Introduction
Cerebrovascular disease is one of the three most
common causes of human mortality, with irreversible sequelae of
severe disability reported in 50–70% of surviving patients
(1). The Chinese diagnostic
guidelines of cerebrovascular diseases in 2010 stated that 7–10% of
males and 5–7% of females aged >65 years presented carotid
artery stenosis of >50% (2). A
retrospective study of symptomatic carotid artery stenosis and
endometrectomy from North America revealed that the annual
incidence of stroke in patients with 60–99% stenosis was 3.2% at a
5-year follow-up (3). In patients
with 60–74% stenosis, the annual incidence of ipsilateral stroke
was 3.0%, while this incidence was elevated to 3.7% in patients
with 75–94% stenosis (4).
Intracranial atherosclerotic stenosis is the most
common cause of ischemic stroke worldwide and also results in the
recurrence of stroke (5). Therefore,
a complete and systematic understanding of the imaging
characteristics and pathogenesis of intracranial artery stenosis
will assist clinical experts in employing more efficient strategies
to prevent and alleviate the induced clinical effects. An
increasing number of modern imaging techniques (6), including magnetic resonance angiography
(MRA), computed tomography angiography, digital subtraction
angiography (DSA), transcranial Doppler (TCD), magnetic resonance
imaging (MRI), diffusion weighted imaging (DWI) and microembolus
(MES) monitoring, are developed to facilitate the inspection of
intracranial artery stenosis and the examination of possible
pathogenesis of the resulted cerebral infarction. Although DSA is
the golden standard of determining the degree of vascular stenosis,
accurate measurement is dependent on the rapid injection of a large
dosage of contrast agents into the artery, which may induce
allergenic reactions (7).
Furthermore, it is an expensive and invasive technique (7). Non-invasive tools, including MRA and
TCD, can be used to detect moderate to severe stenosis (50–99%) as
reliable methods (8). MRA is the
most common strategy for the diagnosis of intracranial artery
stenosis due to its accuracy, inexpensiveness, impersonality,
intuition and non-invasion. It is also convenient in achieving
doctor-patient communication and provides the required conditions
for further DSA examination (9). DWI
is able to diagnose minimal infarction lesions in the cortex and
periventricular regions, but also to discover ultra-early ischemic
alterations in brain tissues and definitely demonstrate the
new-onset infarction lesions (10).
Therefore, DWI has become a favorable method used for the
investigation of the imaging characteristics of infarction
(10). Furthermore, TCD plus MES
monitoring is conducive to the detection of intracranial embolismic
diseases (10).
In the present study, the imaging characteristics
and pathogenesis of intracranial artery stenosis were investigated
in patients with acute cerebral infarction using various modern
imaging tools. DWI was employed to analyze the lesion
characteristics of intracranial artery stenosis in these patients
in detail. Furthermore, TCD, MES monitoring and electrocardiogram
(ECG) alteration techniques were combined to investigate the
pathogenesis of acute cerebral infarction induced by intracranial
artery stenosis.
Materials and methods
Subjects
A total of 84 cases of patients undergoing acute
cerebral infarction during the week prior to admission between
October 2008 and June 2012 in the Department of Neurology at the
First Affiliated Hospital of Nanchang University (Nanchang, China)
were recruited into the current study, subsequent to signing
informed consent forms. The study protocol was reviewed and
approved by the Institutional Ethics Committee of the First
Affiliated Hospital of Nanchang University. The cohort was
comprised of 74 males and 10 females with an age range of 41–80
years and a mean age of 61±9 years. All patients were diagnosed
according to the diagnostic criteria on cerebrovascular diseases
revised by the Fourth Chinese Academic Conference (11). The existences of intracranial artery
stenosis or occlusion, including 77 cases of anterior circulation
artery occlusion (internal carotid artery) and 7 cases of posterior
circulation artery occlusion (vertebrobasilar artery), were
diagnosed according to MRA findings. Patients with cardioembolism,
arteritis, syphilis, concurrent infection, cancer or severe
cardiopulmonary diseases were excluded.
Imaging data
Upon admission all patients were examined by MRI
(Trio3.0T; Siemens AG, Munich, Germany) using the axial T1-weighted
image (T1WI), T2WI, DWI, sagittal T2WI and fluid-attenuated
inversion recovery sequences. DWI was employed to analyze the
morphological features of infarction. Bright hyperintensity
displayed on DWI indicated a fresh infarct lesion. Any observed
changes, which indicated infarction were confirmed by an MRI
radiologist and two senior neurologists.
Infarct location and lesion
morphology
The cases included in the current study included
internal carotid artery stenosis and vertebrobasilar artery
stenosis patients. On the basis of regional division, internal
carotid artery infarctions were divided into cortex infarction,
subcortex infarction, complete infarction, incomplete infarction
that simultaneously implicated the cortex and subcortex, deep
infarction and watershed infarction. Similarly, vertebrobasilar
artery infarctions were divided into cortex infarction, deep
infarction, and infarction that implicated the cortex and deep
vessels. In terms of the morphology distribution, internal carotid
artery and vertebrobasilar artery infarction was recognized as
single or multiple infarction (two or more infarction lesions in
the same or various locations). Regarding the infarct size, lesions
were divided into the small (maximal diameter <20 mm), moderate
(20 mm <maximal diameter <40 mm) and large infarction
(maximal diameter >40 mm).
Sinus bradycardia assessment
All patients received complete bedside ECG
examination within 48 h after hospitalization. Sinus bradycardia in
the patients was defined as a ventricular rate of <60 beats/min
on the ECG.
TCD and MES monitoring
All patients underwent TCD with MES monitoring
within 3 days after hospitalization at a frequency of one time per
day. Related arteries were monitored repeatedly within a short time
(<30 min) using TCD (Digi-Lite system; Rimed Ltd., Ra'anana,
Israel). The TCD device also performed the MES monitoring. The
embolus was observed by physicians using the TCD-8 software for
Multi-DopX4, version 8.00Q (DWL, Sipplingen, Germany) and defined
as either gaseous or solid. The examination results were confirmed
by two professional TCD doctors.
Statistical analysis
A database was established with Excel 2003
(Microsoft Corp., Redmond, WA, USA) and then imported into the SAS
version 9.2 software (SAS Institute Inc., Cary, NC, USA) for
statistical analysis. Qualitative data were analyzed by
χ2-test, while quantitative data were analyzed by
student's t-test for comparison of two independent samples. A
P-value of <0.05 was considered to indicate differences that
were statistically significant.
Results
Infarction types and morphology
The data regarding the infarction types and
morphology of all patients are shown in Table I and Fig.
1. Among the 84 patients, the majority of infarction cases
resulted from internal carotid stenosis (77 patients; 91.7%), while
a small number of cases resulted from vertebral artery stenosis (7
patients; 8.3%). However, the difference in the proportion of
multiple infarctions between the internal carotid stenosis and
vertebral artery stenosis was not statistically significant
(χ2=0.02, P>0.05). Multiple infarction was observed
to be the most common type of infarction in the two stenosis groups
(69.0%).
 | Table I.Infarction types and morphology in 84
cases. |
Table I.
Infarction types and morphology in 84
cases.
|
|
| Infarct
morphology |
|---|
|
|
|
|
|---|
| Infarction site | Cases (%) | Multiple infarction
(%) | Single infarction
(%) |
|---|
| Internal carotid
system | 77 (91.7) | 53 (63.0) | 24 (28.0) |
| Vertebral artery
system | 7
(8.3) | 5 (6.0) | 2 (3.0) |
| Total | 84 | 58 (69.0) | 26 (31.0) |
The specific infarction types and morphology in the
77 cases of infarction in the internal carotid system are
demonstrated in Table II. Typical
images of every infarction type in the internal carotid system are
shown in Fig. 2. The results
indicated that multiple infarction (implicating both the cortex and
centrum ovale, 23.4%) and internal watershed infarction (22.1%)
were the two most common types of infarction in the internal
carotid system. Cortex large single infarction (3.9%), centrum
ovale single infarction (2.6%), and anterior and posterior
watershed infarction (1.3%) were infrequent in internal carotid
stenosis.
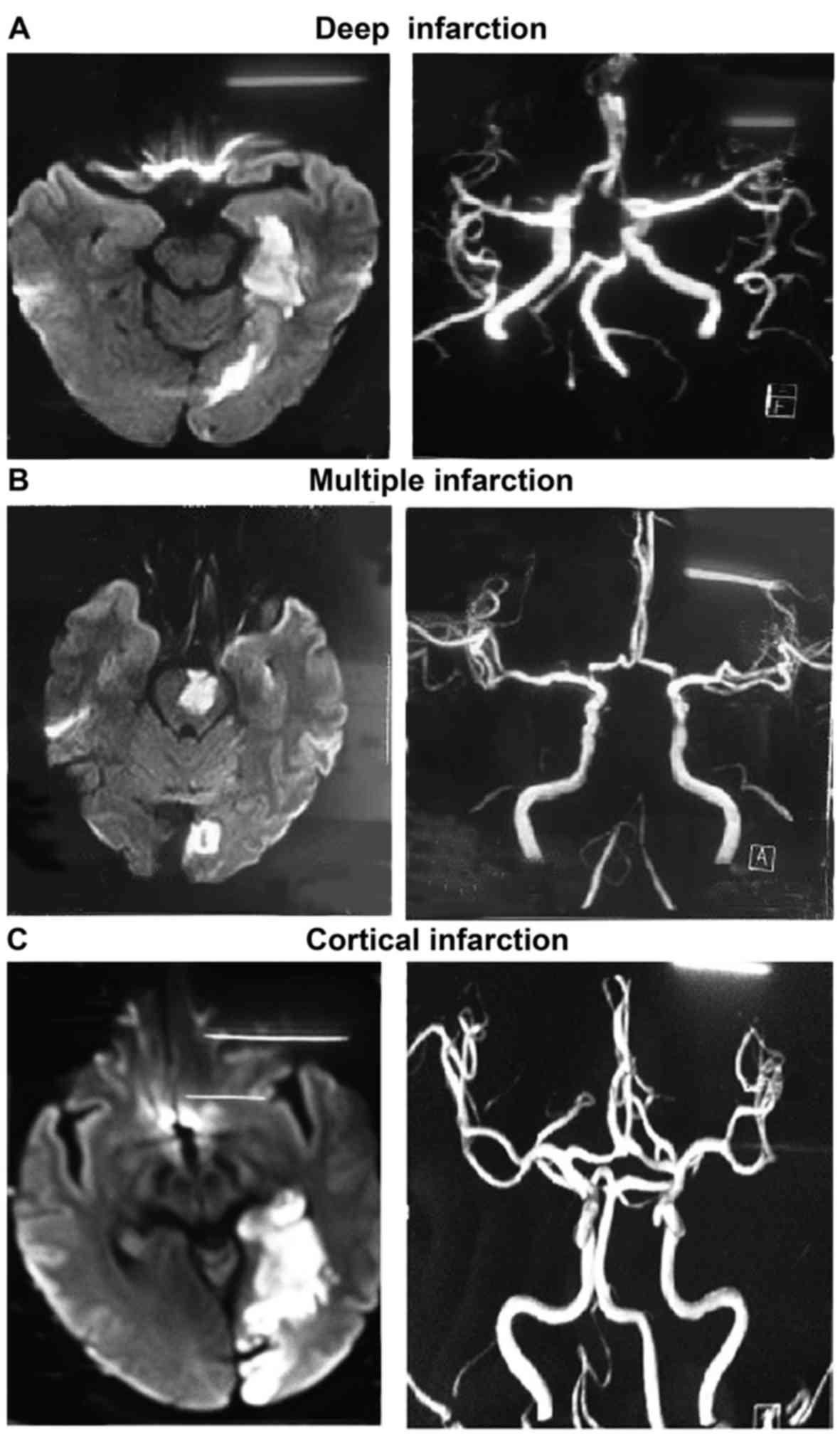
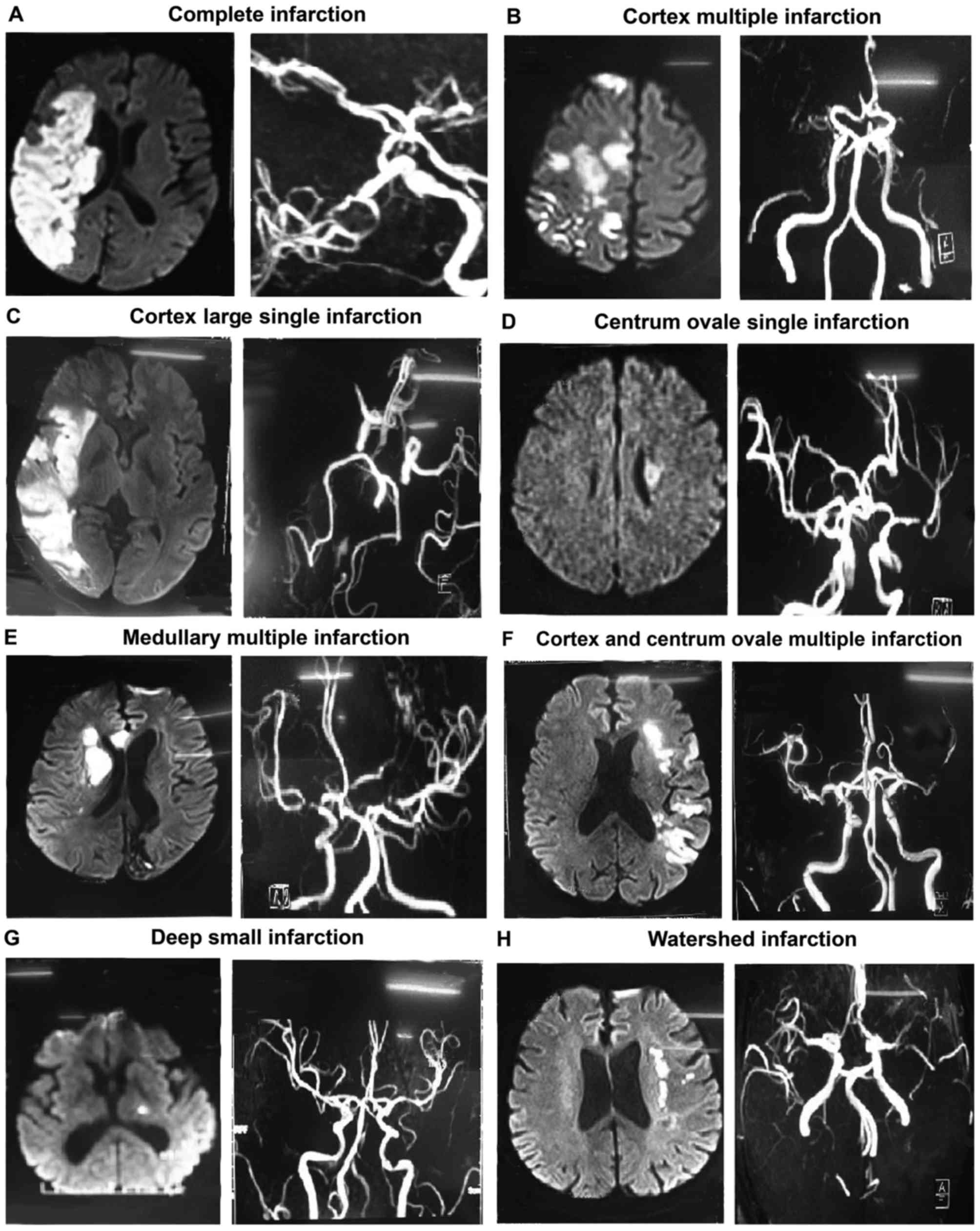 | Figure 2.Typical images of different infarction
types in the internal carotid system. For each type, DWI scans are
shown on the left, and magnetic resonance imaging scans are shown
on the right. (A) Complete infarction, demonstrated by the
occlusion of the right internal carotid artery and right MCA (right
image), and complete infarction of the cortex and perforating
branch in the blood supply area of the right MCA (larger area
infarction) in DWI scan (left image). (B) Cortex multiple
infarction, where the occlusion of bilateral MCA and right anterior
cerebral artery is observed (right image), and DWI demonstrates
multiple cerebral infarction with nonuniform sizes of infarction
lesions and various shapes of round, oval and large nodules in the
right parietal cortex (left image). (C) Cortex large single
infarction, with occlusion of the right MCA (right image), and DWI
demonstrating the cortex infarction in the blood supply area of the
right MCA (left image). (D) Centrum ovale single infarction, with
local stenosis of the left MCA (right image), and the centrum ovale
single infarction observed in DWI (left image). (E) Medullary
multiple infarction, with local stenosis of bilateral MCA (right
image), and multiple infarction in the right medullary area
observed in DWI (left image). (F) Cortex and centrum ovale multiple
infarction, with local stenosis of left MCA (right image), and DWI
showing the multiple infarction in the cortex and the centrum ovale
(left image). (G) Deep small infarction, with local stenosis of
left MCA (right image), and DWI showing the lacunar infarction in
the basal ganglia region (left image). (H) Watershed infarction,
with stenosis of left MCA (right image), and DWI showing the
internal watershed infarction with bunchy infarction lesions around
the left lateral ventricle (left image). DWI, diffusion weighted
imaging; MCA, middle cerebral artery. |
 | Table II.Infarction types and morphology in 77
cases of infarction in the internal carotid system. |
Table II.
Infarction types and morphology in 77
cases of infarction in the internal carotid system.
| Infarction types and
morphology | Cases, n (%) |
|---|
| Complete
infarction | 9 (11.7) |
| Cortex
infarction |
|
|
Multiple | 8 (10.3) |
| Large,
single | 3 (3.9) |
| Centrum ovale
infarction |
|
|
Single | 2 (2.6) |
| Medullary
multiple | 9 (11.7) |
| Multiple
infarctiona | 18 (23.4) |
| Deep small
infarction | 10 (13.0) |
| Watershed
infarction |
|
| Anterior
and posterior | 1 (1.3) |
|
Internal | 17 (22.1) |
Table III depicts
the specific infarction types and morphology in the 7 cases of
infarction in the vertebral artery system. Typical images of each
infarction type in the vertebral artery system are also shown in
Fig. 3. Although the number of cases
was relatively small, a high incidence of multiple infarction was
also observed in these patients.
 | Table III.Infarction types and morphology in 7
cases of infarction in the vertebral artery system. |
Table III.
Infarction types and morphology in 7
cases of infarction in the vertebral artery system.
| Infarction types and
morphology | Cases, n (%) |
|---|
| Deep single
infarction | 1 (14.3) |
| Cortical single
infarction | 1 (14.3) |
| Multiple
infarction | 5 (71.4) |
Sinus bradycardia in different
infarction patterns
The sinus rhythm in patients with different
infarction patterns is demonstrated in Table IV. It was observed that the sinus
bradycardia rate of patients with multiple infarction (31.0%) was
markedly greater in comparison with that in patients with single
infarction (11.5%; χ2=0.01, P<0.05). The sinus
bradycardia rate in all patients was 25.0%.
 | Table IV.Sinus rhythm in patients with
different infarction patterns. |
Table IV.
Sinus rhythm in patients with
different infarction patterns.
|
|
| Sinus
bradycardia |
|---|
|
|
|
|
|---|
| Infarct
morphology | Infarction cases,
n | Cases, n | Rate, % |
|---|
| Multiple
infarction | 58 | 18 | 31.0 |
| Single
infarction | 26 | 3 | 11.5 |
| Total | 84 | 21 | 25.0 |
Embolus in different infarction
patterns
The incidence of embolus in patients with various
patterns of infarction is listed in Table V. The results identified that, as
compared with patients with single infarction (7.7%), the embolus
rate in patients with multiple infarction was notably increased to
~4.7-fold (36.2%; χ2=8.65, P<0.05). The embolus rate
in all patients was 27.4%.
 | Table V.Embolus in patients with different
infarction patterns. |
Table V.
Embolus in patients with different
infarction patterns.
| Infarct
morphology | Infarction cases,
n | Embolus cases, n | Embolus rate, % |
|---|
| Multiple
infarction | 58 | 21 | 36.2 |
| Single
infarction | 26 | 2 | 7.7 |
| Total | 84 | 23 | 27.4 |
Discussion
The imaging features of cerebral infarction caused
by intracranial artery stenosis have been reported in the
literature, however, detailed studies are lacking. In the study by
Chen et al (12), a total of
62 patients were recruited with acute cerebral infarction who met
the following criteria: i) The location of acute infarction was
confirmed by DWI within 1 week after the onset of disease; ii) the
lesion was located in the intracranial segment of the carotid
artery; and iii) the infarction was identified to be induced by the
ipsilateral carotid artery occlusion rather than other causes.
According to the distribution of DWI lesions in these patients, the
infarct types were classified as perforating artery infarct (PAI),
pial infarct (PI), border-zone infarct (BZI) and multiple infarcts,
indicating a combination of the aforementioned types (12). In addition, Kang et al
(13) employed DWI to survey the
infarction types of 35 patients with acute cerebral infarction
induced by internal carotid artery occlusion. The study identified
21 cases of regional infarction (non-watershed infarction),
including 9 cases of cortex multiple infarction, 7 cases of cortex
and deep basal ganglia region multiple infarction, and 5 cases of
single infarction in these sites (13). Others types included 9 cases of
watershed infarction combined with the regional infarction, 1 case
of simple watershed infarction and 4 cases of bilateral cerebral
hemisphere infarction.
Tatu et al (14) reported the criteria for evaluating
the infarction of the middle cerebral artery (MCA) region.
According to the infarct site, volume and distribution demonstrated
on DWI, the cerebral infarction was divided into cortex regional
infarction, deep small infarction, watershed infarction and
multiple infarction (14). The
cortex regional infarction lesion was located in the main branch of
MCA, cortical or medullary blood supply area, and the cortex
regional infarction included the MCA complete, MCA cortical branch,
small cortical and centrum ovale infarctions. The infarction in
patients with MCA occlusive disease (MCAOD) was presented by
imaging as multiple small cortical infarction, which was often
accompanied with centrum ovale infarction (15). In the lenticulostriate artery region,
deep small infarction was defined as ‘giant lacuna’ with a lesion
diameter of >15 mm, striatocapsular infarction and lacunar
infarction (16). Of the MCA
lesions, 30.7% presented lacunar infarction, while lacunar and
striatocapsular infarctions were the most common types of basal
ganglionic infarction (16). A study
focusing on the Chinese and Korean populations also pointed out
that the majority of patients bearing lacunar or deep
striatocapsular infarction had occlusive lesions in the M1 segment
of the ipsilateral MCA or internal carotid artery (17).
Watershed infarction includes the anterior,
posterior and internal watershed infarcts. In recent years,
cerebral watershed infarction has gradually become an important
infarct type of MCAOD. Wong et al has also reported that
chain-type watershed infarction was the most common type in
patients with acute cerebral infarction resulting from MCA
occlusion (18). Watershed
infarction has been identified to be common in severe stenosis or
occlusion of the internal carotid artery (19). Furthermore, progressive aggravation
of neurological impairment in patients with subcortical cerebral
infarction is associated with the atherosclerotic stenosis of the
corresponding arteries; thus, intracranial artery stenosis serves
an important role in the neurological deterioration of patients
with subcortical infarction (20).
Multiple infarction refers to the multiple and
discontinuous non-fused infarction lesion in MCA region. In a
previous study conducted at the Chinese University of Hong Kong
investigated 30 cases of acute stroke by DWI (18), demonstrating that 50% of patients
presented multiple infarction, where the cortical multiple
infarction accounted for 60%; PAI patients accounted for 66.7% of
cases, with 2/3 presenting multiple lesions. In addition, 73% of
patients suffered watershed infarction, and three types of
watershed infarction in the anterior circulation were identified in
MCAOD patients, where the internal border zone was the most
frequently affected site and multifocal distribution with an
arrangement of chain-type was observed (21). Furthermore, the study by Tan and Yang
(22) also reported that multiple
infarction was the most common type in intracranial artery
stenosis.
In agreement with the aforementioned studies, the
present study revealed that the incidence of cerebral infarction,
including multiple and single infarctions, was evidently higher in
the internal carotid system rather than the vertebral artery
system, while multiple infarction (69.0%) was also much more
frequent in comparison with single infarction (31.0%) in the two
artery systems. In particular, multiple infarction implicating both
the cortex and centrum ovale (23.4%) and internal watershed
infarction (22.1%) were the two infarction types with the highest
incidence. Since the literature regarding the imaging
characteristics of cerebral infarction induced by posterior
circulation artery stenosis is limited, the present study enriched
and deepened the knowledge on these features. However, in
comparison with the internal carotid system, the cases involving
the vertebral artery system were significantly lower. Thus, a
large-scale investigation on vertebral artery stenosis should be
performed in the future to obtain more abundant clinical evidence.
Furthermore, the infarction types in carotid artery stenosis
reported herein were different from the previous classification
(12). The classification in the
present study was considered to be more appropriate and specific
compared with the previous classification.
In a previous study, it was confirmed that the
constituents (fibrins and platelet aggregates) of thromboembolism
causing occlusion of the distal artery in patients with internal
carotid artery stenosis were the same as those of the surface
thrombus of atherosclerotic plaques in terms of pathology;
therefore, a mechanism of arterial embolization was proposed
(23). Kang et al (13) considered that the acute cerebral
infarction induced by the occlusion of the internal carotid artery
was multiple infarction, and that arterial embolization was the
leading cause of internal carotid artery stroke. Gao et al
(24) also reported that
intracranial multiple lesions observed on DWI scans often suggested
the possible pathogenesis of embolism. Furthermore, a previous TCD
study reported that MES signals were more likely to be detected in
patients with intracranial artery stenosis that had multiple
lesions (25). In the present study
based on 84 patients with intracranial artery stenosis and cerebral
infarction, it was revealed that the embolus cases in multiple
infarction were notably greater in comparison with those in single
infarction. It was indicated that multiple infarction mainly
resulted from embolization, which was in agreement with the
aforementioned previous studies. Thus, arterial embolization can be
considered as a principle cause of multiple cerebral
infarction.
Chen et al (12) investigated the infarction types and
pathogenesis in 62 patients with intracranial artery stenosis and
observed that hypoperfusion was the main cause of watershed
infarction (12). The corresponding
imaging finding was subcortical white matter with internal
chain-typed distribution or low-density lesion with striped
distribution (26). In the current
study, watershed infarction with a significant majority of internal
watershed infarction accounted for nearly one third of the 58 cases
of multiple infarction. Furthermore, ECG demonstrated that the
incidence of sinus bradycardia in patients with multiple infarction
was significantly higher compared with that in patients with single
infarction; this observation has not been reported in previous
publications, to the best of our knowledge. Thus, it was speculated
that cerebral hypoperfusion induced by bradycardia may be an
important pathogenesis mechanism of watershed infarction or
multiple infarction. Watershed infarcts were usually confluent and
MES was more likely to appear, suggesting that watershed infarction
may be the result of decreased embolus clearance caused by
hypoperfusion in this region (18).
Apart from the multiple infarction, 12 cases of
single infarction in the medullary area and lacunar infarction in
the basal ganglia region were recruited in the present study. In
these patients, the positive rate of embolus and the incidence of
sinus bradycardia on ECG were low. The pathogenesis of single
infarction in the medullary area and lacunar infarction in the
basal ganglia region may be a result of thrombosis induced by de
novo vascular impairment, such as atherosclerosis. Thrombosis
is known as an important pathogenesis of cerebral infarction
(27). For instance, the thrombosis
in MCAOD patients may be associated with the generation of deep
small infarction (28). In
particular, stenosis in the main branch of the MCA may result in
thrombosis in the plaque residues and implicate the ostia of the
perforating artery, thereafter inducing the deep small infarction
in MCAOD patients (28). The single
infarction within the cortex region was also reported to be
connected with the thrombosis of the perforating artery (18).
In conclusion, the present study observed that
cerebral infarction, including multiple and single infarctions, was
evidently more common in the internal carotid system as compared
with the vertebral artery system, with multiple infarction observed
in the majority of cases in the two artery systems. In particular,
multiple infarction implicating the cortex and centrum ovale, and
internal watershed infarction were the most common types. The
pathogenesis of cerebral infarction due to intracranial artery
stenosis included arterial embolization and inadequate
hemoperfusion. Furthermore, sinus bradycardia served an important
role in the generation of cerebral infarction by hypoperfusion.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ashrafi Ahmed SK, Suhail Z and Khambaty Y:
Postembolization infarction in juvenile nasopharyngeal
angiofibroma. J Coll Physicians Surg Pak. 21:115–116.
2011.PubMed/NCBI
|
|
2
|
Guidelines for secondary prevention of
ischemic stroke and transient ischemic attack in China 2010. Chin J
Neurol. 43:154–160. 2010.(In Chinese).
|
|
3
|
Paciaroni M, Eliasziw M, Kappelle LJ,
Finan JW, Ferguson GG and Barnett HJ: Medical complications
associated with carotid endarterectomy. North American Symptomatic
Carotid Endarterectomy Trial (NASCET). Stroke. 30:1759–1763. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferguson GG, Eliasziw M, Barr HW, Clagett
GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW,
Hachinski VC and Barnett HJ: The North American symptomatic carotid
endarterectomy Trial: Surgical results in 1415 patients. Stroke.
30:1751–1758. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koo J: The latest information on
intracranial atherosclerosis: Diagnosis and treatment. Interv
Neurol. 4:48–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin P, Feng JC and Wang SC: Pathogenesis
of cerebral watershed infarction and the compensatory effect of
colleteral ability of the Willis circle. Chin J Cerebrovasc Dis.
5:102–106. 2008.(In Chinese).
|
|
7
|
Ma G, Jiang Z, He J, Liao Y, Zhu M, Huang
Z and Cui F: The value of DSA and CTA for diagnosis of carotid
artery stenosis. Clin Med Eng. 22:535–536. 2015.(In Chinese).
|
|
8
|
Khan M, Naqri L, Bansari A and Kamal AK:
Intracranial atherosclerotic disease. Stroke Res Treat.
2011:2828452011.PubMed/NCBI
|
|
9
|
Deng X, Liu W, Liu J and Zhong L: A study
of MRA for the diagnosis of occlusive and stenotic disorders of
intracranial arteries in ischemic cerebral vascular disease. J
Pract Med Tech. 14:1968–1970. 2007.
|
|
10
|
Qiu CC, Lu H, Wu Q and Yu JY:
Retrospective analysis of clinical value of early diagnosis in
brainstem infarction by magnetic resonance imaging characteristics
with MRI and DWI. Chin J Trauma Disabil Med. 21:22–24. 2013.(In
Chinese).
|
|
11
|
Wu CH and Liu B: The fourth Chinese
academic conference on cerebrovascular disease. Chin Med News.
4:1996.
|
|
12
|
Chen H, Hong H, Liu D, Xu G, Wang Y, Zeng
J, Zhang R and Liu X: Lesion patterns and mechanism of cerebral
infarction caused by severe atherosclerotic intracranial internal
carotid artery stenosis. J Neurol Sci. 307:79–85. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang DW, Chu K, Ko SB, Kwon SJ, Yoon BW
and Roh JK: Lesion patterns and mechanism of ischemia in internal
carotid artery disease: A diffusion-weighted imaging study. Arch
Neurol. 59:1577–1582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tatu L, Moulin T, Bogousslavsky J and
Duvernoy H: Arterial territories of the human brain: Cerebral
hemispheres. Neurology. 50:1699–1708. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu JY, Wei JH and Wang JR: Stroke pattern
analysis in patients with middle cerebral artery occlusive disease.
J Apoplexy Nerv Dis. 22:246–247. 2005.
|
|
16
|
Niizuma K, Shimizu H, Takada S and
Tominaga T: Middle cerebral artery plaque imaging using 3-Tesla
high-resolution MRI. J Clin Neurosci. 15:1137–1141. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bang OY, Heo JH, Kim JY, Park JH and Huh
K: Middle cerebral artery stenosis is a major clinical determinant
in striatocapsular small, deep infarction. Arch Neurol. 59:259–263.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong KS, Gao S, Chan YL, Hansberg T, Lam
WW, Droste DW, Kay R and Ringelstein EB: Mechanisms of acute
cerebral infarctions in patients with middle cerebral artery
stenosis: A diffusion-weighted imaging and microemboli monitoring
study. Ann Neurol. 52:74–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li HF, Zhang X, Zhang Y, Pan XD, Zhao HQ
and Li H: Clinical and neuroradiological features of internal
watershed infarction and the occlusive diseases of carotid artery
system. Neurol Res. 32:1090–1096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hallevi H, Chernyshev OY, EI khoury R,
Soileau MJ, Walker KC, Grotta JC and Savitz SI: Intracranial
atherosclerosis is associated with progression of neurological
deficit in subcortical stroke. Cerebrovasc Dis. 33:64–68. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiao Y, Liu L and Dong ZJ: The subtype of
watershed infarction and the internalcarotid artery/middle cerebral
artery steno-occlusion. Inner Mongolia Med J. 46:1427–1429.
2014.
|
|
22
|
Tan H and Yang Zhi: Types of infarction in
patients with different degrees of middle cerebral artery stenosis
and occlusion. Chin J Nervous Mental Dis. 36:427–429. 2010.
|
|
23
|
Masuda J: A pathologic study of carotid
artery disease as an embolicsource. Jpn J Stroke. 23:347–350. 2001.
View Article : Google Scholar
|
|
24
|
Gao S, Huang J, Huang Y and Li S:
Infarction pathogenesis of atherosclerotic middle cerebral artery
stenosis. Chin J Neurol. 36:155–157. 2003.(In Chinese).
|
|
25
|
Shi MC, Wang SC, Zhou HW, Xing YQ, Cheng
YH, Feng JC and Wu J: Compensatory remodeling in symptomatic middle
cerebral atherosclerotic stenosis: A high-resolution MRI and
microemboli monitoring study. Neurol Res. 34:153–158.
2012.PubMed/NCBI
|
|
26
|
Ryoo S, Park JH, Kim SJ, Kim GM, Chung CS,
Lee KH, Kim JS and Bang OY: Branch occlusive disease: Clinical and
magnetic resonance angiography findings. Neurology. 78:888–896.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katakami N, Takahara M, Kaneto H, Shimizu
I, Ohno K, Ishibashi F, Osonoi T, Kashiwagi A, Kawamori R,
Shimomura I, et al: Accumulation of gene polymorphisms related to
plaque disruption and thrombosis is associated with cerebral
infarction in subjects with type 2 diabetes. Diabetes Care.
33:390–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caplan LR: Intracranial branch
atheromatous disease: A neglected, understudied, and underused
concept. Neurology. 39:1246–1250. 1989. View Article : Google Scholar : PubMed/NCBI
|