Introduction
Rhodomyrtus tomentosa (Aiton) Hassk., a
traditional herb medicine belongs to the family Myrtaceae. It is
native to Southeast Asia and a troublesome invader of native plant
communities in Florida. It is used for treatment of diarrhea
(1), gastrointestinal (2), urinary tract infections (3), anti-inflammation (4) and as an antiseptic wash for wounds
(5). In addition, it is used to
formulate skin whitening, anti-aging and skin beautifying agent
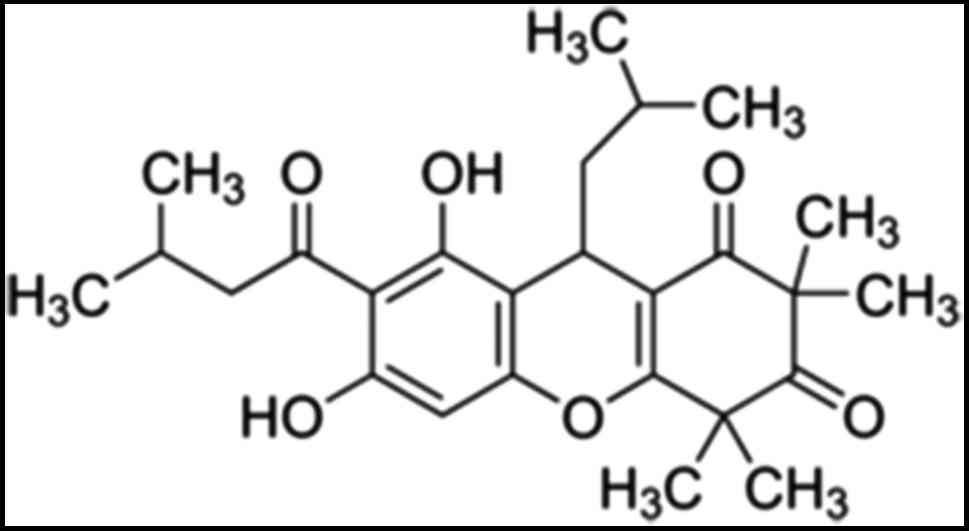
(6). Rhodomyrtone (Fig. 1), a pure compound in
acylphloroglucinol class isolated from Rhodomyrtus tomentosa
leaves. Previous studies have shown that rhodomyrtone displays
antibacterial activity against a wide range of gram-positive
bacteria such as Bacillus subtilis, Enterococcus faecalis,
Staphylococcus aureus, Staphylococcus epidermidis,
Streptococcus spp., and methicillin-resistant Staphylococcus
aureus (MRSA) (7–10). Moreover, some reports indicated that
rhodomyrtone stimulated pro- and anti-inflammatory cytokine
responses (11) and reduced
hyperproliferation and abnormal differentiation of HaCaT cells
(12). However, the anticancer
activity of rhodomyrtone on cancer cells has not been reported.
Skin cancer is the most common type of cancer in the
world, especially in white-skinned individuals. The increasing
incidence rate has been shown worldwide. There are two main types
of skin cancer: Melanoma or malignant melanoma (MM) and
non-melanoma skin cancer (NMSC), including the basal cell
carcinomas (BCCs) and squamous cell carcinomas (SCCs) (13,14). SCC
is the second most common skin cancer, accounting for about 20% of
NMSC cases. It is more common in older people. The major cause of
developing SCC is exposure to UV radiation, which causes cellular
damage (15,16). Current treatments of SCCs consist of
surgery, photodynamic therapy, radiation therapy, chemotherapy or
combination therapy, but these treatments are however
unsatisfactory. Thus, it is necessary to search for a new effective
therapeutic agent to inhibit SCCs.
In this study, we first investigated the effect of
rhomyrtone on cell proliferation and migration of A431 cells. It
was demonstrated that rhodomyrtone effectively inhibited growth and
migration associated with G1 arrest and apoptosis induction in
human epidermoid carcinoma A431 cells.
Materials and methods
Reagents and chemicals
Rhodomyrtone was dissolved in dimethylsulfoxide
(DMSO). MTT (3–4,5-dimethyl-2,5-diphenyl tetrazolium bromide), DMSO
and trypan blue were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Guava Cell Cycle® reagent was purchased from Merck
Millipore (Darmstadt, Germany) and Hoechst 33342 dye was purchased
from Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Rabbit monoclonal antibodies against caspase-7, cleave-PARP,
anti-mouse immunoglobulin G and anti-rabbit immunoglobulin wG
horseradish peroxidase-conjugated secondary antibodies were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA),
and mouse monoclonal antibody against β-actin was obtained from
Merck Millipore.
Cell culture
The human epidermoid carcinoma cells (A431) was
obtained from American Type Culture Collection (Manassas, VA, USA).
A431 cells were maintained as a monolayer in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum, 100 U/ml penicillin G and 100
µg/ml streptomycin (GE Healthcare Life Sciences, Chalfont, UK) and
3.7 g/l sodium bicarbonate into 75 cm2 cell culture
flasks and grown under a 95% humidity, 5% CO2 atmosphere
at 37°C.
Cell viability assay
The effect of rhodomyrtone on cell viability of A431
cells was determined by using MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide)
assay. Cells were seeded in 96-well plates at density of
7.0×103 cells/well and incubated overnight. Then, the
cells were treated with various concentrations (0–100 µg/ml) of
rhodomyrtone for 24 h. After treatment, 0.5 mg/ml MTT solution was
added to each well and the plates were further incubated for 2 h at
37°C. The supernatant was removed and 200 µl DMSO was added to each
well to solubilize water insoluble purple formazan crystals. The
absorbance was measured using an Epoch™ Microplate
spectrophotometer at 570 nm and the percentage of cell survival (%)
was calculated relative to the control. The half maximal inhibitory
concentration (IC50) value was calculated using the
software GraphPad Prism 3.03 (GraphPad Software, Inc., San Diego,
CA, USA). Cell viability assay was performed with three independent
experiments.
Wound healing assay
The effect of rhodomyrtone on cell motility was
determined by wound healing scratch assay (17). A431 cells were seeded in 6-well plate
and allowed to grow until 90% confluence. After that the monolayers
were scratched with a micropipette tip, the cellular debris was
removed by washing with PBS and treated with non-toxic
concentrations (0, 0.5, 1.5 µg/ml) of rhodomyrtone for 12 and 24 h.
The wound area was photographed with an inverted microscope
(Olympus, Tokyo, Japan) and measured by using Image J software.
Apoptotic cells staining with Hoechst
33342 dye
A431 cells were seeded in a 6-well plate at
3×105 cells/well and treated with 15 µg/ml rhodomyrtone
for 0, 3, 6, 9 and 12 h. The cells were then stained with Hoechst
33342 for 15 min. The apoptotic cells were observed using a
fluorescence microscope IX73 model (Olympus) with an ultraviolet
filter.
Cell cycle analysis
A431 cells were seeded in a 6-well plate at density
3×105 cells/well and then treated with 15 µg/ml
rhodomyrtone for 0, 3, 6, 9 and 12 h at 37°C. After treatment,
whole cells were collected and stained with Guava Cell
Cycle® reagent (Merck Millipore). The stained cells were
then sorted and analyzed for DNA content by a Guava easyCyte™ flow
cytometer and GuavaSoft™ software (Merck Millipore).
Western blot analysis
A431 cells were seeded in a 6-well plate at density
3×105 cells/well and then treated with 15 µg/ml
rhodomyrtone for 0, 3, 6 and 9 h at 37°C. After treatment, total
protein was extracted with RIPA lysis buffer (50 mM Tris-HCl, pH
7.4, 1% NP-40, 0.5% C24H39NaO4,
0.1% SDS, 150 western blotting was performed. Then, the membranes
were blocked with 5% skimmed-milk in TBS-Tween buffer for 1 h at
room temperature, and incubated with monoclonal antibody against
cleave-PARP, caspase-7 and β-actin overnight at 4°C. After
incubation with anti-mouse immunoglobulin G or anti-rabbit
immunoglobulin G horseradish peroxidase-conjugated secondary
antibodies, visualization of proteins was developed by using
Immobilon™ Western chemiluminescent HRP substrate (Merck Millipore)
and Chemiluminescent Imaging system (GeneGnome gel documentation;
Synoptics, Ltd., Cambridge, UK).
Statistical analysis
Data are expressed as mean ± SD of three independent
experiments. Statistical analysis was determined by using student's
t-test or one-way analysis of variant (ANOVA). Differences were
considered statistically significant at P<0.05. All statistical
analyses were performed using SPSS 17.0 software (SPSS, Inc.,
Chicago, IL, USA).
Results
Rhodomyrtone inhibited cell
proliferation of A431 cells
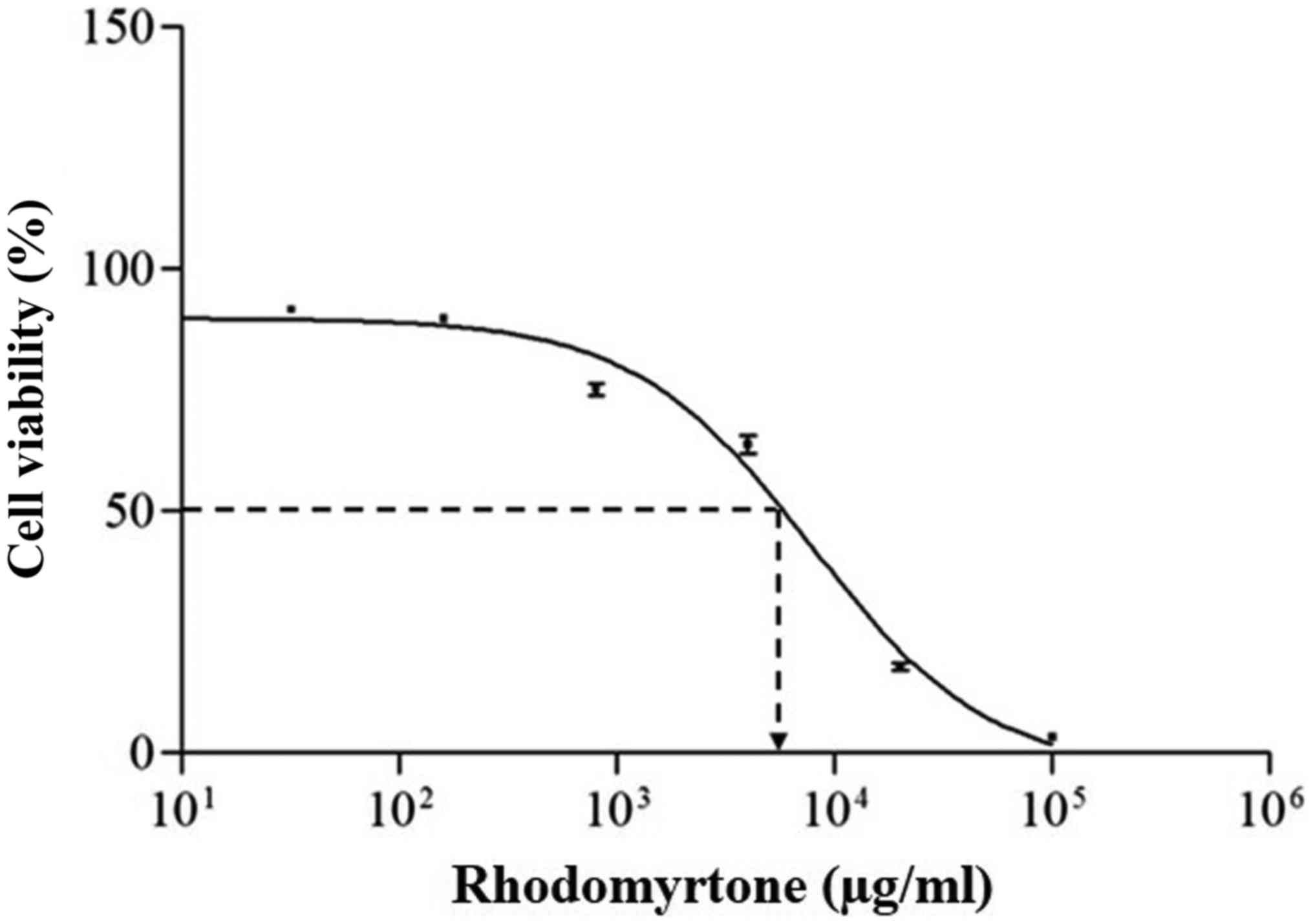
A431 cells were treated with various concentrations
of rhodomyrtone (0–100 µg/ml) for 24 h and cell proliferation was
analyzed by MTT assay. The result showed that rhodomyrtone
inhibited cell proliferation in a dose-dependent manner as shown in
Fig. 2. At high concentrations,
rhodomyrtone significantly inhibited A431 cell proliferation while
at the concentration lower than 3 µg/ml did not. The
IC50 of rhodomyrtone on A431 cells was 8.04±0.11 µg/ml.
This result indicated that rhodomyrtone inhibited cell
proliferation of A431 cells, thus 15 µg/ml rhodomyrtone was
selected for further study of apoptosis induction in A431
cells.
Rhodomyrtone inhibited A431 cell
migration
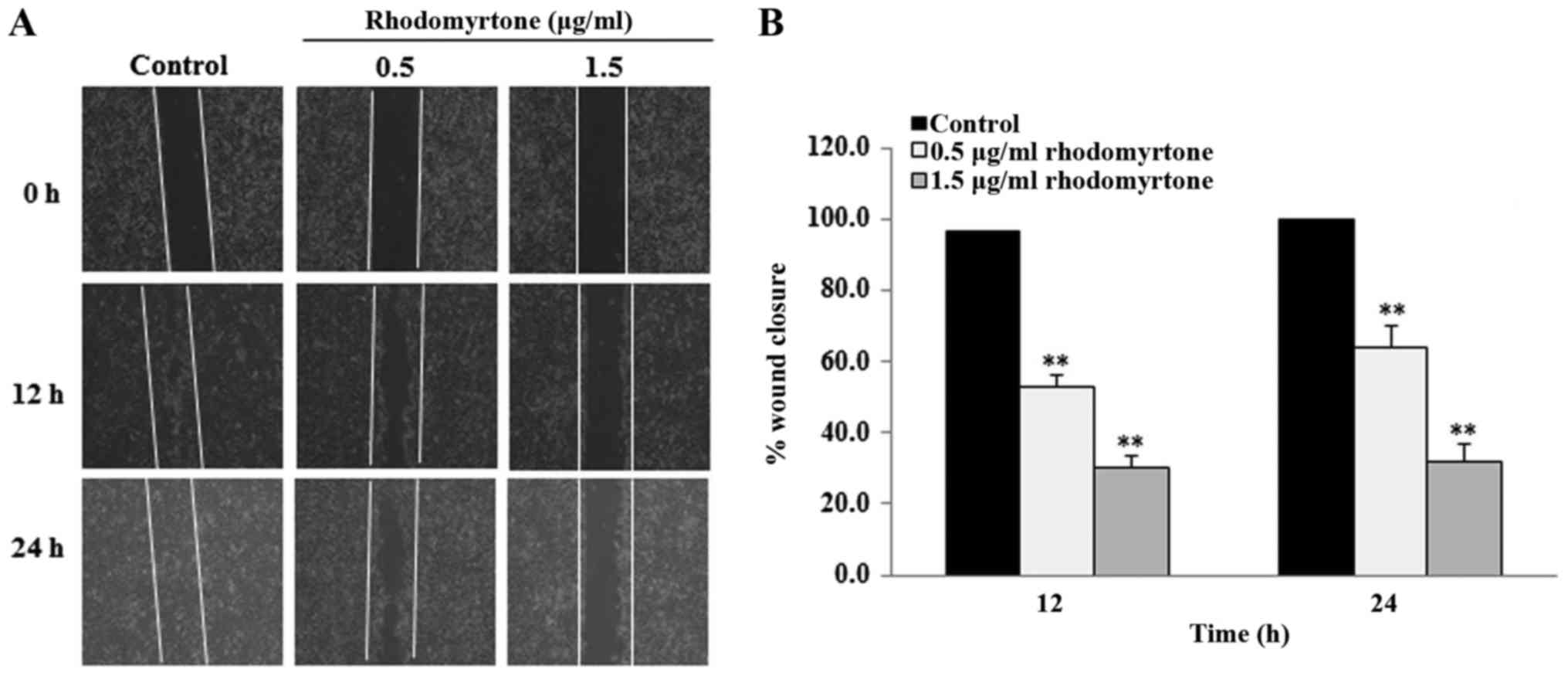
The effect of rhodomyrtone on cell migration was
determined by wound healing assay. The cell monolayers were
scratched with a micropipette tip to create the wound area. After
treatment with non-toxic concentrations (0.5 and 1.5 µg/ml) of
rhodomyrtone for 12 and 24 h, the wound closure was analyzed. The
result showed that rhodomyrtone could reduce migration of A431
cells to the wound area in a time- and dose-dependent manner when
compared with the untreated cells (Fig.
3A and B). Treatment with rhodomyrtone at 0.5, 1.5 µg/ml for 12
and 24 h inhibited 47.3, 36.2, 69.8 and 68.4% of cell migration,
respectively. These results revealed that rhodomyrtone
significantly inhibited the migration of A431 cells
(P<0.01).
Rhodomyrtone induced G1 arrest in A431
cells
To identify the mechanism of growth inhibitory
effect of rhodomyrtone, the cell cycle distribution of A431 cells
were determined by flow cytometry. The results showed that the
percentage of cell population in the G1 phase significantly
increased after treatment with rhodomyrtone (15 µg/ml) for 12 h
(P<0.05). Concomitantly, the percentage of cells in the S phase
significantly decreased after treatment for 9 and 12 h (P<0.05)
(Fig. 4A and B). These results
indicated that rhodomyrtone induced G1 phase arrest in A431
cells.
Rhodomyrtone induced cell apoptosis in
A431 cells
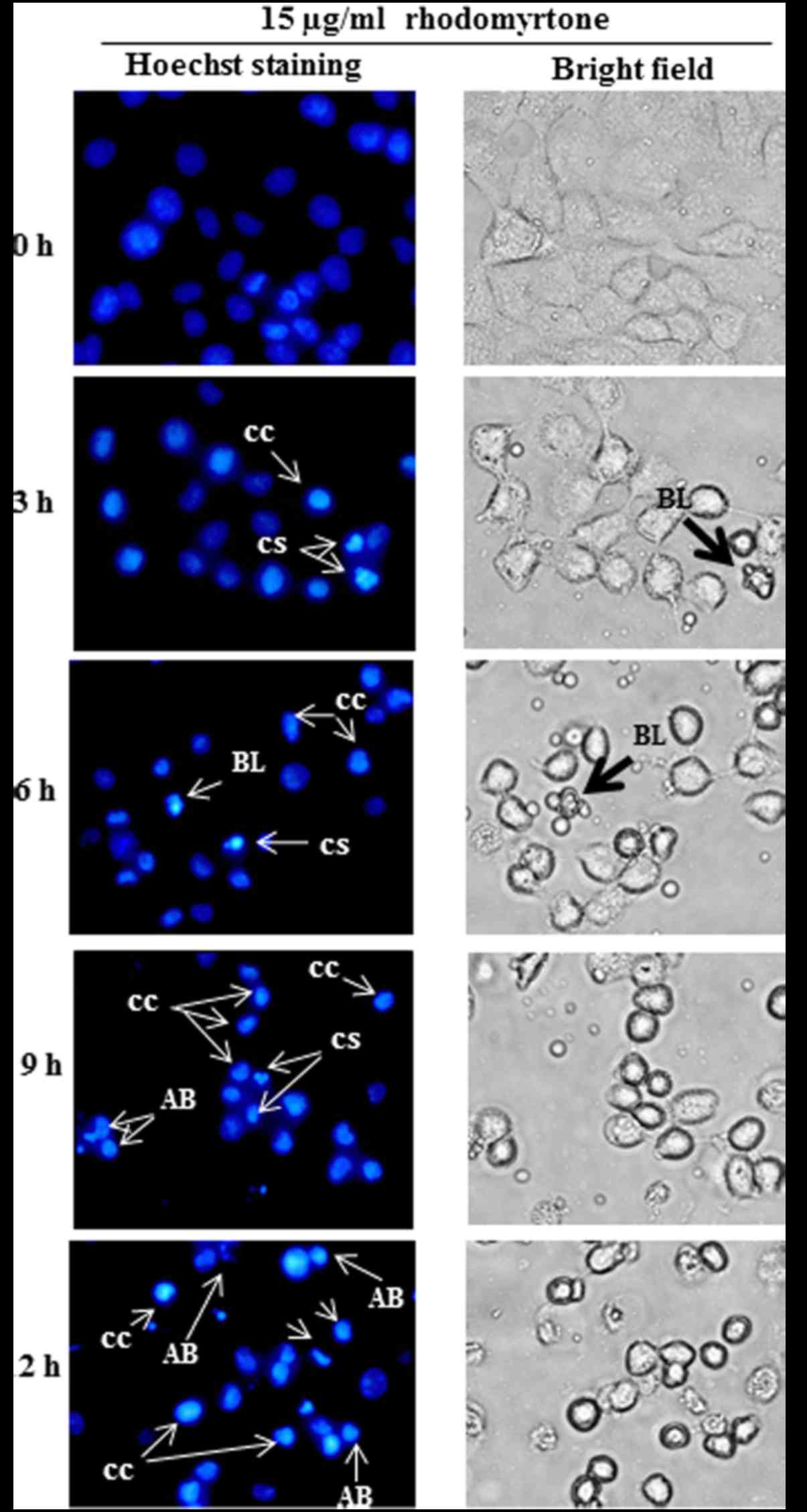
To observe the morphological changes of
treated-cells, Hoechst 33342 staining was performed and observed
under fluorescence microscope. After treatment with 15 µg/ml
rhodomyrtone for 3 and 6 h, the typical characteristic of apoptosis
such as membrane blebbing, chromatin condensation and cell
shrinkage were detected. The treated-cells (9 and 12 h) showed
chromatin condensation and cell shrinkage and apoptotic bodies. The
apoptotic body formation was predominant after treatment with 15
µg/ml rhodomyrtone for 12 h, whereas the untreated-cells (0 h)
showed normal nuclear and cellular morphology (Fig. 5). This result indicated that
rhodomyrtone could inhibit cell proliferation by inducing cellular
apoptosis in A431 cells.
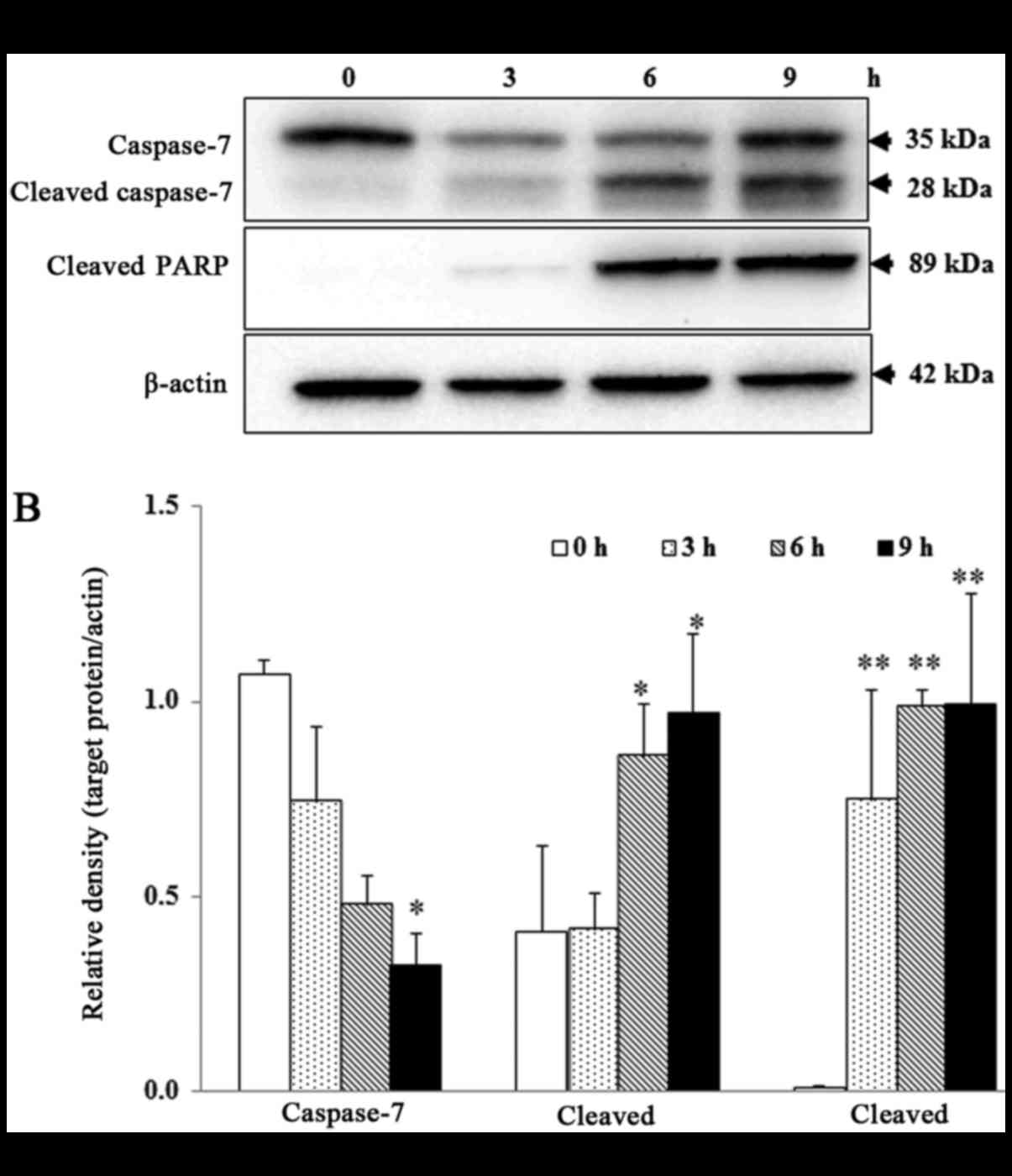
To determine whether rhodomyrtone induce apoptosis
in A431 cells, Western blotting was performed to detect the
activation of caspase-7 and poly ADP-ribose polymerase (PARP)
cleavage, substrate of caspase-7. As shown in Fig. 6A and B, the protein level of cleaved
caspase-7 and cleaved PARP significantly increased, while
pro-caspase-7 significantly decreased in a time-dependent manner
after treatment with 15 µg/ml rhodomyrtone (P<0.05).
Discussions
Rhodomyrtone, an acylphloroglucinol component
derived from the leaves of Rhodomyrtus tomentosa, has been
used as traditional herb medicine for a long time. The roots,
leaves and fruits were used to treat acute and chronic
gastroenteritic, stomachache, dysentery, hepatitis, gynaecopathy,
dysentery and diarrhoea (2,3). Previous reports have shown that
rhodomyrtone exhibited antibacterial activity on gram-positive
bacteria (7–10). However, the effects of rhodomyrtone
on cancer cells have not yet been reported. In this study, we first
demonstrated that rhodomyrtone exhibited an anticancer activity in
epidermoid carcinoma A431 cell.
The induction of apoptosis is one of the important
mechanisms for an anticancer agent. In this study, we investigated
the effects of rhodomyrtone on cell proliferation inhibition, cell
cycle arrest and apoptosis induction. The results showed that
rhodomyrtone dramatically inhibited A431 cell proliferation in a
dose-dependent manner correlated with cell cycle arrest and
apoptosis induction. The IC50 of rhodomyrtone against
A431 cell lines was found to be 8.04±0.11 µg/ml after 24 h
treatment as shown in Fig. 2. The
morphological characteristics of cells undergo apoptosis including
cell shrinkage, chromatin condensation, membrane blebbing and
formation of apoptotic bodies (18).
Our result demonstrated that rhodomytone induced apoptosis in A431
cells as shown in Fig. 5. Apoptosis
can be divided into two major pathways; the extrinsic,
caspase-dependent pathway and the mitochondria-dependent pathway
(19–21). Caspase-dependent pathways include
activation of caspase-8, −9 and −3/7 while mitochondrial pathways
are involved in the efflux of cytochrome c from mitochondria
to the cytoplasm, forming apoptosomes with Apaf-1 and caspase-9,
leading to the activation of caspase-3/7 and apoptosis (22). In our results, we also demonstrated
that rhodomyrtone induced apoptosis in A431 cells via
cleavage of caspase-7 and PARP as shown in Fig. 6A and B. Similar to previous reports
indicating that bioactive compound induced apoptosis in many types
of cancer by activation of caspase and PARP (23–27).
Cell cycle is an important mechanism involved with
cell growth. Cell cycle arrest and apoptosis are the effective
mechanisms that lead to cancer cell death (28). Previous studies showed that natural
bioactive compounds could inhibit human epidermoid carcinoma A431
cells growth by cell cycle arrest and apoptosis induction (24,27,29,30). In
the present study, we also investigated the effect of rhodomyrtone
on cell cycle of A431 cells. Flow cytometric analysis showed that
rhodomyrtone inhibited cell growth of A431 by inducing cell cycle
arrest at G1 phase in a time-dependent manner. G1 phase was
increased from 36.4% up to 51.2% and the cell populations in the S
phase was decreased from 20.4 to 12.0%, implying that cell
proliferation inhibition involved with cell cycle arrest (Fig. 4A and B). Similarly, previous study
reported that shikonin induced cell cycle arrest in G0/G1 phase and
induced apoptosis in A431 cells (24). Although, Soliman et al showed
that some acylphloroglucinol compounds exhibited antiproliferative
activity and inhibited cell cycle progression at S-phase in breast
cancer cell (31). The differences
between these results might be attributed to the bioactive compound
and cell types tested.
Metastasis is a complication of most cancers; it is
one of the leading causes of cancer-related death. Metastasis is a
multistep process leading to the formation of secondary tumors from
the primary tumor, which the cancer cells disseminate from the
primary tumor, migrate through the basement membrane, survive in
the circulatory system, invade into a secondary site and start to
proliferate (32). Cell migration of
cancer cells is an important step in metastasis process. In this
study, wound healing assay was performed to determine the
anti-migration effect of rhodomyrtone on A431 cells. Our result
showed that rhodomyrtone at non-toxic concentrations (0.5 and 1.5
µg/ml) significantly inhibits A431 cell migration in a
dose-dependent manner (Fig. 3A and
B).
In conclusion, we first showed that rhodomyrtone
induced apoptosis in A431 cells via cleavage of caspase-7 and PARP.
Furthermore, rhodomyrtone also caused cell cycle arrest at G1 phase
in a time-dependent manner. We also demonstrated that rhodomyrtone
dramatically inhibited cell migration in A431 cells at a non-toxic
concentration. These finding suggested that rhodomyrtone may be
used as an anticancer agent for skin cancer.
Acknowledgements
We would like to thank the Agricultural Research
Development Agency (Public Organization), Thailand, Research
Division, Faculty of Medicine, and Research Unit in ‘Biological
activities of Bioactive Compounds’, Srinakharinwirot
University.
References
|
1
|
Panthong A, Kanjanapothi D, Taesotiku T
and Taylor WC: Ethnobotanical review of medicinal plants from Thai
traditional books, Part II: Plants with antidiarrheal, laxative and
carminative properties. J Ethnopharmacol. 31:121–156. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei F: Manufacture of oral liquid
containing traditional Chinese medicine extract for treating
gynecopathy [Guangxi Huahong Pharmaceutical Co., Ltd, People's
Republic of China; Shanghai Fosun Pharmaceutical (Group) Co., Ltd],
Faming Zhuanli Shenqing Gongkai Shuomingshu. China Patent
CN1846715. Filed April 13, 2005; issued October 18. 2006.
|
|
3
|
Wei F: Manufacture of traditional Chinese
medicine composition for treating urinary tract infection Gungxi
Huahong Pharmaceutical Co., Ltd, People's Republic of China;
Shanghai Fosun Pharmaceutical (Group) Co., Ltd, Faming Zhuanli
Shenqing Gongkai Shuomingshu. China Patent CN1853687. Filed April
29, 2005; issued November 1. 2006.
|
|
4
|
Panthong A, Kanjanapothi D and Taylor WC:
Ethnobotanical review of medicinal plants from Thai traditional
books, Part I: Plants with anti-inflammatory, anti-asthmatic and
antihypertensive properties. J Ethnopharmacol. 18:213–228. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ong HC and Nordiana M: Malay ethno-medico
botany in Machang, Kelantan, Malaysia. Fitoterapia. 70:502–513.
1999. View Article : Google Scholar
|
|
6
|
Miyake Y and Nojima J: Skin Cosmetic and
Food/Drink for Cosmetrogical Use. Maruzen Pharmaceutical Co., Ltd.,
Hiroshima, Japan. Japan Patent JP2006199678A. Filed December 6,
2005; issued August 3. 2006.
|
|
7
|
Limsuwan S, Trip EN, Kouwen TR, Piersma S,
Hiranrat A, Mahabusarakam W, Voravuthikunchai SP, van Dijl JM and
Kayser O: Rhodomyrtone: A new candidate as natural antibacterial
drug from Rhodomyrtus tomentosa. Phytomedicine. 16:645–651.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Limsuwan S, Hesseling-Meinders A,
Voravuthikunchai SP, van Dijl JM and Kayser O: Potential antibiotic
and anti-infective effects of rhodomyrtone from Rhodomyrtus
tomentosa (Aiton) Hassk. On Streptococcus pyogenes as revealed
by proteomics. Phytomedicine. 18:934–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voravuthikunchai SP, Dolah S and
Charernjiratrakul W: Control of Bacillus cereus in foods by
Rhodomyrtus tomentosa (Ait.) Hassk. leaf extract and its
purified compound. J Food Prot. 73:1907–1912. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saising J, Ongsakul M and Voravuthikunchai
SP: Rhodomyrtus tomentosa (Aiton) Hassk. ethanol extract and
rhodomyrtone: A potential strategy for the treatment of
biofilm-forming staphylococci. J Med Microbiol. 60:1793–1800. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Srisuwan S, Tongtawe P, Srimanote P and
Voravuthikunchai SP: Rhodomyrtone modulates innate immune responses
of THP-1 monocytes to assist in clearing methicillin-resistant
Staphylococcus aureus. PLoS One. 9:e1103212014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chorachoo J, Saeloh D, Srichana T,
Amnuaikit T, Musthafa KS, Sretrirutchai S and Voravuthikunchai SP:
Rhodomyrtone as a potential anti-proliferative and apoptosis
inducing agent in HaCaT keratinocyte cells. Eur J Pharmacol.
772:144–151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scherer D and Kumar R: Genetics of
pigmentation in skin cancer-a review. Mutat Res. 705:141–153. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rigel DS: Cutaneous ultraviolet exposure
and its relationship to the development of skin cancer. J Amer Acad
Dermatol. 58:129–132. 2008. View Article : Google Scholar
|
|
15
|
Afaq F: Natural agents: Cellular and
molecular mechanisms of photoprotection. Arch Biochem Biophys.
50:144–151. 2011. View Article : Google Scholar
|
|
16
|
Bowden GT: Prevention of non-melanoma skin
cancer by targeting ultraviolet-B-light signaling. Nat Rev Cancer.
4:23–35. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu MK, Shih YW, Chien Chang TT, Fang LH,
Huang HC and Chen PS: α-Solanine inhibits human melanoma cell
migration and invasion by reducing matrix metalloproteinase-2/9
activities. Biol Pharm Bull. 33:1685–1691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wyllie AH, Kerr JF and Currie AR: Cell
death: The significance of apoptosis. Int Rev Cytol. 68:251–306.
1980. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 3:719–727. 2005. View Article : Google Scholar
|
|
20
|
Mehmet H: Caspases find a new place to
hide. Nature. 403:29–30. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Y: Mechanisms of caspase activation
and inhibition during apoptosis. Mol Cell. 9:459–470. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tancharoen W, Teeraaungkul S, Krajarng A,
Nilwarangoon S and Watanapokasin R: Apoptosis induction by
rafflesia kerrii meijer flower extract via caspase-dependent and
down-regulation of ERK signaling pathway in epidermoid carcinoma
cells. J Mod Med Chem. 1:37–42. 2013.
|
|
24
|
Tian R, Li Y and Gao M: Shikonin causes
cell-cycle arrest and induces apoptosis by regulating the
EGFR-NF-κB signaling pathway in human epidermoid carcinoma A431
cells. Biosci Rep 35: pii: e00189. 2015. View Article : Google Scholar
|
|
25
|
Zhu Y, Mao Y, Chen H, Lin Y, Hu Z, Wu J,
Xu X, Xu X, Qin J and Xie L: Apigenin promotes apoptosis, inhibits
invasion and induces cell cycle arrest of T24 human bladder cancer
cells. Cancer Cell Int. 13:542013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsang CM, Lau EP, Di K, Cheung PY, Hau PM,
Ching YP, Wong YC, Cheung AL, Wan TS, Tong Y, et al: Berberine
inhibits Rho GTPases and cell migration at low doses but induces G2
arrest and apoptosis at high doses in human cancer cells. Int J Mol
Med. 24:131–138. 2009.PubMed/NCBI
|
|
27
|
Pal CH, Sharma S, Elmets AC, Athar M and
Afaq F: Fisetin inhibits growth, induces G2/M arrest and
apoptosis of human epidermoid carcinoma A431 cells: Role of
mitochondrial membrane potential disruption and consequent caspases
activation. Exp Dermatol. 22:470–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
King KL and Cidlowski JA: Cell cycle
regulation and apoptosis. Annu Rev Physiol. 60:601–617. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li D, Wu LJ, Tashiro S, Onodera S and
Ikejima T: Oridonin-induced A431 cell apoptosis partially through
blockage of the Ras/Raf/ERK signal pathway. J Pharmacol Sci.
103:56–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yim NH, Kim A, Liang C, Cho WK and Ma JY:
Guibitang, a traditional herbal medicine, induces apoptotic death
in A431 cells by regulating the activities of mitogen-activated
protein kinases. BMC Complement Altern Med. 14:3442014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soliman MF, Fathy MM, Salama MM, Al-Abd
MA, Saber RF and El-Halawany AM: Cytotoxic activity of
acylphloroglucinols isolated from the leaves of Eucalyptus
cinerea F. Muell. ex Benth. cultivated in Egypt. Sci Rep.
4:54102014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bravo-Cordero JJ, Hodgson L and Condeelis
J: Directed cell invasion and migration during metastasis. Curr
Opin Cell Biol. 24:277–283. 2012. View Article : Google Scholar : PubMed/NCBI
|