 3rd
week |
1.04±0.13a,c |
0.69±0.10a,c |
45.81±4.82a,c |
27.74±5.20a,c |
| The 4th
week |
1.16±0.14a,c |
0.77±0.16a,c |
42.93±4.42a,c |
23.19±4.91a,c |
| Ator group |
|
Preoperation |
0.66±0.07 |
0.39±0.08 |
81.34±8.33 |
53.06±4.60 |
| The 1st
week |
0.93±0.13a–c |
0.76±0.15a–c |
51.80±6.97a–c |
36.08±4.12a–c |
| The 2nd
week |
0.98±0.10a–c |
0.78±0.13a–c |
56.15±8.61a–c |
36.14±5.19a–c |
| The 3rd
week |
0.86±0.07a,c,
d |
0.62±0.10a,c,
d |
64.06±7.64a–d |
43.31±4.21a–d |
| The 4th
week |
0.78±0.07a,c,
d |
0.52±0.10a,c,
d |
73.19±5.68a,d |
48.44±3.58a,d |
Within-group comparisons at different
time-points
LVEDD and LVESD in Ator group and MI group were
increased after operation, but LVEF and LVFS were decreased. The
differences were statistically significant (p<0.05). After Ator
treatment, LVEDD and LVESD in Ator group were decreased at the 4th
week after operation compared with those at the 1st and 2nd week
after operation, but LVEF and LVFS in Ator group were increased
compared with those at the 1st, 2nd and 3rd week after operation.
The differences were statistically significant (p<0.05). At the
4th week, LVEDD and LVESD in MI group were increased, but LVEF and
LVFS were decreased after operation compared with those at the 1st
and 2nd week after operation. The differences were statistically
significant (p<0.05). There were no statistically significant
differences in conventional ultrasound indexes between normal group
and sham-operation group before and after operation
(p>0.05).
Between-group comparisons at the same
time-points
Compared with those in normal group and
sham-operation group at the corresponding time-points, LVEDD and
LVESD in Ator group and MI group were increased, but LVEF and LVFS
were decreased (p<0.05). After Ator treatment, compared with
those in MI group at the 3rd and 4th week after operation, LVEDD
and LVESD in Ator group were decreased, but LVEF and LVFS were
increased. The differences were statistically significant
(p<0.05). There were no statistically significant differences in
the conventional ultrasound indexes between normal group and
sham-operation group at the same time-points (p>0.05).
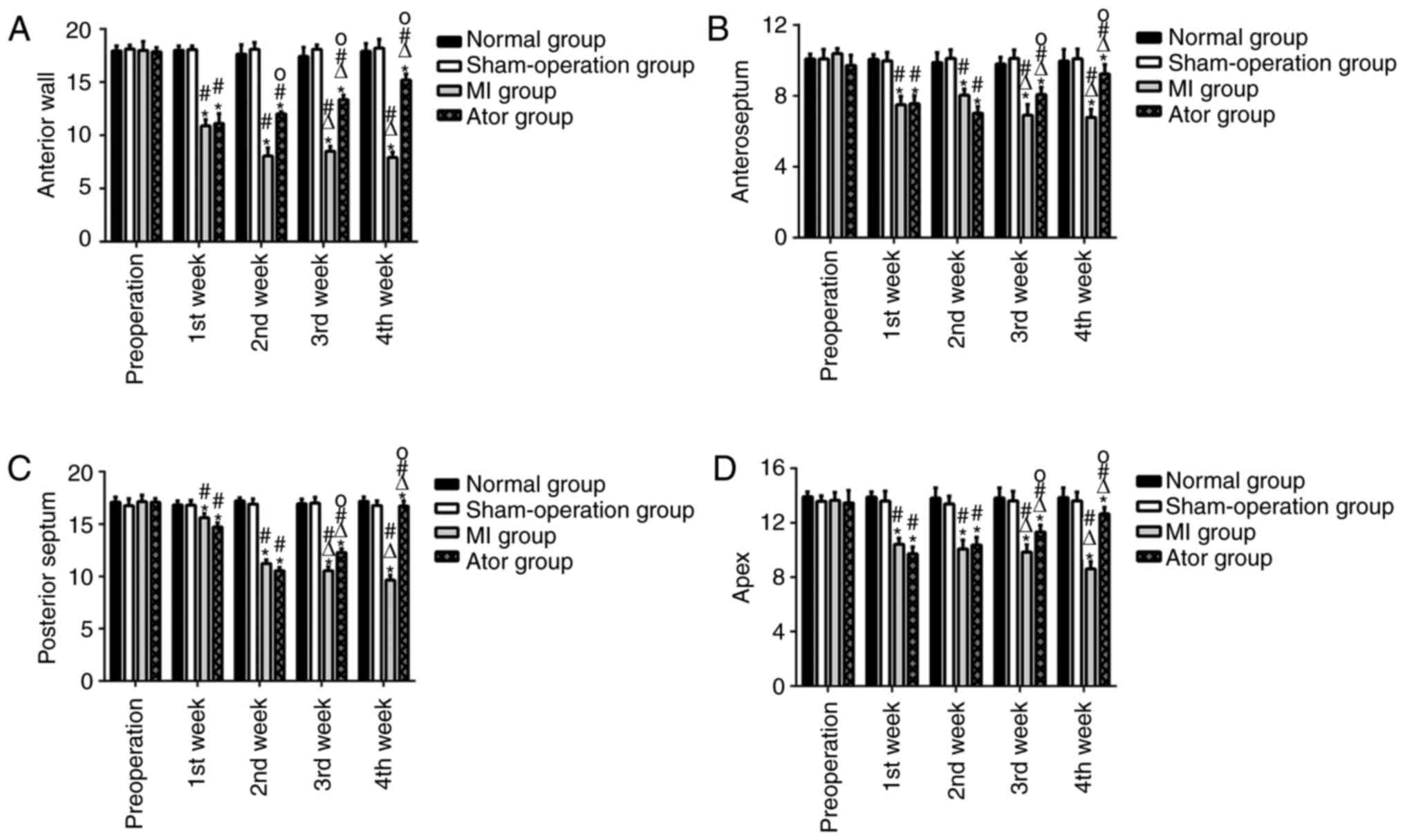
Comparison of 2D-STI detection indexes (Table II and Fig. 5)
 | Table II.Comparisons of radial strain of seven
left ventricular segments of the four groups (mean ± SD). |
Table II.
Comparisons of radial strain of seven
left ventricular segments of the four groups (mean ± SD).
| Group | Anterior wall | Lateral wall | Posterior wall | Inferior wall | Anteroseptum | Posterior
septum | Apex |
|---|
| Normal group |
|
Preoperation |
17.94±0.46 |
20.46±1.15 |
19.32±0.05 |
8.50±0.59 |
10.08±0.27 |
17.11±0.49 |
13.92±0.35 |
| The 1st
week |
18.01±0.40 |
20.43±0.74 |
19.32±0.06 |
8.70±0.75 |
10.06±0.27 |
16.84±0.39 |
13.89±0.39 |
| The 2nd
week |
17.64±0.89 |
20.43±0.48 |
19.25±0.71 |
8.31±0.84 |
9.88±0.56 |
17.20±0.31 |
13.81±0.75 |
| The 3rd
week |
17.43±0.85 |
20.60±0.34 |
19.54±0.50 |
8.94±0.79 |
9.80±0.38 |
16.95±0.44 |
13.82±0.74 |
| The 4th
week |
17.90±0.75 |
20.50±0.42 |
19.35±0.51 |
8.39±1.11 |
9.96±0.66 |
17.17±0.44 |
13.84±0.72 |
| Sham-operation
group |
|
Preoperation |
18.13±0.35 |
20.58±0.48 |
19.13±0.59 |
8.23±0.63 |
10.09±0.54 |
16.76±0.66 |
13.59±0.39 |
| The 1st
week |
18.07±0.34 |
20.37±0.60 |
19.57±0.59 |
8.51±1.08 |
9.97±0.48 |
16.80±0.47 |
13.59±0.74 |
| The 2nd
week |
18.10±0.63 |
20.25±0.70 |
19.53±0.64 |
8.35±0.55 |
10.12±0.48 |
16.91±0.49 |
13.37±0.59 |
| The 3rd
week |
18.10±0.41 |
20.38±0.44 |
19.26±0.82 |
8.31±1.04 |
10.11±0.49 |
17.00±0.56 |
13.61±0.71 |
| The 4th
week |
18.21±0.83 |
20.52±0.66 |
19.06±0.49 |
8.22±0.82 |
10.09±0.56 |
16.77±0.45 |
13.61±0.65 |
| MI group |
|
Preoperation |
17.98±0.84 |
20.58±0.36 |
19.42±0.62 |
8.12±0.75 |
10.39±0.29 |
17.15±0.63 |
13.65±0.69 |
| The 1st
week |
10.90±0.60a,c |
20.50±0.62 |
9.06±0.64 |
8.19±0.59 |
7.50±0.48a,c |
15.62±0.42a,c |
10.42±0.44a,c |
| The 2nd
week |
8.06±0.76a,c |
20.12±0.83 |
19.52±0.79 |
8.23±0.71 |
8.03±0.35a,c |
11.22±0.35a,c |
10.07±0.65a,c |
| The 3rd
week |
8.50±0.48a–c |
20.47±0.39 |
19.40±0.76 |
8.08±1.18 |
6.90±0.61a–c |
10.54±0.37a–c |
9.85±0.61a–c |
| The 4th
week |
7.93±0.50a–c |
20.47±0.59 |
19.44±0.21 |
8.10±0.91 |
6.78±0.46a–c |
9.64±0.47a–c |
8.61±0.52a–c |
| Ator group |
|
Preoperation |
17.86±0.40 |
20.56±0.44 |
19.13±0.46 |
8.59±0.99 |
9.71±0.58 |
17.09±0.35 |
13.46±0.91 |
| The 1st
week |
11.12±0.92a,c |
20.38±0.51 |
19.01±0.59 |
8.53±0.94 |
7.56±0.46a,c |
14.73±0.40a,c |
9.72±0.49a,c |
| The 2nd
week |
12.02±0.21a,c,
d |
20.63±0.16 |
19.04±0.57 |
8.51±0.98 |
7.02±0.38a,c |
10.54±0.31a,c |
10.37±0.56a,c |
| The 3rd
week |
13.35±0.42a–d |
20.61±0.45 |
19.35±0.60 |
8.28±1.02 |
8.08±0.41a–d |
12.27±0.38a–d |
11.33±0.49a–d |
| The 4th
week |
15.20±0.55a–d |
20.55±0.55 |
19.14±0.82 |
8.53±0.89 |
9.23±0.53a–d |
16.72±0.51a–d |
12.64±0.48a–d |
Within-group comparisons at different
time-points
The radial, circumferential and longitudinal
myocardial systolic peak velocities, strain and strain rates of
left ventricular anterior, anteroseptal, posteroseptal walls and
apex in Ator group and MI group after operation were decreased
significantly and the differences were statistically significant
(p<0.05). The radial, circumferential and longitudinal
myocardial systolic peak velocities, strain and strain rates of
left ventricular lateral, posterior, inferior walls had no
significant changes (p>0.05). At the 3rd and 4th week after
operation, the radial, circumferential and longitudinal myocardial
systolic peak velocities, strain and strain rates of left
ventricular anterior, anteroseptal, posteroseptal walls and apex in
Ator group were increased compared with those at the 1st and 2nd
week after operation (p<0.05), while they were decreased in MI
group compared with those at the 1st and 2nd week after operation
(p<0.05). There were no statistically significant differences in
the radial, circumferential and longitudinal myocardial systolic
peak velocities, strain and strain rates in the 7 segments of left
ventricular in normal group and sham-operation group after
operation compared with those before operation (p>0.05).
Between-group comparisons at the same
time-points
Compared with those in normal group and
sham-operation group at the 1st week after operation, the radial,
circumferential and longitudinal myocardial systolic peak
velocities, strain and strain rates of left ventricular anterior,
anteroseptal, posteroseptal walls and apex in Ator group and MI
group were decreased, and the differences were statistically
significant (p<0.05); there were no statistically significant
differences in the radial, circumferential and longitudinal
myocardial systolic peak velocities, strain and strain rates of
left ventricular anterior, anteroseptal, posteroseptal walls and
apex at the 1st week after operation between MI group and Ator
group (p>0.05), but at the 3rd and 4th week, there were
significant increase and the differences were statistically
significant (p<0.05). There were no statistically significant
differences in the radial, circumferential and longitudinal
myocardial systolic peak velocities, strain and strain rates of
left ventricular lateral, posterior, inferior walls among the four
groups before and after operation at corresponding time-points
(p>0.05).
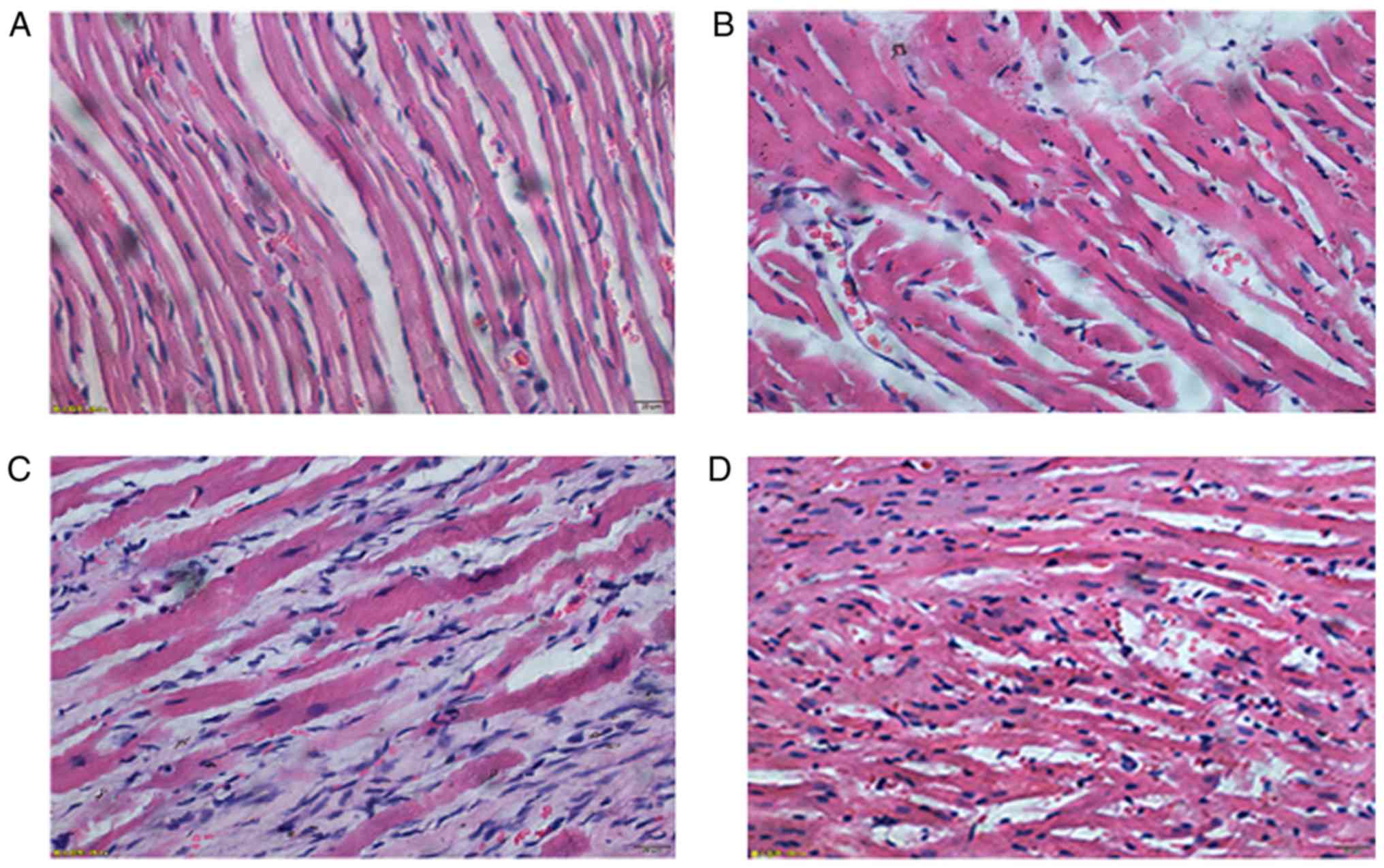
Pathological examination results of
left ventricular anterior myocardium
In normal group, the structure of myocardial cells
were clear, tissues were arranged neatly and stained evenly, and
there was no obvious inflammatory cell infiltration in
intermuscular space. In sham-operation group, the structure of
myocardial cells was arranged less neatly, and there were a few
necrotic myocardium and fibrous hyperplasia. In MI group, there
were massive ischemic necrosis of myocardial cells, fibrosis in
some areas and fracture of partial muscle fibers in the few
remaining cardiac fibers; massive inflammatory cell infiltration
could be seen in intermuscular space. In Ator group, there were a
few necrotic myocardium cells and fibrotic areas; a little
inflammatory cell infiltration still could be seen in intermuscular
space (Fig. 6).
Discussion
After MI, inflammation, myocardial loss, cardiac
remodeling and other factors lead to the weakened myocardial motion
and decrease the cardiac function; the accurate assessment of
myocardial motor function is of great significance in evaluating
the degree of myocardial injury, choosing the therapeutic regimen
and assessing the treatment effect.
Myocardial motion can be divided into the
longitudinal, radial and circumferential motion (12,13).
2D-STI technique can automatically identify and track the spatial
motion of acoustic spots of myocardial tissues in the region of
interest frame by frame. Moreover, it can qualitatively and
quantitatively describe the movement speed, strain, strain rate and
rotation angle of myocardial tissues in the three motor directions;
this technique is not obviously angle-dependent and not susceptible
to the surrounding tissues and myocardium. It is accurate, rapid
and sensitive when assessing the myocardial movement and
deformation, so in recent years it has become a newly-developed
technique to evaluate the myocardial function, especially the
cardiac systolic and diastolic functions in the pathological state
(14,15).
Among the drugs commonly-used in the treatment of
MI, the statin drug is a kind of 3-hydroxy-3-methyl glutaryl
coenzyme A (HMG-COA) reductase inhibitor that can competitively
inhibit the activity of rate-limiting enzyme HMG reductase during
cholesterol synthesis, inhibit the cholesterol synthesis, and
reduce the low-density lipoprotein (LDL) level (16). After applied to MI, the statin drug
can prevent the secondary myocardial infarction and other
cardiovascular events by regulating blood lipid levels.
In this study, Ator was administered to MI rats for
4 weeks; the left ventricular myocardial systolic motion was
monitored using the 2D-STI technique, and the myocardial
pathological changes were observed via H&E staining. The
results showed that after Ator treatment, LVEDD and LVESD were
decreased at the 3rd and 4th week after operation compared with MI
group at the same time-points, while LVEF and LVFS were increased;
LVEDD and LVESD were decreased at the 4th week compared with those
at the 1st and 2nd week after operation, while LVEF and LVFS were
increased compared with those at the 1st, 2nd and 3rd week after
operation. The differences were statistically significant
(p<0.05), which meant the M-mode echocardiogram detection index
were improved significantly. It showed that Ator can improve left
ventricular systolic function in MI rats. Besides, the anterior,
anteroseptal, posteroseptal walls and apex which were LAD blood
supplying area, radial, circumferential and longitudinal systolic
peak velocities, strains, and strain rates were increased
significantly at the 3rd and 4th week after operation compared with
those at the 1st and 2nd week after operation and those in MI group
at the same time, which was further proved that Ator can
effectively improve the left ventricular systolic function in MI
rats. Its mechanism may be related with that Ator can significantly
reduce the level of C-reactive protein (CRP) and serum amyloid
protein A (17), promote the
neutrophil apoptosis (18), reduce
the inflammatory response, reduce the depletion of glutathione
peroxidase (GSH-Px) and superoxide dismutase (SOD) (19), resist the oxidative stress and
protect cells. Furthermore, myocardial tissues H&E staining
showed that the inflammatory response of myocardial tissues and
fibroplasia was alleviated after Ator treatment, further proving
that Ator can alleviate myocardial inflammatory response and
myocardial fibrosis.
In this experiment, MI rats were used as the objects
of study, and the improvement of cardiac function was observed
using the single medication mode, which eliminated the effect of
confounding factors in clinical combined medication therapy of
coronary heart disease on the evaluation of drug therapeutic
effect. At the same time, the accurate and sensitive 2D-STI
technique improved the reliability of the experimental result.
However, there were also some limitations in this experiment: i)
the dose of Ator used in this experiment was 10 mg/kg/day, and the
dose was single, so the drug concentration gradient needs to be
increased to explore the appropriate concentration and side effects
of Ator; ii) Ator is the lipid-lowering drug, but the objects of
this study were the healthy SD rats, so whether the effect of
statin drugs on cardiac function will be affected remain unknown
yet if the observed objects have dyslipidemia; iii) the mechanism
of Ator in improving the left ventricular systolic function after
MI remains to be further investigated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, MX, JY and MS participated in the design of the
experiments. YH, MX and ML performed the experiments. YW, SL and JX
extracted the data. MX, YH and LG analyzed the data. YH, MX
prepared the initial draft, and MX, YH, JY and XS prepared the
final manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The animal experimental program was approved by the
Ethics Committee of Xiangyang No. 1 People's Hospital, Hubei
University of Medicine (Xiangyang, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lai HM, Aronow WS, Mercando AD, Kalen P,
Desai HD, Gandhi K, Sharma M, Amin H and Lai TM: The impact of
statin therapy on long-term cardiovascular outcomes in an
outpatient cardiology practice. Med Sci Monit. 17:CR683–CR686.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao X, Chang G, Liu J, Sun G, Liu L, Qin
S and Zhang D: Simvastatin ameliorates ventricular remodeling via
the TGF-β1 signaling pathway in rats following myocardial
infarction. Mol Med Rep. 13:5093–5101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reichert K, do Carmo Pereira HR, Torina
Galluce A, de Carvalho Diógenes D, Sposito Carvalho A, de Souza
Vilarinho KA, da Mota Silveira-Filho L, de Oliveira Martins PP and
Petrucci O: Atorvastatin improves ventricular remodeling after
myocardial infarction by interfering with collagen metabolism. PLoS
One. 11:e01668452016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin JH, Connelly KA, Boyle A, Kompa A,
Zhang Y, Kelly D, Gilbert RE and Krum H: Effect of atorvastatin on
cardiac remodelling and mortality in rats following hyperglycemia
and myocardial infarction. Int J Cardiol. 143:353–360. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang XL, Sanganalmath SK, Sato H, Bi Q,
Hunt G, Vincent RJ, Peng Y, Shirk G, Dawn B and Bolli R:
Atorvastatin therapy during the peri-infarct period attenuates left
ventricular dysfunction and remodeling after myocardial infarction.
PLoS One. 6:e253202011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mihaylova B, Emberson J, Blackwell L,
Keech A, Simes J, Barnes EH, Voysey M, Gray A, Collins R and
Baigent C: Cholesterol Treatment Trialists' (CTT) Collaborators:
The effects of lowering LDL cholesterol with statin therapy in
people at low risk of vascular disease: Meta-analysis of individual
data from 27 randomised trials. Lancet. 380:581–590. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song T, Liu J, Tao X and Deng JG:
Protection effect of atorvastatin in cerebral ischemia-reperfusion
injury rats by blocking the mitochondrial permeability transition
pore. Genet Mol Res. 13:10632–10642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Whitehead NP, Kim MJ, Bible KL, Adams ME
and Froehner SC: A new therapeutic effect of simvastatin revealed
by functional improvement in muscular dystrophy. Proc Natl Acad Sci
USA. 112:12864–12869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee J, Hong EM, Jang JA, Park SW, Koh DH,
Choi MH, Jang HJ and Kae SH: Simvastatin induces apoptosis and
suppresses insulin-like growth factor 1 receptor in bile duct
cancer cells. Gut Liver. 10:310–317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Neukamm A, Høiseth AD, Einvik G, Lehmann
S, Hagve TA, Søyseth V and Omland T: Rosuvastatin treatment in
stable chronic obstructive pulmonary disease (RODEO): A randomized
controlled trial. J Intern Med. 278:59–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M, Li Z, Zhang X, Xie X, Zhang Y,
Wang X and Hou Y: Rosuvastatin attenuates atrial structural
remodelling in rats with myocardial infarction through the
inhibition of the p38 MAPK signalling pathway. Heart Lung Circ.
24:386–394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Waldman LK, Nosan D, Villarreal F and
Covell JW: Relation between transmural deformation and local
myofiber direction in canine left ventricle. Circ Res. 63:550–562.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li L, Craft M, Hsu HH, Zhang M, Klas B,
Danford DA and Kutty S: Left ventricular rotational and twist
mechanics in the human fetal heart. J Am Soc Echocardiogr.
30(773–780): e12017.
|
|
14
|
Amundsen BH, Helle-Valle T, Edvardsen T,
Torp H, Crosby J, Lyseggen E, Støylen A, Ihlen H, Lima JA, Smiseth
OA, et al: Noninvasive myocardial strain measurement by speckle
tracking echocardiography: Validation against sonomicrometry and
tagged magnetic resonance imaging. J Am Coll Cardiol. 47:789–793.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aurich M, Keller M, Greiner S, Steen H,
Siepen Aus dem F, Riffel J, Katus HA, Buss SJ and Mereles D: Left
ventricular mechanics assessed by two-dimensional echocardiography
and cardiac magnetic resonance imaging: Comparison of
high-resolution speckle tracking and feature tracking. Eur Heart J
Cardiovasc Imaging. 17:1370–1378. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sirtori CR: The pharmacology of statins.
Pharmacol Res. 88:3–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinlay S, Schwartz GG, Olsson AG, Rifai N,
Leslie SJ, Sasiela WJ, Szarek M, Libby P and Ganz P: Myocardial
ischemia reduction with aggressive cholesterol lowering study
investigators: High-dose atorvastatin enhances the decline in
inflammatory markers in patients with acute coronary syndromes in
the MIRACL study. Circulation. 108:1560–1566. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mandal P, Chalmers JD, Graham C, Harley C,
Sidhu MK, Doherty C, Govan JW, Sethi T, Davidson DJ, Rossi AG, et
al: Atorvastatin as a stable treatment in bronchiectasis: A
randomised controlled trial. Lancet Respir Med. 2:455–463. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nazli Y, Colak N, Alpay MF, Uysal S,
Uzunlar AK and Cakir O: Neuroprotective effect of atorvastatin in
spinal cord ischemia-reperfusion injury. Clinics (Sao Paulo).
70:52–60. 2015. View Article : Google Scholar : PubMed/NCBI
|